Breaking Down Barriers to Detection and Care in Early-Age-Onset Colorectal Cancer in Canada
Abstract
:1. Introduction
- Increase awareness of the importance of EAOCRC, especially among primary care and the general public;
- Promote the earlier detection of EAOCRC in populations under age 50;
- Invest in research to optimize management pathways in EAOCRC.
1.1. Symposium Goals
1.2. Symposium Organization
1.3. Participants
1.4. Agenda
1.5. Emerging Themes
- The need for improvement in the early detection of CRC in younger populations;
- The application of leading models of care to meet the unique needs in the treatment of younger CRC patients.
2. Breaking Down the Barriers to the Early Detection of CRC in Younger Populations
2.1. CRC Screening Programs in Canada
2.2. Unique Needs of Younger Patients for Early Detection
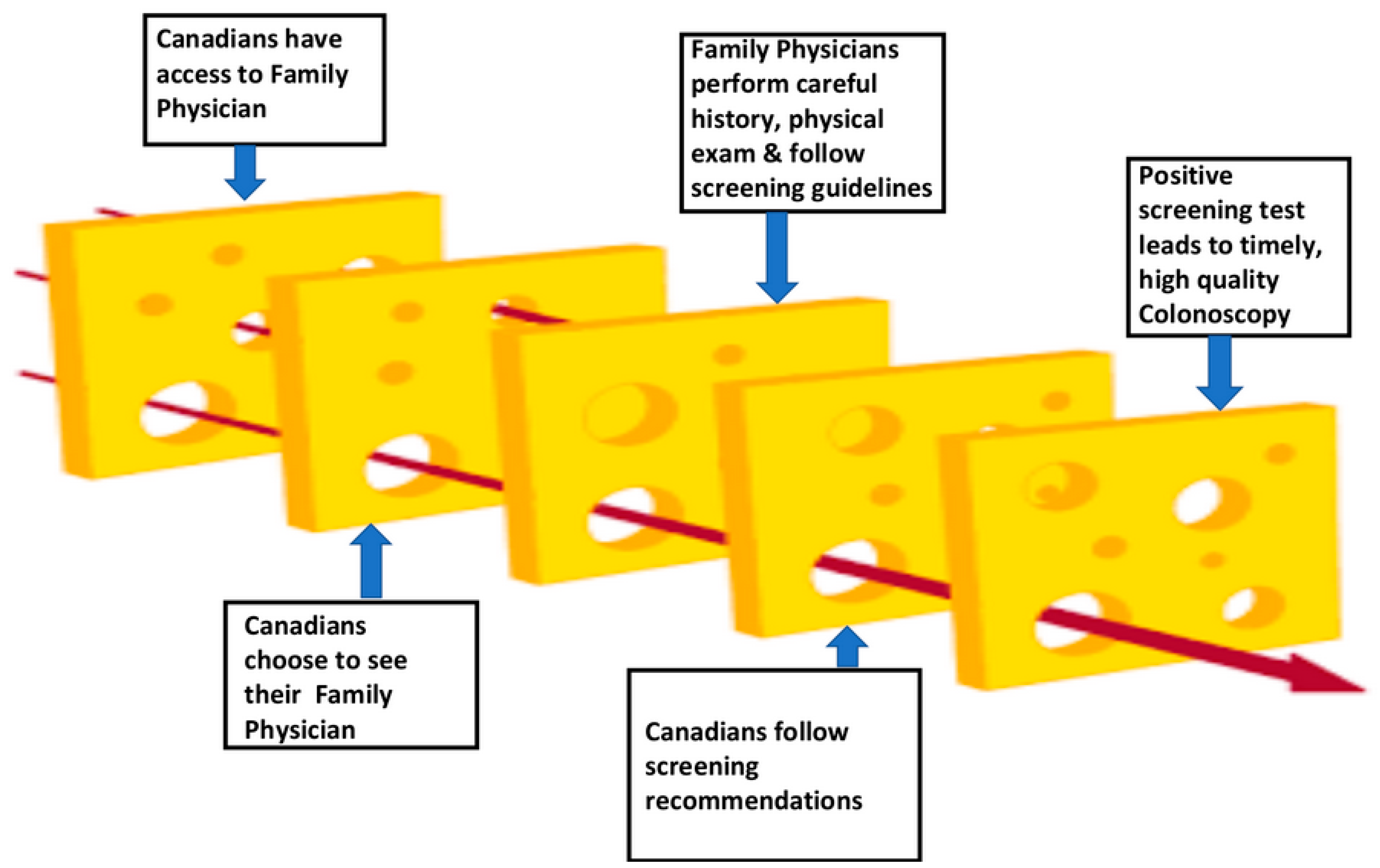
2.3. How Well Does the Current Crc Screening System Work for Canadians under Age 50?
Gaps in the Current System
2.4. Support for Reducing the CRC Screening Age to 45 in Canada
2.5. Future Advances: Promising Novel Screening Techniques
2.6. Advocating for Change: Lessons Learned from the U.S. Experience
3. Meeting the Unique Treatment Needs of Younger CRC Patients
3.1. Different Needs of EAOCRC Patients
3.2. Leading Practices in the United States and Canada
- The recognition of the very different needs of patients under age 50;
- Multidisciplinary management and research;
- A broad scope of medical and psychosocial services (cancer treatment, mental health, sexual health and fertility, genetics, social work);
- Dedicated resources to educate and coordinate services after referral;
- Collaboration between the centre and family physicians;
- Championship of the creation and ongoing operation of the program.
3.3. Checklist of Programs and Services for EAOCRC Patients
- Utilize a value-based healthcare approach to identify and prioritize the unique needs of younger patients across the continuum of care from prevention to treatment and survivorship/end-of-life.
- Implement multidisciplinary care pathways and integrated research programs
- ○
