Harnessing Real-World Evidence to Advance Cancer Research
Abstract
:1. Introduction
2. Conventional Clinical Trials and Real-World Data Studies
2.1. Clinical Trials
- Clinical trial participants are highly selected and may not be representative of the broader patient population, as only 3% of patients with cancer are enrolled in RCTs [8,9]. Older patients aged 65 years and above are consistently under-represented in clinical trials, even though cancers are overwhelmingly diagnosed in older adults [9,10,11,12,13,14]. There is also under-representation of patients from racial and ethnic minorities, those with socioeconomic disadvantage, and patients with complex health problems; therefore, RCTs may lack information about treatment tolerability and efficacy in patients with multiple co-morbidities and poor performance status [9,15,16,17,18].
- Patient care provided in clinical trials does not necessarily represent routine clinical practice [6,19]. Clinical trial participants typically receive more intensive monitoring than patients in routine practice, which may influence outcomes. There is evidence that clinical trial participants benefit from the ‘trial effect’ or ‘protocol/Hawthorne effect’, in which the clinical trial participation in itself may have a positive effect on outcomes due to more intensive care [20,21]. This is supported by the fact that patients who are referred for clinical trial participation at specialist centres often have better survival outcomes than those who are not [22,23].
2.2. Real-World Data
2.2.1. Examples of Real-World Data
2.2.2. Strengths of Real-World Data Research
2.2.3. Limitations of Real-World Data Research
3. Potential Uses of Real-World Data in Oncology Research
- Studying patients that are under-represented in clinical trials: For example, in oncology, the under-representation of older adults in clinical trials leads a relative shortage of evidence to guide their care [9,10,11,12,13,14,35]. Real-world data offer opportunities to study the extent of and factors contributing to evidence gaps for these patients, and to gain insights into their management and outcomes.
- Examining cancer therapy use and outcomes: While RCTs offer evidence of what is achievable under favourable circumstances, they do not necessarily provide a reliable indication of outcomes of patients who receive the same interventions in less controlled circumstances [19,34]. Real-world data offers opportunities to examine how routine care differs from clinical trials and trial evidence-based guidelines. Differences in patients, practice and providers often leads to patients in routine practice having shorter survival and higher rates of treatment toxicity compared to clinical trial participants [36,37]. This difference between outcomes of patients selected to participate in trials (efficacy) and outcomes when the same treatment is applied in real-world practice (effectiveness) is referred to as the efficacy-effectiveness gap [38,39].
- Rare cancers: It is challenging to generate evidence to guide the care of patients with rare cancers due to difficulty in accruing sufficient participants to RCTs to have adequate statistical power to detect differences in outcomes. Observational studies using real-world data are increasingly recognized as a means to advance research into rare cancers by improving the understanding of their natural history, evaluating clinical practice, establishing standards of care, and generating hypotheses for testing in clinical trials [40,41].
- Rare and long-term toxicities: While RCTs have limited ability to provide information on rare and late treatment toxicities, real-world data research often include data from larger numbers of patients collected over longer periods of time and hence could provide this information.
- Health economic evaluation: Health economic evaluation is used to model anticipated costs associated with adoption of new cancer medicines and is often used by health technology assessment bodies to determine funding of and access to treatments. However, these predictive models and estimates are often based on assumptions that may not accurately reflect the true costs of health interventions in the real world. Real-world data can enable estimation of actual health care use and costs to support health economic evaluation.
