Abstract
Aim: The immune system plays an important role in tumor development and treatment. In this study, we aimed to determine the relationships among the expressions of PD-L1, CD3, CD8, MMR proteins, clinicopathological features, and prognosis of CRC. Methods: Immunohistochemistry was used to determine the expression of PD-L1, CD3, and CD8 in 771 patients with CRC. Results: The expression of PD-L1 in TC was related to the right colon, adenocarcinoma, and dMMR, and in IC, it was related to younger CRC patients and the TNM stage. The expression of CD3 and CD8 in tumor-infiltrating lymphocytes was related to lymph node metastasis and the TNM stage. The expression of PD-L1 in TC and IC was correlated with the infiltration of CD3+ and CD8+ lymphocytes. Univariate survival analysis showed that the expression of PD-L1 in TC, IC, and dMMR was related to a better prognosis. Multivariate survival analysis showed that age, TNM stage, and dMMR were independent prognostic factors for CRC. The OS of the chemotherapy was significantly higher than that of the non-chemotherapy in III-IV TNM stage patients; CRC patients with positive PD-L1 expression in TC or IC and dMMR did not benefit from chemotherapy. Conclusions: PD-L1 expression in TC and IC was closely related to the density of CD3 and CD8 infiltration in tumor-infiltrating lymphocytes. The expression of CD3 and CD8 in tumor-infiltrating lymphocytes and the expression of PD-L1 in IC were linked to the TNM stage of CRC patients. PD-L1 expression in TC and IC and MMR status may act as an important biomarker for guiding the postoperative treatment of III-IV TNM stage CRC patients.
1. Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed malignancies and the leading cause of death for cancer patients in China [1]. The prognosis of CRC patients has markedly improved due to advances in surgical techniques, radiotherapy, and chemotherapy. However, the recurrence and metastasis rates in CRC patients remain high. It is necessary to further explore novel therapies for CRC treatment, and one possibility is an immunotherapeutic approach to correct abnormal immunity [2].
Immunotherapy has achieved remarkable results in the field of tumor therapy by reactivating the ability of the host immune system to kill malignant tumor cells. The most widely research are immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen-4 (CTLA-4), which have shown significant benefits in the treatment of advanced CRC [3]. Currently, ICIs, such as PD-1 inhibitors nivolumab and pembrolizumab, have been approved for the treatment of metastatic cancers with DNA mismatch repair defects (dMMR) and high microsatellite instability (MSI-H) [4]. The KEYNOTE-164 (NCT02460198) clinical trial confirmed the efficacy of pembrolizumab in patients with dMMR/MSI-H metastatic CRC, leading to the first entry of the era of immunotherapy for dMMR/MSI-H metastatic CRC [5]. In addition, recent studies have found that first-line use of nivolumab combined with low-dose ipilimumab has strong and long-lasting clinical benefits and good tolerance in dMMR/MSI-H metastatic CRC patients [6]. In recent years, immunotherapy has become a research hotspot in the field of tumor therapy, and various targeted immune microenvironment treatment strategies, including ICIs, have achieved certain results in CRC. However, it cannot be ignored that the efficacy of immunotherapy in CRC still needs further improvement.
The PD-1/PD-L1 axis serves as an immune checkpoint, is upregulated in many tumors and their microenvironments, and plays an important role in tumor immune escape by suppressing T helper 1 cell (Th1)-type cytotoxic immune responses [7]. The combination of PD-L1 and programmed death-ligand 2 (PD-L2) with PD-1 induces antigen-stimulated lymphocyte proliferation and the down-regulation of cytokines (such as IFN-γ and IL-2), leading to lymphocyte loss and immune tolerance [8,9,10]. Studies have reported that malignant tumors were controlled by interfering with PD-1/PD-L1 interaction to restore the ability of the innate immune system [11,12,13,14]. The detection of PD-L1 protein expression via immunohistochemistry has a certain guiding value for the treatment of ICIs [15,16,17,18]. However, the results of studies on the relationships between PD-L1 and clinical characteristics and prognosis are not fully consistent [19,20,21].
In recent years, the important role of the immune system in tumor development and treatment has been recognized. Cluster of differentiation 3 (CD3) and T cell receptor (TCR) form a TCR/CD3 complex that plays a key role in T cell stimulation signal transduction and T cell activation [22]. Studies have shown that CD3+ tumor-infiltrating lymphocytes have better survival rates in hepatocellular carcinoma [23]. Infiltrating cytotoxic T cells that were positive for the transmembrane glycoprotein cluster of differentiation 8 (CD8) were a prognostic factor in CRC [24]. In addition, CD8+ T cells directly attack cancer cells and play an important role in anti-tumor immunity [25,26]. However, continuous exposure to tumor antigens can lead to lymphocyte failure, lymphocyte dysfunction, and poor clinical outcomes [27,28]. Studies have shown that the expression of PD-L1 is often accompanied by the infiltration of tumor-infiltrating lymphocytes in CRC cells [16]. Other studies reported that the expression of PD-L1 on tumor cells might contribute to negative regulation against tumor-infiltrating lymphocytes in non-small cell lung cancer [29]. Deeply exploring the functional characteristics and key molecules of different types of immune cells in the immune microenvironment of colorectal cancer, discovering more therapeutic targets with clinical application value, and exploring better combination therapy strategies will help further improve the effectiveness of immunotherapy while guiding clinical physicians to provide more personalized treatment for colorectal cancer patients.
Mismatch repair (MMR) proteins play an important role in finding and repairing DNA mismatch and maintaining genome stability [30]. The abnormal function of MMR proteins is mainly caused by the deletion of MLH1, MSH2, PMS2, and MSH6. Microsatellite instability (MSI) is caused by the loss of function of DNA MMR proteins. Its molecular characteristics are considered to be important gene markers, which account for 10%~20% of all colorectal cancer cases [31]. Current research shows that the MMR protein status is related to the occurrence, prognosis, and immunotherapy of CRC [32,33]. Therefore, in the present study, we investigate the clinical relevance and prognostic significance of PD-L1, CD3, CD8, and MMR protein expression in CRC.
2. Material and Methods
2.1. Patients and Tissue Specimens
A total of 771 human primary CRC tissue specimens from 2005 to 2015 without preoperative chemotherapy, radiotherapy, and immunotherapy were collected in this study, including 734 colorectal adenocarcinomas and 37 colorectal mucinous adenocarcinomas. Of all patients, 430 were males and 341 were females, with ages ranging from 17 to 93 years and a median age of 61 years. All patients were diagnosed with CRC by two pathologists according to the World Health Organization histological tumor classification criteria. There were 79 cases with high histologic grades and 692 cases with low histologic grades. There were 363 cases with lymph node metastasis and 408 cases without lymph node metastasis. From these, 335 and 436 cases exhibited I–II TNM stages and III–IV TNM stages, respectively. Postoperative patients with III–IV TNM stage underwent 5-fluorouracil-based chemotherapy. The patients were beginning follow-up after surgery, using a combination of outpatient review, hospitalization, and telephone communication. Follow-up was performed once every six months. Complete follow-up data were obtained in 569 cases.