- Surgical, radiation, oncology treatments;
- ○
- Fertility and sexual health;
- ○
- Mental health;
- ○
- Genetic and biomarker testing;
- ○
- Family supports (child care, financial support);
- ○
- Education and collaboration with primary care and patient organizations;
- ○
- Access to research studies.
- Provide dedicated resources to educate patients and staff, to coordinate and navigate services after referral, and to liaise with external partners.
- Champion the value of a specialized unit serving younger CRC patients.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vera, M.A.; Gill, S.; Ashamalla, S.; Schiller, D.; Brenner, D.R.; Wong, C.; Wildgoose, P.; Esplen, M.J.; Lieu, C.; Fitzpatrick, R.; et al. Early-age-onset colorectal cancer in Canada: Evidence, issues and calls to action. Curr. Oncol. 2022, 29, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Statistics Advisory Committee; Canadian Cancer Society; Statistics Canada; Public Health Agency of Canada. Canadian Cancer Statistics 2021; Canadian Cancer Society: Toronto, ON, Canada, 2021; Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2021-statistics/2021-pdf-en-final.pdf (accessed on 3 January 2023).
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. CMAJ 2016, 188, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Canadian Partnership Against Cancer. Canadian Strategy for Cancer Control 2019–2029; Canadian Partnership Against Cancer: Toronto, ON, Canada, 2019; Available online: https://www.partnershipagainstcancer.ca/cancer-strategy/ (accessed on 3 January 2023).
- Brenner, D.R.; Heer, E.; Sutherland, R.L.; Ruan, Y.; Tinmouth, J.; Heitman, S.J.; Hilsden, R.J. National trends in colorectal cancer incidence among older and younger adults in Canada. JAMA Netw. Open 2019, 2, e198090. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jema, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Colorectal Cancer Alliance. “Never Too Young” Survey Report 2020; Colorectal Cancer Alliance: Washington, DC, USA, 2019; Available online: https://www.ccalliance.org/about/never-too-young/survey (accessed on 30 December 2022).