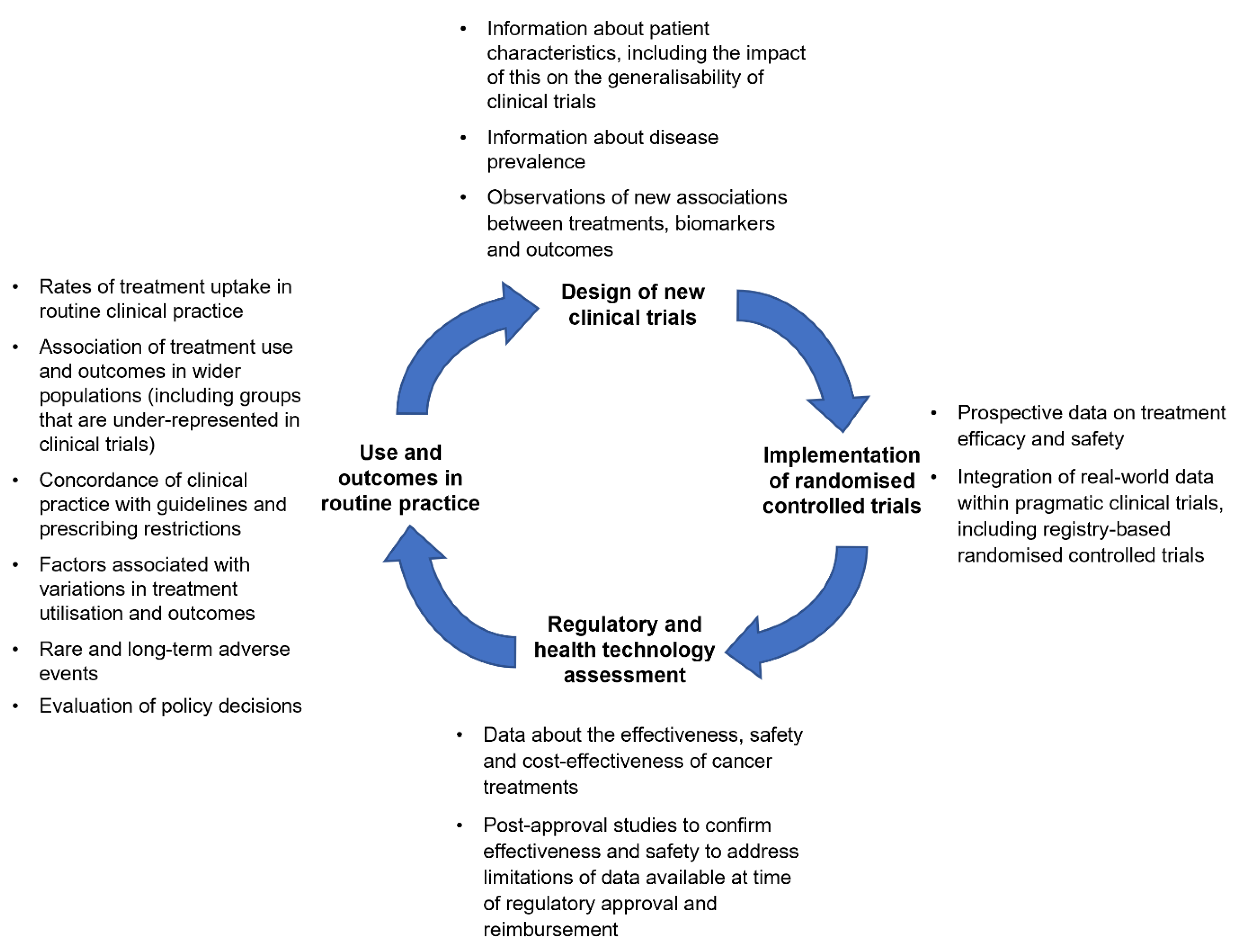
4. A Framework for Clinical Trials and Real-World Evidence in Oncology Research
4.1. Complementary Roles for Clinical Trials and Real-World Evidence
4.1.1. Using Real-World Evidence to Extend Clinical Trial Evidence
4.1.2. Using Real-World Evidence to Support Clinical Trial Design
4.1.3. Integration of Real-World Evidence in Clinical Trials
4.2. Real-World Evidence to Support Population-Level Decision-Making
5. Considerations for Harnessing Real-World Evidence for Cancer Medicines Research
5.1. Heterogeneity of Real-World Data
5.2. Promoting the Quality and Reliability of Real-World Evidence
5.3. Concerns about the Reliability of Real-World Evidence
5.3.1. Strategies to Promote the Reliability and Credibility of Real-World Evidence
5.3.2. Guidance for Conducting Real-World Evidence Studies
6. The Potential of Real-World Data to Advance Cancer Medicine Research around the World
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. The Cancer Atlas; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Elkin, E.B.; Bach, P.B. Cancer’s next frontier: Addressing high and increasing costs. JAMA 2010, 303, 1086–1087. [Google Scholar] [CrossRef]
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef]
- Sibbald, B.; Roland, M. Understanding controlled trials. Why are randomised controlled trials important? Br. Med. J. 1998, 316, 201. [Google Scholar] [CrossRef]
- Collins, R.; Bowman, L.; Landray, M.; Peto, R. The magic of randomization versus the myth of real-world evidence. N. Engl. J. Med. 2020, 382, 674–678. [Google Scholar] [CrossRef]
- Booth, C.; Tannock, I. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br. J. Cancer 2014, 110, 551. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.M. Generalizing the Results of Cancer Clinical Trials. J. Clin. Oncol. 2009, 28, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Cain, D.F. National cancer institute sponsored cooperative clinical trials. Cancer 1990, 65, 2376–2382. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, L.F.; Unger, J.M.; Crowley, J.J.; Coltman, C.A.J.; Albain, K.S. Underrepresentation of Patients 65 Years of Age or Older in Cancer-Treatment Trials. N. Engl. J. Med. 1999, 341, 2061–2067. [Google Scholar] [CrossRef]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef]