2.2. Tissue Microarray and Immunohistochemistry
Tissue microarray (TMA) was constructed using the specimens from paraffin-embedded blocks of CRC primary tumors. Each case in the TMA was represented in 5 cores, 1 mm in size for each core. Immunoreaction for PD-L1 (SP142), CD3 (LN10), and CD8 (SP16) was purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China. Antigen–antibody reactions were visualized using a Ventana OptiView™ Amplification kit, followed by a Ventana OptiView™ DAB IHC Detection Kit (Ventana, AZ, USA). Counterstaining was performed using Ventana Hematoxylin II, followed by bluing reagent. Immunohistochemistry was performed using the BenchMark® ULTRA (Ventana, AZ, USA). Positive and negative controls were stained concurrently and showed appropriate immunostaining.
2.3. Immunoreactivity Evaluation
Immunohistochemical staining of PD-L1 in tumor cells (TC) and immune cells (IC) was performed using SP142, while immunohistochemical staining of tumor-infiltrating lymphocytes was performed using CD3 and CD8. A positive (+) expression of PD-L1 was given if ≥1% of TC or IC showed convincing cell membrane staining. A negative (−) expression of PD-L1 is defined as <1% of TC or IC showing cell membrane staining. A positive (+) expression of CD3 and CD8 was given if ≥5% of tumor-infiltrating lymphocytes showed convincing cell membrane staining, while <5% of tumor-infiltrating lymphocyte stainings were negative (−). The result was judged by two pathologists who were blinded to the clinical patient data.
2.4. MMR Protein Detection
MMR protein antibodies MLH1 (ES05), PMS2 (EP51), MSH2 (RED2), and MSH6 (EP49) working solutions were purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China. The four antibodies are all nuclear staining. Among the four antibodies, as long as one antibody staining result is negative, it is called mismatch repair deficient (dMMR), which is characterized by high-frequency microsatellite instability (MSI-H). Those with positive expression of all four antibodies were judged to be mismatch repair proficient (pMMR), which is characterized by low levels of microsatellite instability (MSI-L) or microsatellite stability (MSS).
2.5. Statistical Analysis
Data were analyzed using the SPSS 17.0 software package (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA). Two sets of measurement data were compared using χ2 test. Prognosis was estimated by the Kaplan–Meier, univariate, and Cox proportional hazard regression analysis methods. p values less than 0.05 were considered statistically significant.
3. Result
3.1. Expression of PD-L1, CD3/CD8 Proteins, and MMR in 771 CRCs
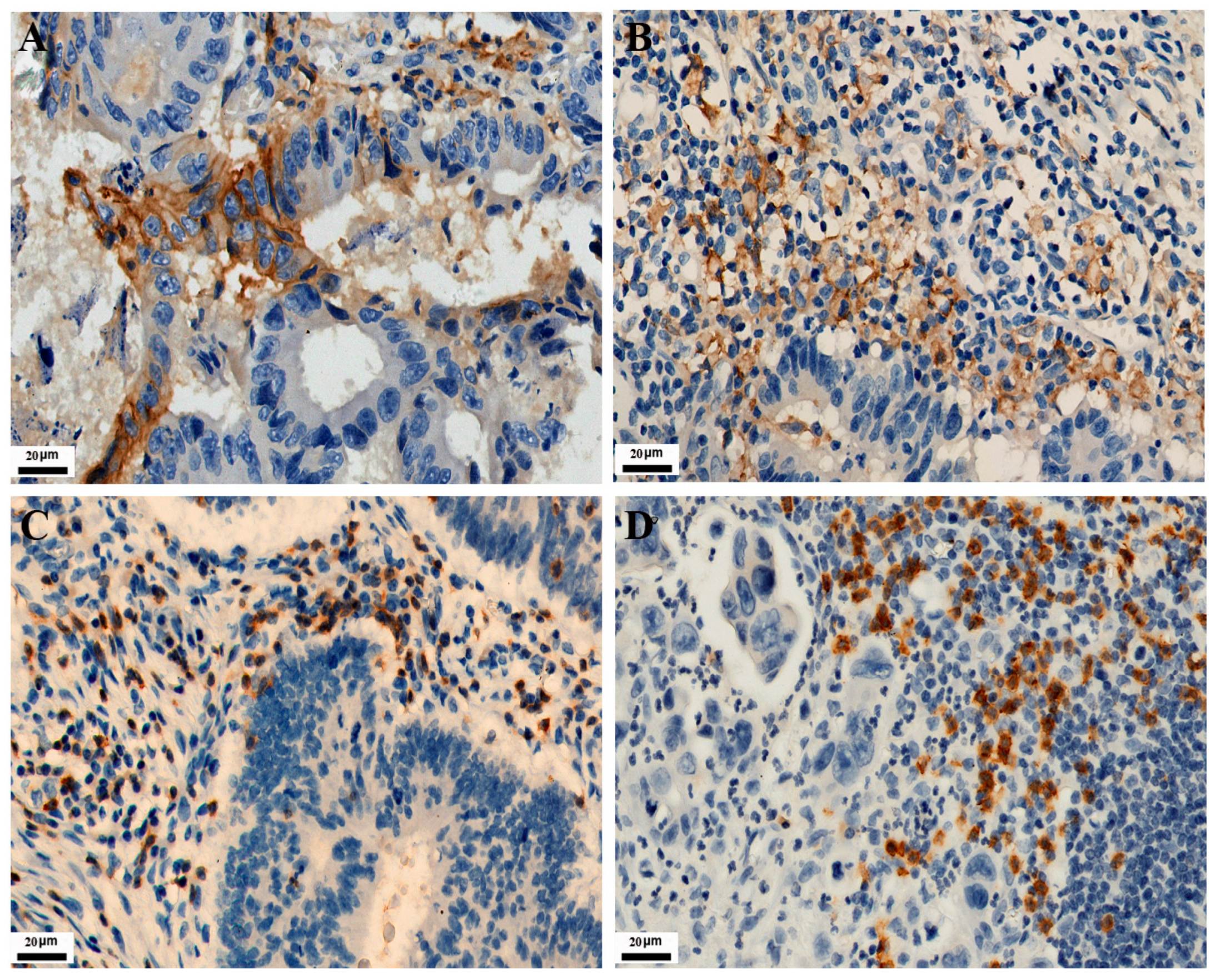
As shown in Figure 1, PD-L1 expression in TC and IC is localized on the cell membrane. Among the 771 CRC cases, the positive expression number of PD-L1 in TC was 120, and the positive expression number of PD-L1 in IC was 282. Their positive expression rates were 15.6% and 36.6%, respectively. The expressions of CD3 and CD8 in tumor-infiltrating lymphocytes were localized on the cell membrane. Their positive expression number was 138 and 204, with positive expression rates of 17.9% and 26.5%, respectively. As shown in Figure 2, MMR protein expression in TC is localized on the nuclear. Immunohistochemical staining showed 93 positive cases, with an incidence of dMMR of 12.1% in the patients.