- O’Sullivan, D.E.; Hilsden, R.J.; Ruanc, Y.; Forbes, N.; Heitman, S.J.; Brenner, D.R. The incidence of young-onset colorectal cancer in Canada continues to increase. Cancer Epidemiol. 2020, 69, 101828. [Google Scholar] [CrossRef]
- Loomans-Kropp, H.A.; Umar, A. Increasing incidence of colorectal cancer in young adults. J. Cancer Epidemiol. 2019, 2019, 9841295. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Ruan, Y.; Cheung, W.Y.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Early-onset colorectal cancer incidence, staging, and mortality in Canada: Implications for population-based screening. Am. J. Gastroenterol. 2022, 117, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Reason, J. Human error: Models and management. BMJ 2000, 320, 768–770. [Google Scholar] [CrossRef]
- Korzinski, D. Doc Deficits: Half of Canadians Either Can’t Find a Doctor or Can’t Get a Timely Appointment with the One They Have; Angus Reid Institute [Internet]: Vancouver, BC, Canada, 2022; Available online: https://angusreid.org/canada-health-care-family-doctors-shortage (accessed on 3 January 2023).
- Scott, R.B.; Rangel, L.E.; Osler, T.M.; Hyman, N.H. Rectal cancer in patients under the age of 50 years: The delayed diagnosis. Am. J. Surg. 2016, 211, 1014–1018. [Google Scholar] [CrossRef]
- Colorectal Cancer Resource & Action Network (CCRAN). CCRAN EAOCRC Symposium [Internet]; Colorectal Cancer Resource & Action Network: Toronto, ON, Canada, 2022; Available online: https://videos.ccran.org/EAOCRC_Symposium/ (accessed on 17 August 2023).
- Roos, V.H.; Mangas-Sanjuan, C.; Rodriguez-Girondo, M.; Medina-Prado, L.; Steyerberg, E.W.; Bossuyt, P.M.M.; Dekker, E.; Jover, R.; van Leerdam, M.E. Effects of family history on relative and absolute risks for colorectal cancer: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk factors for early-onset colorectal cancer: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 20, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Murphy, C.C.; Lieu, C.H.; Hampel, H. Early age onset colorectal cancer. Adv. Cancer Res. 2021, 151, 1–37. [Google Scholar] [PubMed]
- Carroll, J.C.; Permaul, J.A.; Semotiuk, K.; Yung, E.M.; Blaine, S.; Dicks, E.; Warner, E.; Rothenmund, H.; Esplen, M.J.; Moineddin, R.; et al. Hereditary colorectal cancer screening: A 10-year longitudinal cohort study following an educational intervention. Prev. Med. Rep. 2020, 20, 101189. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.T.; Ahnen, D.J.; Schroy, P.C., III; Hampel, H.; Baxter, N.; Boland, C.R.; Burt, R.W.; Butterly, L.; Doerr, M.; Doroshenk, M.; et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: A state-of-the-science review. Cancer 2016, 122, 2633–2645. [Google Scholar] [CrossRef]
- Darvishian, M.; Moustaqim-Barrette, A.; Awadalla, P.; Bhatti, P.; Broet, P.; McDonald, K.; Murphy, R.A.; Skead, K.; Urquhart, R.; Vena, J.; et al. Provincial variation in colorectal cancer screening adherence in Canada; evidence from the Canadian Partnership for Tomorrow’s Health. Front Oncol. 2023, 13, 1113907. [Google Scholar] [CrossRef]
- Solbak, N.M.; Xu, J.-Y.; Vena, J.E.; Al Rajabi, A.; Vaseghi, S.; Whelan, H.K.; McGregor, S.E. Patterns and predictors of adherence to colorectal cancer screening recommendations in Alberta’s Tomorrow Project participants stratified by risk. BMC Public Health 2018, 18, 177. [Google Scholar] [CrossRef]
- Canadian Partnership Against Cancer. Wait Time to Follow-Up Colonoscopy. Available online: https://www.partnershipagainstcancer.ca/topics/colorectal-indicators-2017-2018/indicators/wait-times/ (accessed on 14 January 2023).
- Del Giudice, M.E.; Vella, E.T.; Hey, A.; Simunovic, M.; Harris, W.; Levitt, C. Guideline for referral of patients with suspected colorectal cancer by family physicians and other primary care providers. Can. Fam. Physician 2014, 60, 717–723. [Google Scholar]
- Alberta Health Services. High Risk Rectal Bleeding Pathway for Colorectal Cancer (CRC) Diagnosis. Available online: https://www.albertahealthservices.ca/assets/about/scn/ahs-scn-cancer-high-risk-rectal-bleeding-pathway.pdf (accessed on 19 June 2023).