- Trimble, E.L.; Cain, D.; Ungerleider, R.S.; Friedman, M.A.; Carter, C.L.; Freidlin, B. Representation of older patients in cancer treatment trials. Cancer 1994, 74, 2208–2214. [Google Scholar] [CrossRef]
- Talarico, L.; Chen, G.; Pazdur, R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J. Clin. Oncol. 2004, 22, 4626–4631. [Google Scholar] [CrossRef]
- Singh, H.; Kanapuru, B.; Smith, C.; Fashoyin-Aje, L.A.; Myers, A.; Kim, G.; Pazdur, R. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the U.S. Food and Drug Administration. J. Clin. Oncol. 2017, 35, 10009. [Google Scholar] [CrossRef]
- Gross, C.P.; Filardo, G.; Mayne, S.T.; Krumholz, H.M. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer 2005, 103, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Gralow, J.R.; Albain, K.S.; Ramsey, S.D.; Hershman, D.L. Patient income level and cancer clinical trial participation: A prospective survey study. JAMA Oncol. 2016, 2, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Cooksley, C.; Bekele, B.N.; Frumovitz, M.; Avritscher, E.B.C.; Sun, C.; Bodurka, D.C. Generalizability of cancer clinical trial results. Cancer 2006, 106, 2452–2458. [Google Scholar] [CrossRef]
- Sarfati, D.; Koczwara, B.; Jackson, C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 2016, 66, 337–350. [Google Scholar] [CrossRef]
- Black, N. Why we need observational studies to evaluate the effectiveness of health care. Br. Med. J. 1996, 312, 1215–1218. [Google Scholar] [CrossRef]
- Braunholtz, D.A.; Edwards, S.J.L.; Lilford, R.J. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J. Clin. Epidemiol. 2001, 54, 217–224. [Google Scholar] [CrossRef]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne Effect: A randomised, controlled trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Stiller, C.A. Centralised treatment, entry to trials and survival. Br. J. Cancer 1994, 70, 352. [Google Scholar] [CrossRef] [PubMed]
- Schwentner, L.; Van Ewijk, R.; Kurzeder, C.; Hoffmann, I.; König, J.; Kreienberg, R.; Blettner, M.; Wöckel, A. Participation in adjuvant clinical breast cancer trials: Does study participation improve survival compared to guideline adherent adjuvant treatment? A retrospective multi-centre cohort study of 9433 patients. Eur. J. Cancer 2013, 49, 553–563. [Google Scholar] [CrossRef]
- Kerali, H. Why Are Oncology Clinical Trials Taking Longer? Available online: https://www.clinicaltrialsarena.com/news/why-are-oncology-clinical-trials-taking-longer-4698649-2/ (accessed on 27 April 2021).