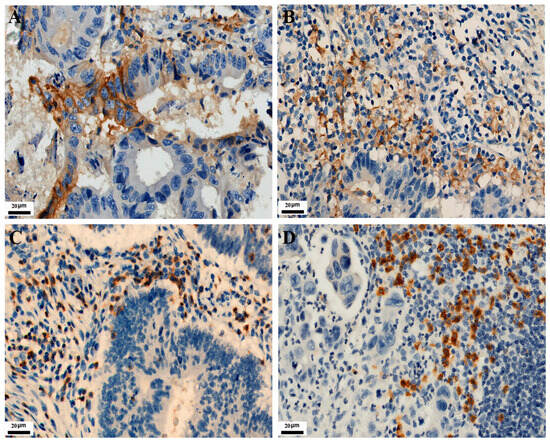
Figure 1.
Representative images of IHC staining for PD-L1, CD3, and CD8 expression in CRC (IHC, 400×). Positive PD-L1 staining is shown in TC and IC (A,B), respectively. Positive CD3 and CD8 staining are shown in tumor-infiltrating lymphocytes (C,D), respectively.
3.2. The Correlation of PD-L1 and CD3/CD8 Expression with MMR Proteins Expression and Clinicopathological Parameters in 771 CRCs
As shown in Table 1, PD-L1 expression in TC mostly occurred in the right colon, adenocarcinoma, and dMMR (p < 0.05) and had no significant correlation with patient age, gender, histological grade, lymph node metastasis, and TNM stage (p > 0.05). The expression of PD-L1 in IC was related to the younger CRC patients and I-II TNM stage of the tumor (p < 0.05) but was not significantly related to gender, tumor location, histological grade, histological type, lymph node metastasis, and MMR protein expression (p > 0.05). The expressions of CD3 and CD8 were significantly related to lymph node metastasis and the III-IV TNM stage (p < 0.05), and the expression of CD8 in the right colon is higher than that in the left colon (p < 0.05), while there was no significant correlation between the expression of both and age, gender, histological grade, histological type, and MMR proteins expression (p > 0.05). In addition, the high expression of PD-L1 in TC and IC was related to the high expression of CD3 and CD8 (p < 0.05), and the high expression of CD3 was also significantly related to the high expression of CD8 (p < 0.05).

Table 1.
Expression of PD-L1, CD3, and CD8 and their correlation with clinicopathological parameters and MMR status in 771 CRC.
3.3. The Relationship among the Expression of PD-L1, CD3, CD8, and MMR and the Prognosis of Postoperative CRC Patients
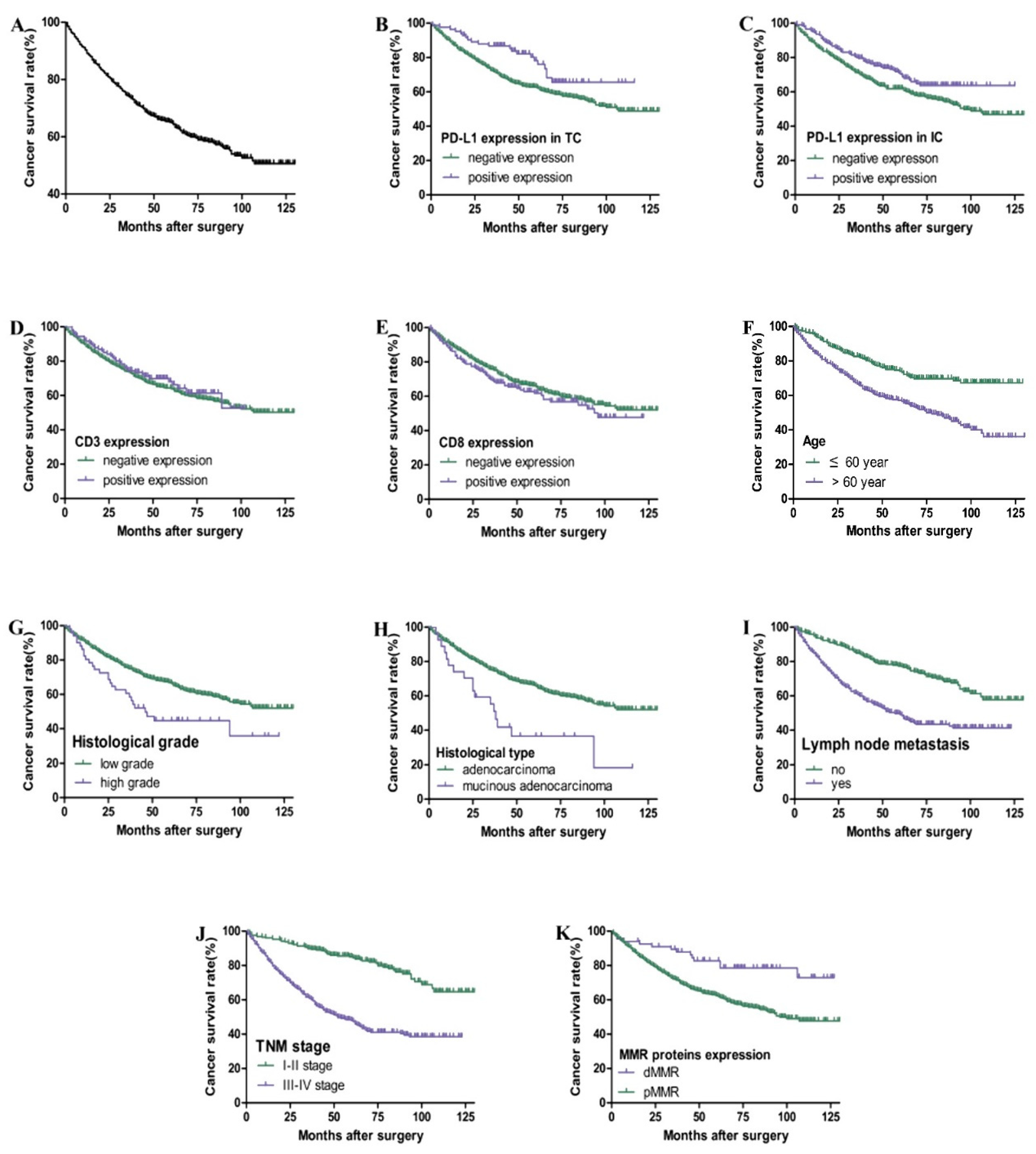
As shown in Table 2 and Figure 3, the total 3-year and 5-year survival rates for 569 cases of postoperative CRC patients are 74% and 65%, respectively. Kaplan–Meier survival curve and Cox univariate survival analysis showed that the positive expression of PD-L1 in TC (HR = 0.572), IC (HR = 0.696), and dMMR (HR = 0.412) was associated with a better prognosis. The patients with a higher age (HR = 2.091), higher histological grade (HR = 1.770), mucinous adenocarcinoma (HR = 2.247), lymph node metastasis (HR = 2.360), and III-IV TNM stage (HR = 3.503) had a worse prognosis (p < 0.05). However, there was no significant correlation among gender, tumor location, CD3 and CD8 expression, and prognosis (p > 0.05). Cox multivariate regression analysis showed that age, TNM stage, and dMMR were independent prognostic factors (HR = 2.237, 4.437, and 0.429. p all <0.05).