- Alberta Health Services. Iron Deficiency Anemia (IDA) Pathway for Colorectal Cancer Diagnosis. Available online: https://www.albertahealthservices.ca/assets/about/scn/ahs-scn-cancer-iron-deficiency-anemia-pathway.pdf (accessed on 19 June 2023).
- Cancer Care Ontario. Colorectal Cancer Screening Recommendations Summary [Internet]; Cancer Care Ontario: Toronto, ON, Canada, 2019; Available online: https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/screening/resources-healthcare-providers/colorectal-cancer-screening-summary (accessed on 15 April 2023).
- Juul, F.E.; Cross, A.J.; Schoen, R.E.; Senore, C.; Pinsky, P.; Miller, E.; Segnan, N.; Wooldrage, K.; Wieszczy-Szczepanik, P.; Armaroli, P.; et al. 15-Year benefits of sigmoidoscopy screening on colorectal cancer incidence and mortality. A pooled analysis of randomized trials. Ann. Int. Med. 2022, 175, 1525–1533. [Google Scholar] [CrossRef]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.P.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.S.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. Colorectal cancer screening: An updated modeling study for the US Preventive Services Task Force. JAMA 2021, 325, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Peterse, E.F.P.; Meester, R.G.S.; Siegel, R.L.; Chen, J.C.; Dwyer, A.; Ahnen, D.J.; Smith, R.A.; Zauber, A.G.; Lansdorp-Vogelaar, I. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018, 124, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Kalyta, A.; Ruan, Y.; Telford, J.J.; De Vera, M.A.; Peacock, S.; Brown, C.J.; Donnellan, F.; Gill, S.; Brenner, D.B.; Loree, J.M. Association of reducing the recommended colorectal cancer screening age with cancer incidence, mortality, and costs in Canada using OncoSim. JAMA Oncol. 2023. [Google Scholar] [CrossRef]
- Brenner, D.R.; O’Sullivan, D.E.; Hilsden, R.J. Implications of the United States recommendations for early-age-at-onset colorectal cancer screening in Canada. Prev. Med. 2022, 155, 106923. [Google Scholar] [CrossRef] [PubMed]
- Azad, N.S.; Leeds, I.L.; Wanjau, W.; Eun, J.S.; Shin, E.J.; Padula, W.V. Cost-utility of colorectal cancer screening at 40 years old for average-risk patients. Prev. Med. 2020, 133, 106003. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.; Loree, J.M.; Kasi, P.M.; Parikh, A.R. Using circulating tumor DNA in colorectal cancer: Current and evolving practices. J. Clin. Oncol. 2022, 40, 2846–2857. [Google Scholar] [CrossRef]
- Yu, I.S.; Aubin, F.; Goodwin, R.; Loree, J.M.; Mather, C.; Sheffield, B.S.; Snow, S.; Gill, S. Tumor biomarker testing for metastatic colorectal cancer: A Canadian consensus practice guideline. Ther. Adv. Med. Oncol. 2022, 14, 1–29. [Google Scholar] [CrossRef]
- Aisner, D.L.; Riely, G.J. Non–small cell lung cancer: Recommendations for biomarker testing and treatment. Presentation. J Natl. Compr. Canc. Netw. 2021, 19, 610–613. [Google Scholar] [CrossRef]
- Tivey, A.; Britton, F.; Scott, J.A.; Rothwell, D.; Lorigan, P.; Lee, R. Circulating tumour DNA in melanoma-clinic ready? Curr. Oncol. Rep. 2022, 24, 363–373. [Google Scholar] [CrossRef]
- Zwezerijnen-Jiwa, F.H.; Sivov, H.; Paizs, P.; Zafeiropoulou, K.; Kinross, J. A systematic review of microbiome-derived biomarkers for early colorectal cancer detection. Neoplasia 2023, 36, 100868. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for colorectal cancer. US Preventive Services Task Force recommendation statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Howren, A.; Sayre, E.C.; Cheng, V.; Oveisi, N.; McTaggart-Cowan, H.; Peacock, S.; De Vera, M.A. Risk of anxiety and depression after diagnosis of young-onset colorectal cancer: A population-based cohort study. Curr. Oncol. 2022, 29, 3072–3081. [Google Scholar] [CrossRef] [PubMed]