- Kim, C.; Prasad, V. Strength of validation for surrogate end points used in the US Food and Drug Administration’s approval of oncology drugs. Mayo. Clin. Proc. 2016, 91, 713–725. [Google Scholar] [CrossRef]
- Kemp, R.; Prasad, V. Surrogate endpoints in oncology: When are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Garrison, L.P., Jr.; Neumann, P.J.; Erickson, P.; Marshall, D.; Mullins, C.D. Using real-world data for coverage and payment decisions: The ISPOR Real-World Data Task Force report. Value Health 2007, 10, 326–335. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Framework for FDA’s Real-World Evidence Program; United States Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Jarow, J.P.; LaVange, L.; Woodcock, J. Multidimensional evidence generation and FDA regulatory decision making: Defining and using “real-world” data. JAMA 2017, 318, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Bellows, B.K.; Kuo, K.-L.; Biltaji, E.; Singhal, M.; Jiao, T.; Cheng, Y.; McAdam-Marx, C. Real-world evidence in pain research: A review of data sources. J. Pain Palliat. Care Pharmacother. 2014, 28, 294–304. [Google Scholar] [CrossRef]
- Parkin, D.M. The evolution of the population-based cancer registry. Nat. Rev. Cancer 2006, 6, 603–612. [Google Scholar] [CrossRef]
- Nabhan, C.; Klink, A.; Prasad, V. Real-world evidence—What does it really mean? JAMA Oncol. 2019, 5, 781–783. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Levit, L.A.; Dale, W.; Mohile, S.G.; Muss, H.B.; Fehrenbacher, L.; Magnuson, A.; Lichtman, S.M.; Bruinooge, S.S.; Soto-Perez-de-Celis, E. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J. Clin. Oncol. 2015, 33, 3826–3833. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.; Vera-Badillo, F.; Wang, L.; Attalla, M.; De Gouveia, P.; Leibowitz-Amit, R.; Knox, J.; Moore, M.; Sridhar, S.; Joshua, A. Translating clinical trials to clinical practice: Outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann. Oncol. 2013, 24, 2972–2977. [Google Scholar] [CrossRef] [PubMed]
- Green, A.K.; Curry, M.; Trivedi, N.; Bach, P.B.; Mailankody, S. Assessment of outcomes associated with the use of newly approved oncology drugs in Medicare beneficiaries. JAMA Netw. Open 2021, 4, e210030. [Google Scholar] [CrossRef]
- Templeton, A.J.; Booth, C.M.; Tannock, I.F. Informing patients about expected outcomes: The efficacy-effectiveness gap. J. Clin. Oncol. 2020, 38, 1651–1654. [Google Scholar] [CrossRef]
- Sargent, D. What constitutes reasonable evidence of efficacy and effectiveness to guide oncology treatment decisions? Oncologist 2010, 15, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Billingham, L.; Malottki, K.; Steven, N. Research methods to change clinical practice for patients with rare cancers. Lancet Oncol. 2016, 17, e70–e80. [Google Scholar] [CrossRef]
- Casali, P.G.; Bruzzi, P.; Bogaerts, J.; Blay, J.Y.; Aapro, M.; Adamous, A.; Berruti, A.; Blay, J.Y.; Bogaerts, J.; Bressington, J.; et al. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: A European consensus position paper. Ann. Oncol. 2015, 26, 300–306. [Google Scholar] [CrossRef]
- Karim, S.; Booth, C.M. Effectiveness in the absence of efficacy: Cautionary tales from real-world evidence. J. Clin. Oncol. 2019, 37, 1047–1050. [Google Scholar] [CrossRef]
- Pencina, M.J.; Rockhold, F.W.; D’Agostino, R.B., Sr. Deriving real-world insights from real-world data: Biostatistics to the rescue. Ann. Intern. Med. 2018, 169, 401–402. [Google Scholar] [CrossRef]
- Bosco, J.L.F.; Silliman, R.A.; Thwin, S.S.; Geiger, A.M.; Buist, D.S.M.; Prout, M.N.; Yood, M.U.; Haque, R.; Wei, F.; Lash, T.L. A most stubborn bias: No adjustment method fully resolves confounding by indication in observational studies. J. Clin. Epidemiol. 2010, 63, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Prasad, V. Are observational, real-world studies suitable to make cancer treatment recommendations? JAMA Netw. Open 2020, 3, e2012119. [Google Scholar] [CrossRef]
- Tannock, I.F.; Amir, E.; Booth, C.M.; Niraula, S.; Ocana, A.; Seruga, B.; Templeton, A.J.; Vera-Badillo, F. Relevance of randomised controlled trials in oncology. Lancet Oncol. 2016, 17, e560–e567. [Google Scholar] [CrossRef]
- Friends of Oncology Research. Blueprint for Breakthrough: Exploring Utility of Real World Evidence (RWE). Available online: https://www.focr.org/events/blueprint-breakthrough-exploring-utility-real-world-evidence-rwe (accessed on 13 February 2020).
- Seruga, B.; Sterling, L.; Wang, L.; Tannock, I.F. Reporting of serious adverse drug reactions of targeted anticancer agents in pivotal phase III clinical trials. J. Clin. Oncol. 2011, 29, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Sentinel Initiative Website. Available online: https://www.sentinelinitiative.org/ (accessed on 29 January 2021).