Table 2.
Univariate and multivariate analysis of variables on overall survival in 569 CRC.
Table 2.
Univariate and multivariate analysis of variables on overall survival in 569 CRC.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 2.091 | 1.582–2.764 | 0.000 * | 2.237 | 1.685–2.968 | 0.000 * |
| Gender | 0.886 | 0.681–1.152 | 0.365 | 0.916 | 0.803–1.488 | 0.571 |
| Tumor location | 1.114 | 0.832–1.492 | 0.467 | 1.093 | 0.803–1.488 | 0.571 |
| Histological grade | 1.770 | 1.191–2.631 | 0.005 * | 1.405 | 0.753–2.624 | 0.285 |
| Histological type | 2.247 | 1.368–3.689 | 0.001 * | 1.321 | 0.609–2.864 | 0.481 |
| Lymph node metastasis | 2.360 | 1.809–3.080 | 0.000 * | 0.774 | 0.531–1.128 | 0.182 |
| TNM stage | 3.503 | 2.587–4.744 | 0.000 * | 4.437 | 2.898–6.792 | 0.000 * |
| CD3 | 0.892 | 0.624–1.275 | 0.530 | 0.814 | 0.551–1.202 | 0.300 |
| CD8 | 1.172 | 0.876–1.568 | 0.285 | 1.217 | 0.888–1.666 | 0.222 |
| PD-L1 in TC | 0.572 | 0.365–0.897 | 0.015 * | 0.713 | 0.439–1.158 | 0.172 |
| PD-L1 in IC | 0.696 | 0.520–0.932 | 0.015 * | 0.826 | 0.595–1.148 | 0.256 |
| MMR proteins expression | 0.412 | 0.240–0.709 | 0.001 * | 0.429 | 0.245–0.750 | 0.003 * |
* p < 0.05.
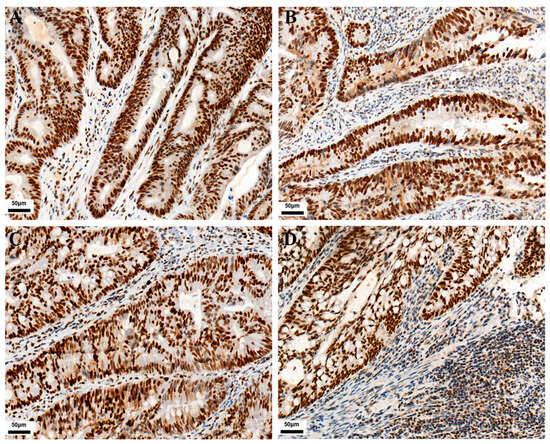
Figure 2.
Representative images of MMR immunohistochemical staining in CRC (IHC, 200×). (A) MLH1 positive expression. (B) MSH2 positive expression. (C) MSH6 positive expression. (D) PMS2 positive expression.
Figure 2.
Representative images of MMR immunohistochemical staining in CRC (IHC, 200×). (A) MLH1 positive expression. (B) MSH2 positive expression. (C) MSH6 positive expression. (D) PMS2 positive expression.