| Session | Speakers |
|---|---|
| Day 1: Breaking down the barriers | |
| Symposium opening | Moderator: Monika Slovinec D’Angelo, Health Scientist, Conference Board of Canada Ms. Filomena Servidio-Italiano, President & CEO, CCRAN |
| Key learnings from CCRAN’s June 2021 Symposium | Mary A. De Vera, Epidemiologist, University of British Columbia Darren Brenner, Molecular Epidemiologist, University of Calgary |
| Breaking down the barriers to timely detection of colorectal cancer | Moderator: Monika Slovinec D’Angelo, Panel: Ms. Stephanie Florian, Widow of CRC patient, British Columbia Dr. Dan Schiller, Colorectal Surgical Oncologist, University of Alberta Ms. Julie Savard, Endoscopy Nurse Clinician, Jewish General Hospital, Montreal Dr. Lisa Del Giudice, Family Physician, Sunnybrook Health Sciences Centre, Toronto |
| Identifying and addressing the needs of younger crc patients | Moderator: Dr. Petra Wildgoose, Lead, Young Adult Colorectal Cancer Program, Sunnybrook Health Sciences Centre Panel: Mr. Marcelino Dolores, Patient Expert Dr. Mary Jane Esplen, Psychosocial Oncologist, University of Toronto Dr. Michael Raphael, GI Medical Oncologist Sunnybrook Health Sciences Center Dr. David Gurau, Reproductive Endocrinologist, Generation Fertility |
| Integrative therapies to address treatment-induced toxicities | Dr. Eric Marsden, Director, Marsden Centre for Excellence in Integrative Medicine |
| Promoting timely detection of CRC: what can we learn from the American experience? | Moderator: Dr. Robin McGee, Psychologist and Stage 4 Rectal Cancer Patient Panel: Mr. Andrew Spiegel, Executive Director, Global Colon Cancer Association (US) Ms. Martha Raymond, Executive Director, GI Cancers Alliance Inc. (US) Ms. Becky Selig, Director of Patient Education and Research, Fight Colorectal Cancer (US) Mr. Jason Gisser, Board Member, AYA Canada Ms. Dani Taylor, Manager of Programs and Partnerships, Young Adult Cancer Canada (YACC) Ms. Teresa Norris, President, HPV Global Action |
| Advancements in colorectal cancer treatments | Moderator: Dr. Scott Berry, GI Medical Oncologist, Kingston Health Sciences Centre Panel: Dr. Chris Lieu, GI Medical Oncologist, University of Colorado Cancer Center Dr. Kim Ma, Department of Haematology-Oncology, Jewish General Hospital, Montreal Dr. Eric Chen, GI Medical Oncologist, Princess Margaret Cancer Centre, Toronto Mr. Steve Slack, Rectal Cancer Patient |
| Day 2: Optimizing colorectal cancer care and outcomes | |
| What do we know and what have we learned from the June 2021 Symposium? | Dr. Mary A. De Vera, Epidemiologist, University of British Columbia Dr. Clarence Wong, Gastroenterologist, University of Alberta |
| Improving the EAOCRC patient care pathway | Moderator: Dr. Monika Slovinec D’Angelo, Health Scientist, Conference Board of Canada Panel: Ms. Hayley Painter, Young Adult mCRC Survivor Dr. Dan Schiller, Colorectal Surgical Oncologist, University of Alberta Ms. Julie Savard, Endoscopy Nurse Clinician, Jewish General Hospital, Montreal Dr. Anna Wilkinson, Family Physician, Ottawa Academic Family Health Team |