- Hodson, R. Precision oncology. Nature 2020, 585, S1. [Google Scholar] [CrossRef]
- Agarwala, V.; Khozin, S.; Singal, G.; O’Connell, C.; Kuk, D.; Li, G.; Gossai, A.; Miller, V.; Abernethy, A.P. Real-world evidence in support of precision medicine: Clinico-genomic cancer data as a case study. Health Aff. 2018, 37, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.J.; Bell, J.L.; Bruce, J.P.; Doherty, G.J.; Galvin, M.; Green, M.F.; Hunter-Zinck, H.; Kumari, P.; Lenoue-Newton, M.L.; Li, M.M. AACR Project GENIE: 100,000 cases and beyond. Cancer Discov. 2022, 12, 2044–2057. [Google Scholar] [CrossRef]
- Kohno, T.; Kato, M.; Kohsaka, S.; Sudo, T.; Tamai, I.; Shiraishi, Y.; Okuma, Y.; Ogasawara, D.; Suzuki, T.; Yoshida, T. C-CAT: The National Datacenter for Cancer Genomic Medicine in Japan. Cancer Discov. 2022, 12, 2509–2515. [Google Scholar] [CrossRef]
- Schilsky, R.L. Finding the evidence in real-world evidence: Moving from data to information to knowledge. J. Am. Coll. Surg. 2017, 224, 1–7. [Google Scholar] [CrossRef]
- Gyawali, B.; Parsad, S.; Feinberg, B.A.; Nabhan, C. Real-world evidence and randomized studies in the precision oncology era: The right balance. JCO Precis. Oncol. 2017, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.B.; Faridi, K.F.; Butala, N.M.; Zhao, Y.; Tamez, H.; Valsdottir, L.R.; Brennan, J.M.; Shen, C.; Popma, J.J.; Kazi, D.S.; et al. Use of administrative claims to assess outcomes and treatment effect in randomized clinical trials for transcatheter aortic valve replacement. Circulation 2020, 142, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Faridi, K.F.; Tamez, H.; Strom, J.B.; Song, Y.; Butala, N.M.; Shen, C.; Popma, J.J.; Kazi, D.S.; Yeh, R.W. Use of administrative claims data to estimate treatment effects for 30 versus 12 months of dual antiplatelet therapy after percutaneous coronary intervention. Circulation 2020, 142, 306–308. [Google Scholar] [CrossRef]
- Konstam, M.A. Real world data as trial end points. Circulation 2020, 142, 214–216. [Google Scholar] [CrossRef]
- Ford, I.; Norrie, J. Pragmatic Trials. N. Engl. J. Med. 2016, 375, 454–463. [Google Scholar] [CrossRef]
- Ramsberg, J.; Neovius, M. Register or electronic health records enriched randomized pragmatic trials: The future of clinical effectiveness and cost-effectiveness trials? Nord. J. Health Econ. 2017, 5, 62–76. [Google Scholar]
- Corrigan-Curay, J.; Sacks, L.; Woodcock, J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA 2018, 320, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Karanatsios, B.; Prang, K.-H.; Verbunt, E.; Yeung, J.M.; Kelaher, M.; Gibbs, P. Defining key design elements of registry-based randomised controlled trials: A scoping review. Trials 2020, 21, 552. [Google Scholar] [CrossRef]
- Erlinge, D.; Omerovic, E.; Fröbert, O.; Linder, R.; Danielewicz, M.; Hamid, M.; Swahn, E.; Henareh, L.; Wagner, H.; Hårdhammar, P.; et al. Bivalirudin versus heparin monotherapy in myocardial infarction. N. Engl. J. Med. 2017, 377, 1132–1142. [Google Scholar] [CrossRef]
- Mathes, T.; Buehn, S.; Prengel, P.; Pieper, D. Registry-based randomized controlled trials merged the strength of randomized controlled trails and observational studies and give rise to more pragmatic trials. J. Clin. Epidemiol. 2018, 93, 120–127. [Google Scholar] [CrossRef]
- Lauer, M.S.; D’Agostino, R.B., Sr. The randomized registry trial—The next disruptive technology in clinical research? N. Engl. J. Med. 2013, 369, 1579. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.; Srinathan, S.K.; McAuley, D.F.; Mrkobrada, M.; Levine, O.; Ribic, C.; Molnar, A.O.; Dattani, N.D.; Burke, A.; Guyatt, G.; et al. The statistical significance of randomized controlled trial results is frequently fragile: A case for a Fragility Index. J. Clin. Epidemiol. 2014, 67, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Desnoyers, A.; Wilson, B.E.; Nadler, M.B.; Amir, E. Fragility index of trials supporting approval of anti-cancer drugs in common solid tumours. Cancer Treat Rev. 2021, 94, 102167. [Google Scholar] [CrossRef]