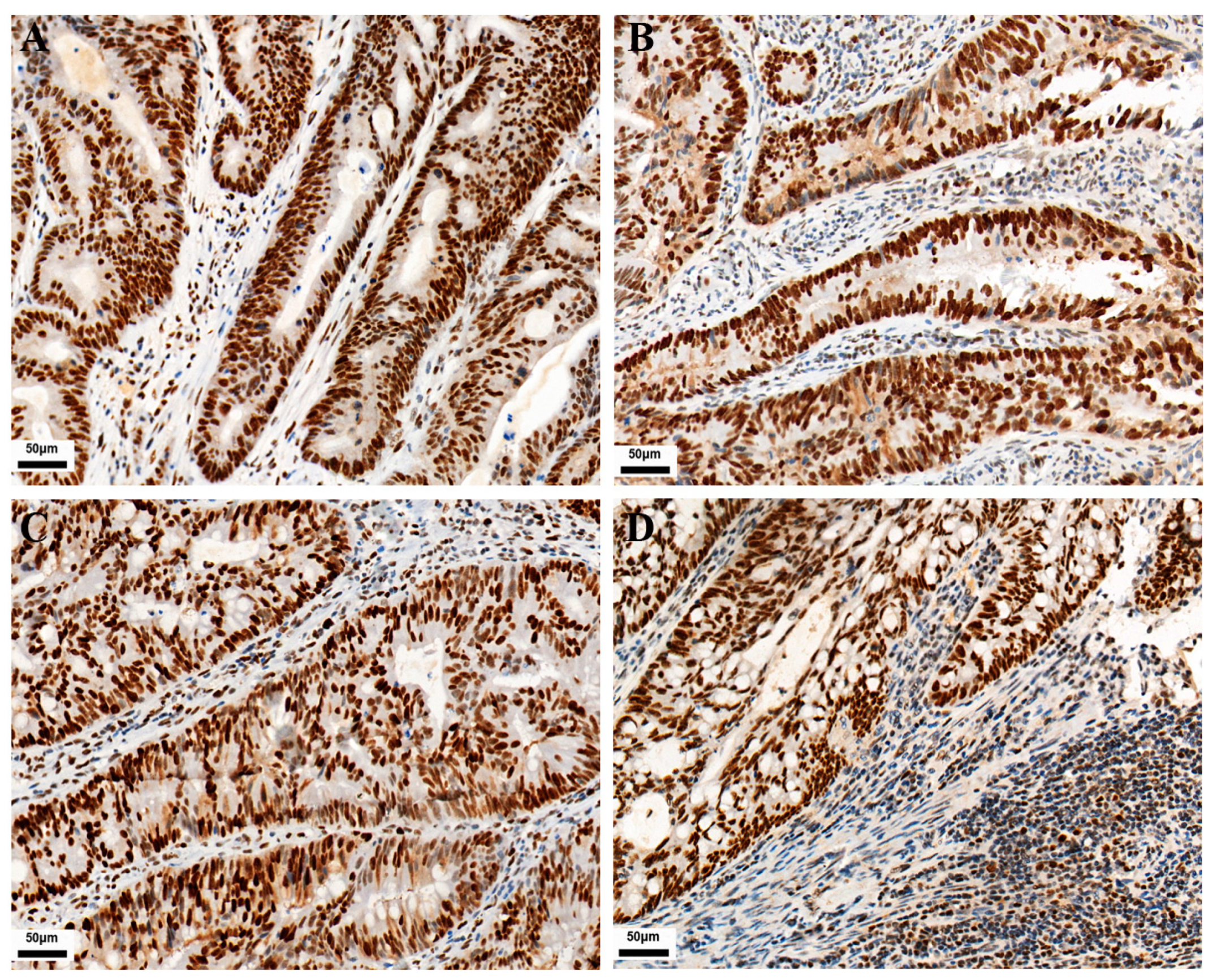
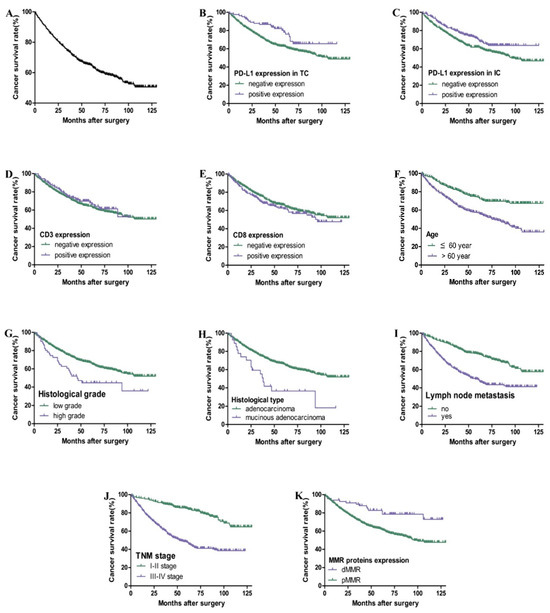
Figure 3.
Kaplan–Meier overall survival curves of PD-L1, CD3, and CD8 expression and clinicopathological parameters in CRC patients. Overall survival rate of patients with CRC (A). Patients with positive expression of PD-L1 in TC (B) and IC (C) had better overall survival (p < 0.05). There is no significant correlation between CD3 (D) and CD8 (E) expression and prognosis (p > 0.05). Patients with higher age, higher histological grade, mucinous adenocarcinoma, lymph node metastasis, III–IV TNM stage, and pMMR had poor overall survival (F–K) (p < 0.05).
Figure 3.
Kaplan–Meier overall survival curves of PD-L1, CD3, and CD8 expression and clinicopathological parameters in CRC patients. Overall survival rate of patients with CRC (A). Patients with positive expression of PD-L1 in TC (B) and IC (C) had better overall survival (p < 0.05). There is no significant correlation between CD3 (D) and CD8 (E) expression and prognosis (p > 0.05). Patients with higher age, higher histological grade, mucinous adenocarcinoma, lymph node metastasis, III–IV TNM stage, and pMMR had poor overall survival (F–K) (p < 0.05).
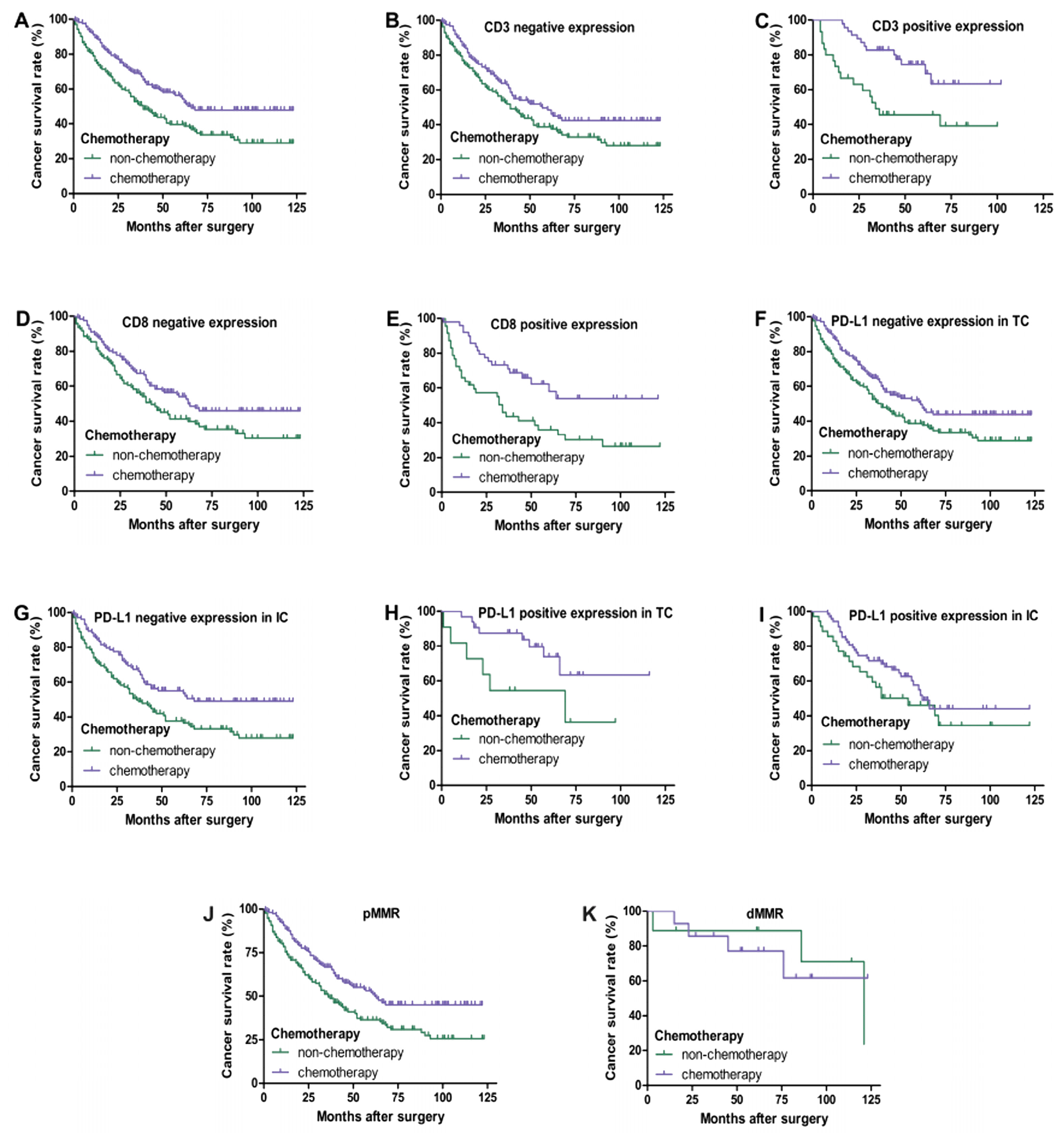
In III-IV TNM stage CRC patients, as shown in Figure 4, univariate survival analysis showed that chemotherapy improved the overall survival rate of patients (p < 0.05). Regardless of the positive or negative expression of CD3 and CD8 in tumor-infiltrating lymphocytes, chemotherapy significantly improved the survival rate of patients (p < 0.05). However, chemotherapy of patients with a positive expression of PD-L1 in TC and IC and dMMR had no significant correlation with prognosis (p > 0.05), while chemotherapy of patients with a negative expression of PD-L1 in TC and IC and pMMR had a better prognosis (p < 0.05).
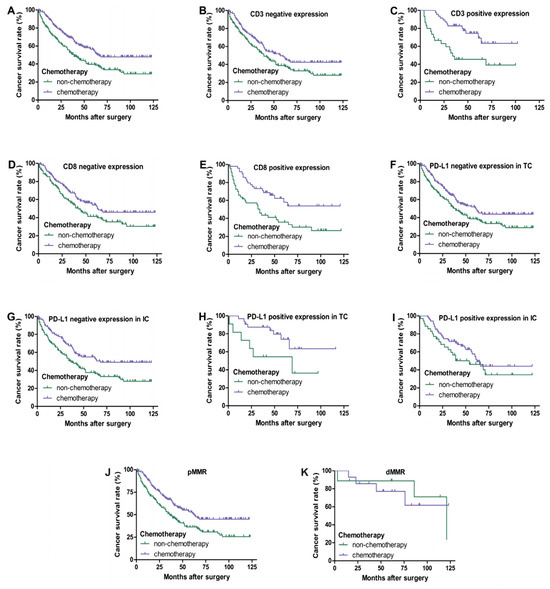
Figure 4.
Kaplan–Meier overall survival curves of PD-L1, CD3, and CD8 expression in III–IV TNM stage CRC patients with chemotherapy. Patients with chemotherapy had a better overall survival (A) (p < 0.05). Regardless of whether CD3 and CD8 are negative (B,D) or positive (C,E) expression, patients with chemotherapy always have a better prognosis (p < 0.05). Patients who received chemotherapy with negative expression of PD-L1 in TC (F) and IC (G) had a better prognosis (p < 0.05). No significant difference in overall survival between patients with chemotherapy and non-chemotherapy of PD-L1 positive expression in TC (H) (p = 0.055) and IC (I) (p > 0.05). Patients who received chemotherapy with pMMR had a better prognosis (J) (p < 0.05). There was no significant difference in overall survival between patients with chemotherapy and non-chemotherapy of dMMR (K) (p > 0.05).
4. Discussion