| Defining value and building system capacity for timely detection of EAOCRC | Moderator: Dr. Jill Tinmouth, Lead Scientist, ColonCancerCheck Program, Sunnybrook Research Institute, Toronto Panel: Dr. Petra Wildgoose, Lead, Young Adult Colorectal Cancer Program, Sunnybrook Health Sciences Centre, Toronto Dr. Yooj Ko, Medical Oncologist, Sunnybrook Health Sciences Centre, Toronto Ms. Eva Villalba, Executive Director, Quebec Cancer Coalition Mr. Jason Sutherland, Economist, Centre for Health Services and Policy Research, Vancouver Dr. Ian Bookman, Gastroenterologist, St. Joseph’s Health Centre, Hamilton Mr. Fred Horne, Horne and Associates, Edmonton |
| Best practices for systematically improving management of EAOCRC | Moderator: Dr. Sharlene Gill, GI Medical Oncologist, BC Cancer Agency Panel: Dr. Petra Wildgoose, Lead, Young Adult Colorectal Cancer Program, Sunnybrook Health Sciences Centre, Toronto Dr. Kimmie Ng, GI Medical Oncologist, Dana-Farber Cancer Institute, Boston Dr. Robin Mendelsohn, Gastroenterologist, Memorial Sloan Kettering Cancer Center, New York City Dr. Aparna Parikh, GI Oncologist, Massachusetts General Hospital Cancer Center, Boston Dr. Cathy Eng, GI Medical Oncologist, Vanderbilt-Ingram Cancer Centre, Nashville |
| Accessing innovations in CRC diagnostics and treatment | Moderator: Dr. Michael Raphael, GI Medical Oncologist, Sunnybrook Health Sciences Centre, Toronto Panel: Dr. Arvind Dasari, Medical Oncologist, MD Anderson Cancer Centre, Houston Dr. Aaron Pollett, Anatomic Pathologist, Mount Sinai Hospital, Toronto Dr. Stephanie Snow, Medical Oncologist, QEII Health Sciences Centre, Halifax Dr. Clarence Wong, Gastroenterologist, University of Alberta, Edmonton Dr. José Perea, Colorectal Surgeon, Jimenez Diaz Foundation University Hospital, Madrid, Spain Mr. Bill McGinley, Stage IV Colorectal Cancer Patient, Toronto |
| Key Lessons from U.S. Advocacy Experience | Canadian Policy Opportunities |
|---|---|
Data are crucial to support policy change:
| Conduct broad cost–benefit analyses:
|
Collaboration is essential:
| Advocate with pan-Canadian bodies and provinces
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raphael, M.J.; Wildgoose, P.; Servidio-Italiano, F.; De Vera, M.A.; Brenner, D.; D’Angelo, M.S.; McGee, R.; Berry, S.; Wong, C.; Gill, S. Breaking Down Barriers to Detection and Care in Early-Age-Onset Colorectal Cancer in Canada. Curr. Oncol. 2023, 30, 9392-9405. https://doi.org/10.3390/curroncol30110680
Raphael MJ, Wildgoose P, Servidio-Italiano F, De Vera MA, Brenner D, D’Angelo MS, McGee R, Berry S, Wong C, Gill S. Breaking Down Barriers to Detection and Care in Early-Age-Onset Colorectal Cancer in Canada. Current Oncology. 2023; 30(11):9392-9405. https://doi.org/10.3390/curroncol30110680
Chicago/Turabian StyleRaphael, Michael J., Petra Wildgoose, Filomena Servidio-Italiano, Mary A. De Vera, Darren Brenner, Monika Slovinec D’Angelo, Robin McGee, Scott Berry, Clarence Wong, and Sharlene Gill. 2023. "Breaking Down Barriers to Detection and Care in Early-Age-Onset Colorectal Cancer in Canada" Current Oncology 30, no. 11: 9392-9405. https://doi.org/10.3390/curroncol30110680
APA StyleRaphael, M. J., Wildgoose, P., Servidio-Italiano, F., De Vera, M. A., Brenner, D., D’Angelo, M. S., McGee, R., Berry, S., Wong, C., & Gill, S. (2023). Breaking Down Barriers to Detection and Care in Early-Age-Onset Colorectal Cancer in Canada. Current Oncology, 30(11), 9392-9405. https://doi.org/10.3390/curroncol30110680