- Beaulieu-Jones, B.K.; Finlayson, S.G.; Yuan, W.; Altman, R.B.; Kohane, I.S.; Prasad, V.; Yu, K.-H. Examining the use of real-world evidence in the regulatory process. Clin. Pharmacol. Ther. 2019, 107, 843–852. [Google Scholar] [CrossRef]
- Ladanie, A.; Schmitt, A.M.; Speich, B.; Naudet, F.; Agarwal, A.; Pereira, T.V.; Sclafani, F.; Herbrand, A.K.; Briel, M.; Martin-Liberal, J.; et al. Clinical trial evidence supporting US Food and Drug Administration approval of novel cancer therapies between 2000 and 2016. JAMA Netw. Open 2020, 3, e2024406. [Google Scholar] [CrossRef]
- Beaver, J.A.; Howie, L.J.; Pelosof, L.; Kim, T.; Liu, J.; Goldberg, K.B.; Sridhara, R.; Blumenthal, G.M.; Farrell, A.T.; Keegan, P.; et al. A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: A review. JAMA Oncol. 2018, 4, 849–856. [Google Scholar] [CrossRef]
- Fu, M.; Naci, H.; Booth, C.M.; Gyawali, B.; Cosgrove, A.; Toh, S.; Xu, Z.; Guan, X.; Ross-Degnan, D.; Wagner, A.K. Real-world use of and spending on new oral targeted cancer drugs in the US, 2011–2018. JAMA Intern. Med. 2021, 181, 1596–1604. [Google Scholar] [CrossRef]
- Cipriani, A.; Ioannidis, J.P.; Rothwell, P.M.; Glasziou, P.; Li, T.; Hernandez, A.F.; Tomlinson, A.; Simes, J.; Naci, H. Generating comparative evidence on new drugs and devices after approval. Lancet 2020, 395, 998–1010. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern. Med. 2019, 179, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.S. Real-world data for efficient health technology assessment. Eur. J. Cancer 2017, 79, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Tadrous, M.; Ahuja, T.; Ghosh, B.; Kropp, R. Developing a Canadian real-world evidence action plan across the drug life cycle. Healthc. Policy 2020, 15, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Makady, A.; Ham, R.T.; de Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. Policies for use of real-world data in health technology assessment (HTA): A comparative study of six HTA Agencies. Value Health 2017, 20, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Value Health 2017, 20, 1003–1008. [Google Scholar] [CrossRef]
- Gill, J.; Prasad, V. Improving observational studies in the era of big data. Lancet 2018, 392, 716–717. [Google Scholar] [CrossRef]
- Goodman, S.N.; Schneeweiss, S.; Baiocchi, M. Using design thinking to differentiate useful from misleading evidence in observational research. JAMA 2017, 317, 705–707. [Google Scholar] [CrossRef]
- Oehrlein, E.M.; Graff, J.S.; Harris, J.; Perfetto, E.M. Patient-community perspectives on real-world evidence: Enhancing engagement, understanding, and trust. Patient-Patient-Cent. Outcomes Res. 2019, 12, 375–381. [Google Scholar] [CrossRef]
- Girman, C.J. Real world evidence components that drive credibility and believability. In Proceedings of the 37th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Virtual, 23 August 2021. [Google Scholar]
- Miksad, R.A.; Abernethy, A.P. Harnessing the power of real-world evidence (RWE): A checklist to ensure regulatory-grade data quality. Clin. Pharmacol. Ther. 2018, 103, 202–205. [Google Scholar] [CrossRef]
- Franklin, J.M.; Schneeweiss, S. When and how can real world data analyses substitute for randomized controlled trials? Clin. Pharmacol. Ther. 2017, 102, 924–933. [Google Scholar] [CrossRef]
- Khozin, S.; Blumenthal, G.M.; Pazdur, R. Real-world data for clinical evidence generation in oncology. J. Natl. Cancer Inst. 2017, 109, djx187. [Google Scholar] [CrossRef]
- Panagiotou, O.A.; Heller, R. Inferential challenges for real-world evidence in the era of routinely collected health data: Many researchers, many more hypotheses, a single database. JAMA Oncol. 2021, 7, 1605–1607. [Google Scholar] [CrossRef] [PubMed]
- Real-World Evidence Transparency Initiative. Real-World Evidence Registry. Centre for Open Science. Available online: https://osf.io/registries/rwe/discover (accessed on 10 November 2021).