PD-L1 is expressed in a variety of tumor cells and lymphocytes of the tumor microenvironment. PD-L1 binding with PD-1 induces immune suppression and enables tumor cells to obtain immune escape; this is one of the mechanisms for tumor tolerance [34,35,36,37]. Blockade of PD1/PD-L1 signaling inhibits the immune escape of tumor cells, and the body’s own T cells play an anti-tumor role and achieve the purpose of immunotherapy [38,39]. Studies have shown that the positive expression rate of PD-L1 in TC was 12.5%, and the positive expression rate in IC was 29.8% in CRC [16]. In addition, the positive expression rates of PD-L1 in salivary duct carcinoma TC and IC were 32% and 66%, respectively [40]. In the present study, the positive expression rates of PD-L1 in TC and IC of CRC were 15.6% and 36.6%, respectively. These studies indicated that PD-L1 has different expression rates in different antibodies, and PD-L1 has a higher expression rate in tumor IC compared with TC. Our results showed that the positive expression of PD-L1 in TC was more likely to occur in right colon, adenocarcinoma, and dMMR patients. Some studies found that PD-L1 was more expressed in TC in MSI-H CRC patients than in MSS [41,42]. Inaguma et al. recently also confirmed that high PD-L1 expression in TC was significantly associated with BRAF-mutated and MMR-deficient status in CRC, typically located in the right or transverse colon [43]. Our result indicated that the expression of PD-L1 in IC was associated with young CRC patients and the I-II TNM stage. Kim et al. showed that the expression of PD-L1 in IC is related to the I-II TNM stage [16]. These findings indicated that PD-L1 expression in IC is closely linked to tumor progression in CRC.
CD3 and CD8 are important biomarkers of T lymphocytes. Many studies have reported the clinical significance and prognosis value of its expression in tumor-infiltrating immune cells of CRC. Won-Suk Lee et al. found that the positive expression rate of CD3 in tumor-infiltrating lymphocytes of CRC was 59% [44]. Huang et al. showed that the expression rate of CD8+TIL in CRC was 33% and closely related to lymph node metastasis and III-IV TNM stage [45]. In the present study, our results show that the positive expression rates of CD3 and CD8 in tumor-infiltrating lymphocytes were 17.9% and 26.5%, respectively, and both significantly correlated with lymph node metastasis and III-IV TNM stage, suggesting that CD3 and CD8 in TILs of CRC is related to tumor progression. Our results indicate that the expression of CD8 in the right colon is higher than that in the left colon. Zhang et al. observed [46] that compared with left-side CRC, the infiltration of CD8+T cells in right-side CRC was enhanced, which is consistent with our research results. It has been reported that in some cases, right CRC and left CRC exhibit various biological and clinical differences, including embryonic origin, microbiota load, vascular supply, and main physiological functions [47,48], which may affect somatic mutations and immunophenotypes between two different disease locations, thereby affecting the selection of molecular targeted drugs or immunotherapy methods.
Our study showed that the expressions of PD-L1 in TC and IC are both correlated with the expression of CD3 and CD8 in tumor-infiltrating lymphocytes. Tomohiro Kikuchi et al. identified a subpopulation of patients with pMMR-CRC who were positive for PD-L1in TC and who had levels of elevated CD8+ and CD4+ TILs infiltration similar to those dMMR-CRC patients [49]. Kim et al. found that PD-L1 expression in TC and IC of CRC were accompanied by increased infiltration of CD3+ TILs [16], and Wang et al. showed that the expression of PD-L1 in TC of CRC was positively associated with CD8 + TILs density [50]. In summary, the high expression of PD-L1 in TC and IC is accompanied by increased expression of CD3 and CD8 in tumor-infiltrating lymphocytes. This may partly be due to the fact that activated T cells can secrete cytokines such as interferon-γ, thereby inducing the expression of PD-L1 in tumor cells and TILs in the immune microenvironment [51].
Previous studies found that the relationship between PD-L1 expression and prognosis in CRC is controversial and not fully consistent. Zhu et al. found that the low expression of PD-L1 in TC has better overall survival in CRC [21]. Conversely, Li et al. [19] found that higher expressions of PD-L1 in TC correlate with better prognosis of CRC patients. Also, the results of Soo Jung Lee et al. showed that patients with high expression of PD-L1 in IC have better prognosis for colon cancer [52]. In the present study, we found that the positive expressions of PD-L1 in TC and IC were associated with better prognosis, where PD-L1 expression in IC was found to play an important role in tumor immune escape and influence tumor progression and was thereby correlated with a favorable prognosis [52]. In our study, no significant correlation was found between the positive expression of CD3 and CD8 in CRC patients and prognosis. Nosho et al. found that in the multivariate model, CD3+ and CD8+ cell density was not associated with survival in CRC [53]. In addition, our findings showed that dMMR patients had a better prognosis than pMMR patients, which was consistent with the result reported by Qin et al., who reported that colon cancer displaying dMMR had a better 5-year overall survival (OS) rate and disease-free survival (DFS) rate than the patients with pMMR [54]. Our results suggest that the prognosis of CRC patients with a positive expression of PD-L1 in TC is better than the negative, which may partly be attributed to the positive expression of PD-L1 in TC being more likely to occur in dMMR as well as the positive expression of PD-L1 in IC significantly correlating to the early TNM stage.
5-Fu and its derivatives are commonly used in the chemotherapy of CRC. In the present study, we evaluated the predictive value of CD3/CD8/PD-L1 protein expressions of CRC in adjuvant chemotherapy. Being consistent with Li et al.’s results [55], our study also found that chemotherapy significantly improved the survival time of III-IV TNM stage CRC patients. Patients with negative expression of PD-L1 in TC or IC and pMMR benefit from the chemotherapy. Our results show whether the positive expression of CD3 and CD8 in tumor-infiltrating lymphocytes does or not does not affect the chemotherapy effect of CRC patients. However, CRC patients with positive PD-L1 expression in TC or IC and dMMR did not benefit from chemotherapy. For CRC patients with positive PD-L1 expression and dMMR status, immune checkpoint inhibition may be a potentially more effective treatment strategy [33,42,56]. Therefore, our results suggest that the expression of PD-L1 and MMR status may act as an important biomarker for guidance in the postoperative treatment of CRC patients with the III-IV TNM stage.
In conclusion, PD-L1 expression in TC and IC was closely related to the density of CD3 and CD8 infiltration in tumor-infiltrating lymphocytes. The expression of CD3 and CD8 in tumor-infiltrating lymphocytes and the expression of PD-L1 in IC were linked to the TNM stage of CRC patients. PD-L1 expression in TC and IC and MMR status may act as an important biomarker for guidance postoperative treatment of III-IV TNM stage CRC patients.
Author Contributions
Conceptualization, K.L.; validation, J.L. (Jihong Liu), F.L., S.W. and B.L.; formal analysis, J.L. (Jihong Liu), J.L. (Jinbang Li) and F.L.; investigation, J.L. (Jihong Liu), F.L., S.W. and B.L.; data curation, J.L. (Jihong Liu) and F.L.; writing—original draft preparation, J.L. (Jihong Liu), J.L. (Jinbang Li) and K.L.; writing—review and editing, K.L.; visualization, J.L. (Jihong Liu), J.L. (Jinbang Li) and F.L.; supervision, K.L.; project administration, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by special funds for clinical research from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (QYRYCRC2023008).
Institutional Review Board Statement
The Ethics Committees of the Qingyuan People’s Hospital approved the study (IRB-2023-024).
Informed Consent Statement
The Ethics Committees waived the patients’ informed consent due to the retrospective nature of the study.
Data Availability Statement
Data generated for the current study are available from the corresponding author upon reasonable request.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CD3: cluster of differentiation 3; CD8: cluster of differentiation 8; CRC: colorectal cancer; IHC: immunohistochemistry; TC: tumor cells; IC: immune cells; PD-L1: programmed death-ligand 1; PD-1: programmed death-1; Th1: T helper 1; ICIs: immune checkpoint inhibitors; MMR: mismatch repair; MSI: microsatellite instability; TMA: tissue microarray; dMMR: mismatch repair deficient; pMMR: mismatch repair proficient; MSI-H: high-frequency microsatellite instability; MSI-L: low levels of microsatellite instability; MSS: microsatellite stable; OS: overall survival.