- Wang, S.V.; Pinheiro, S.; Hua, W.; Arlett, P.; Uyama, Y.; Berlin, J.A.; Bartels, D.B.; Kahler, K.H.; Bessette, L.G.; Schneeweiss, S. STaRT-RWE: Structured template for planning and reporting on the implementation of real world evidence studies. Br. Med. J. 2021, 372, m4856. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; Committee, R.W. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices; United States Food and Drug Administration: Silver Spring, MD, USA, 2017. [Google Scholar]
- European Medicines Agency. Guideline on Registry-Based Studies: Draft; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- United States Food and Drug Administration. Best Practices for Conducting and Reporting Pharmacoepidemiologic Safety Studies Using Electronic Healthcare Data; United States Food and Drug Administration: Silver Spring, MD, USA, 2013. [Google Scholar]
- Breckenridge, A.M.; Breckenridge, R.A.; Peck, C.C. Report on the current status of the use of real-world data (RWD) and real-world evidence (RWE) in drug development and regulation. Br. J. Clin. Pharmacol. 2019, 85, 1874–1877. [Google Scholar] [CrossRef] [PubMed]
- Birkett, D.J.; Mitchell, A.S.; McManus, P. A cost-effectiveness approach to drug subsidy and pricing in Australia. Health Aff. 2001, 20, 104–114. [Google Scholar] [CrossRef]
- Henry, D.; Stehlik, P.; Camacho, X.; Pearson, S.-A. Access to routinely collected data for population health research: Experiences in Canada and Australia. Aust. N. Z. J. Public Health 2018, 42, 430–433. [Google Scholar] [CrossRef]
- Mues, K.E.; Liede, A.; Liu, J.; Wetmore, J.B.; Zaha, R.; Bradbury, B.D.; Collins, A.J.; Gilbertson, D.T. Use of the Medicare database in epidemiologic and health services research: A valuable source of real-world evidence on the older and disabled populations in the US. Clin. Epidemiol. 2017, 9, 267–277. [Google Scholar] [CrossRef]
- Adamson, D.M.; Chang, S.; Hansen, L.G. Health Research Data for the Real World: The MarketScan Databases; Thompson Healthcare: New York, NY, USA, 2008. [Google Scholar]
- Ma, X.; Long, L.; Moon, S.; Adamson, B.J.S.; Baxi, S.S. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. Medrxiv 2020. [Google Scholar] [CrossRef]
- Guttmann, A. The SPOR Canadian Data Platform: Opportunity for multi-provincial research. Can. Med. Assoc. J. 2019, 191, E1091–E1092. [Google Scholar] [CrossRef]
- Suissa, S.; Henry, D.; Caetano, P.; Dormuth, C.R.; Ernst, P.; Hemmelgarn, B.; LeLorier, J.; Levy, A.; Martens, P.J.; Paterson, J.M. CNODES: The Canadian network for observational drug effect studies. Open Med. 2012, 6, e134. [Google Scholar]
- Wettermark, B.; Zoëga, H.; Furu, K.; Korhonen, M.; Hallas, J.; Nørgaard, M.; Almarsdottir, A.; Andersen, M.; Andersson Sundell, K.; Bergman, U.; et al. The Nordic prescription databases as a resource for pharmacoepidemiological research—A literature review. Pharmacoepidemiol. Drug Saf. 2013, 22, 691–699. [Google Scholar] [CrossRef]
- Bright, C.J.; Lawton, S.; Benson, S.; Bomb, M.; Dodwell, D.; Henson, K.E.; McPhail, S.; Miller, L.; Rashbass, J.; Turnbull, A.; et al. Data resource profile: The Systemic Anti-Cancer Therapy (SACT) dataset. Int. J. Epidemiol. 2019, 49, 15–15l. [Google Scholar] [CrossRef] [PubMed]
- Enerly, E.; Holmstrøm, L.; Skog, A.; Knudsen, K.O.; Nygård, J.F.; Møller, B.; Ursin, G. INSPIRE: A new opportunity for cancer pharmacoepidemiology research. Norsk Epidemiol. 2021, 29, 29–33. [Google Scholar] [CrossRef]
- National Cancer Institute. Overview of the SEER Program. 2020.
- Enewold, L.; Parsons, H.; Zhao, L.; Bott, D.; Rivera, D.R.; Barrett, M.J.; Virnig, B.A.; Warren, J.L. Updated overview of the SEER-Medicare data: Enhanced content and applications. J. Natl. Cancer Inst. Monogr. 2020, 2020, 3–13. [Google Scholar]
| Strengths | Limitations | |
|---|---|---|
| Clinical trials |
|
|
| Real-world data studies |
|
|
| Type of Real-World Data | Types of Outcomes and Measures | Strengths | Limitations | Potential Applications |
|---|---|---|---|---|
| Electronic medical records | Patient-level clinical information, e.g., vital signs, primary diagnosis, medical history Medicine prescriptions Laboratory and imaging test results | Detailed patient-level clinical information Longitudinal data capture | Unstructured data is not easy to query and capture for research Does not include data on whether treatment orders (e.g., medicine prescriptions) were carried out | Understanding patient and disease characteristics, treatment and outcomes in routine clinical practice |
| Health administrative data | Medicine dispensing records Health service utilization (e.g., diagnostic tests, imaging, physician visits, hospital admission, emergency department use) Financial data | Large numbers of patients Near-complete history of a patient’s health care resource utilization and costs provided by a given payer (e.g., universal health system) Longitudinal data capture Representative of the population | Lack of clinical data Important clinical endpoints (e.g., progression, death) often unavailable Data quality issues | Measurement of health care utilization and costs Identify outcomes of patients with rare events |
| Cancer registries | Date of cancer diagnosis Location, histology and staging of cancer Demographic details of patients | Specific and uniform capture of clinically rich and defined patient characteristics and outcomes | Data is limited to the specific disease that the registry is designed to capture | Understanding the natural history of a disease Studying the changes in incidence and prevalence of conditions over time |
| Health surveys | Self-reported health status Health-related behaviours | Representative of the general population, if correctly weighted | Self-reported data may be affected by recall bias | Understanding the health and sociodemographic characteristics of a population |
| Patient-generated data | Health statistics (e.g., heart rate, step count, activity levels) Patient-reported outcomes Quality of life data | Capture detailed and longitudinal information including patient-reported outcomes Data contains patient-centric outcomes Does not rely on data reported by providers | Populations may not be representative Capture of limited outcomes | Direct measurement of patient symptoms, activity and other outcomes |
| Social media | Patient-reported outcomes and experiences | Unfiltered reporting of outcomes and experiences by patients | Usually limited to qualitative data Data not uniformly reported by patients Difficult to confirm clinical outcomes and authenticity | Understanding patient experiences (e.g., factors affecting treatment adherence) |
Concerns regarding real-world evidence research
|
Strategies for improving the quality and acceptability of real-world evidence research
|
| Country | Universal Health Care System | Nationwide Coverage | Routine Collection of Cancer Medicine Data | Datasets for Facilitating Real-World Cancer Medicine Research |
|---|---|---|---|---|
| Australia | Yes | Yes—Commonwealth health system components (PBS, MBS) | Yes—all PBS-subsidised cancer medicines given in outpatient settings | Yes—DVA data collection |
| Canada | Yes | No—data collected in province-based health systems | Yes | Yes—SPOR to coordinate cross-jurisdictional research (but not cancer specific) |
| UK | Yes | Yes | No | Yes—SACT database collecting mandatory reporting of cancer treatment from NHS hospitals |
| Nordic countries | Yes | Yes | No | Yes—INSPIRE to collect cancer medicine data as part of Cancer Registry in Norway |
| USA (SEER-Medicare) | No | No—SEER registries cover 47.9% of the USA population [104], 96% of SEER patients aged ≥ 65 years can be matched to Medicare data [105] | Yes—Medicare data includes claims for episodes of systemic cancer treatment. | Yes—SEER-Medicare, CancerLinQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Pearson, S.-A.; Simes, R.J.; Chua, B.H. Harnessing Real-World Evidence to Advance Cancer Research. Curr. Oncol. 2023, 30, 1844-1859. https://doi.org/10.3390/curroncol30020143
Tang M, Pearson S-A, Simes RJ, Chua BH. Harnessing Real-World Evidence to Advance Cancer Research. Current Oncology. 2023; 30(2):1844-1859. https://doi.org/10.3390/curroncol30020143
Chicago/Turabian StyleTang, Monica, Sallie-Anne Pearson, Robert J. Simes, and Boon H. Chua. 2023. "Harnessing Real-World Evidence to Advance Cancer Research" Current Oncology 30, no. 2: 1844-1859. https://doi.org/10.3390/curroncol30020143