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Koudougou, C.; Bonneville, M.; Matysiak-Budnik, T.; Touchefeu, Y. Review article: Antitumoural immunity in colorectal cancer–current and potential future implications in clinical practice. Aliment. Pharmacol. Ther. 2013, 38, 3–15. [Google Scholar] [CrossRef]
- Weng, J.; Li, S.; Zhu, Z.; Liu, Q.; Zhang, R.; Yang, Y.; Li, X. Exploring immunotherapy in colorectal cancer. J. Hematol. Oncol. 2022, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loroit, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.L.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, H.E.; Cho, N.Y.; Lee, H.S.; Kang, G.H. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br. J. Cancer 2016, 115, 490–496. [Google Scholar] [CrossRef]
- Masugi, Y.; Nishihara, R.; Yang, J.; Mima, K.; Da Silva, A.; Shi, Y.; Inamura, K.; Cao, Y.; Song, M.; Nowak, J.A.; et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut 2017, 66, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Dai, W.; Cai, G.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 2016, 15, 55. [Google Scholar] [CrossRef]
- Lee, K.S.; Kwak, Y.; Ahn, S.; Shin, E.; Oh, H.K.; Kim, D.W.; Kang, S.B.; Choe, G.; Kim, W.H.; Lee, H.S. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol. Immunother. 2017, 66, 927–939. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, H.; Huang, Z.; Li, S.; Zhu, X.; He, J.; Yang, J.; Yu, X.; Yi, X. Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9351–9359. [Google Scholar] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.-Z.; Pan, K.; Zhao, J.J.; Chen, J.-G.; Li, J.J.; Lv, L.; Wang, D.D.; Zheng, H.X.; Jiang, S.S.; Zhang, X.F.; et al. Decreased expression of interleukin-36α correlates with poor prognosis in hepatocellular carcinoma. Cancer Immunol. Immunother. 2013, 62, 1675–1685. [Google Scholar] [CrossRef]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar]
- Deschoolmeester, V.; Baay, M.; Lardon, F.; Pauwels, P.; Peeters, M. Immune Cells in Colorectal Cancer: Prognostic Relevance and Role of MSI. Cancer Microenviron. 2011, 4, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Hsiao, J.R.; Chang, K.C.; Wu, Y.H.; Su, I.J.; Jin, Y.T.; Chang, Y. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod. Pathol. 2010, 23, 1393–1403. [Google Scholar] [CrossRef]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their pd-1 expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [CrossRef]
- Modrich, P. Mechanisms in E. coli and Human Mismatch Repair (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 8490–8501. [Google Scholar] [CrossRef]
- Zaanan, A.; Meunier, K.; Sangar, F.; Fléjou, J.F.; Praz, F. Microsatellite instability in colorectal cancer: From molecular oncogenic mechanisms to clinical implications. Cell. Oncol. 2011, 34, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sinha, S.; Paul, R.N. The impact of microsatellite stability status in colorectal cancer. Curr. Probl. Cancer 2018, 42, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Hermel, D.J.; Sigal, D. The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer. J. Pers. Med. 2019, 9, 5. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; von Pawel, J.; Park, K.; Rittmeyer, A.; Gandara, D.R.; Aix, S.P.; Han, J.Y.; Gadgeel, S.M.; Hida, T.; Cortinovis, D.L.; et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1156–1170. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Xu, B.; Jungbluth, A.A.; Frosina, D.; Alzumaili, B.; Aleynick, N.; Slodkowska, E.; Higgins, K.; Ho, A.; Morris, L.; Ghossein, R.; et al. The immune microenvironment and expression of PD-L1, PD-1, PRAME and MHC I in salivary duct carcinoma. Histopathology 2019, 75, 672–682. [Google Scholar] [CrossRef]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S.; et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, S.; Lasota, J.; Wang, Z.; Felisiak-Golabek, A.; Ikeda, H.; Miettinen, M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod. Pathol. 2017, 30, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Park, S.; Lee, W.Y.; Yun, S.H.; Chun, H.-K. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 2010, 116, 5188–5199. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chiang, S.F.; Ke, T.W.; Chen, T.-W.; You, Y.S.; Chen, W.T.L.; Chao, K.S.C. Clinical significance of programmed death 1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell infiltration in stage II-III colorectal cancer. Sci. Rep. 2018, 8, 15658. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Dai, Y.; Cheng, J.N.; Gong, Z.; Feng, Y.; Sun, C.; Jia, Q.; Zhu, B. Immune Landscape of Colorectal Cancer Tumor Microenvironment from Different Primary Tumor Location. Front. Immunol. 2018, 9, 1578. [Google Scholar] [CrossRef]
- Minoo, P.; Zlobec, I.; Peterson, M.; Terracciano, L.; Lugli, A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int. J. Oncol. 2010, 37, 707–718. [Google Scholar] [CrossRef]
- Noble, A.; Durant, L.; Dilke, S.M.; Man, R.; Martin, I.; Patel, R.; Hoyles, L.; Pring, E.T.; Latchford, A.; Clark, S.K.; et al. Altered Mucosal Immune-Microbiota Interactions in Familial Adenomatous Polyposis. Clin. Transl. Gastroenterol. 2022, 13, e00428. [Google Scholar] [CrossRef]
- Kikuchi, T.; Mimura, K.; Okayama, H.; Nakayama, Y.; Saito, K.; Yamada, L.; Endo, E.; Sakamoto, W.; Fujita, S.; Endo, H.; et al. A subset of patients with MSS/MSI-low-colorectal cancer showed increased CD8(+) TILs together with up-regulated IFN-γ. Oncol. Lett. 2019, 18, 5977–5985. [Google Scholar] [CrossRef]
- Wang, W.; Jing, H.; Liu, J.; Bu, D.; Zhang, Y.; Zhu, T.; Lu, K.; Xu, Y.; Cheng, M.; Liu, J.; et al. Correlation between schistosomiasis and CD8+ T cell and stromal PD-L1 as well as the different prognostic role of CD8+ T cell and PD-L1 in schistosomal-associated colorectal cancer and non-schistosomal-associated colorectal cancer. World J. Surg. Oncol. 2021, 19, 321. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Srivastava, R.M.; Trivedi, S.; Lei, Y.; Chandran, U.; Seethala, R.R.; Freeman, G.J.; Ferris, R.L. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016, 76, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jun, S.Y.; Lee, I.H.; Kang, B.W.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.S.; Yoon, G.; Kim, J.G. CD274, LAG3, and IDO1 expressions in tumor-infiltrating immune cells as prognostic biomarker for patients with MSI-high colon cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Ying, J.; Lyu, N.; Guo, L.; Zhi, W.; Zhou, A.; Wang, J. Correlations between DNA mismatch repair (MMR) and prognosis and prediction of treatment efficacy in stage II/II colon cancer. Zhonghua Zhong Liu Za Zhi 2014, 36, 844–848. [Google Scholar]
- Li, Y.; Zhao, L.; Güngör, C.; Tan, F.; Zhou, Z.; Li, C.; Song, X.; Wang, D.; Pei, Q.; Liu, W. The main contributor to the upswing of survival in locally advanced colorectal cancer: An analysis of the SEER database. Ther. Adv. Gastroenterol. 2019, 12, 1756284819862154. [Google Scholar] [CrossRef]
- Yaghoubi, N.; Soltani, A.; Ghazvini, K.; Hassanian, S.M.; Hashemy, S.I. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed. Pharmacother. 2019, 110, 312–318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).