Vertebral Primary Bone Lesions: Review of Management Options
Abstract
:1. Introduction
2. Assessment of Patients with Vertebral Lesions
2.1. Incidence Rates
2.2. Clinical Considerations
2.3. Imaging Considerations
3. Treatment of Patients with Vertebral Lesions
3.1. Primary Benign Tumors
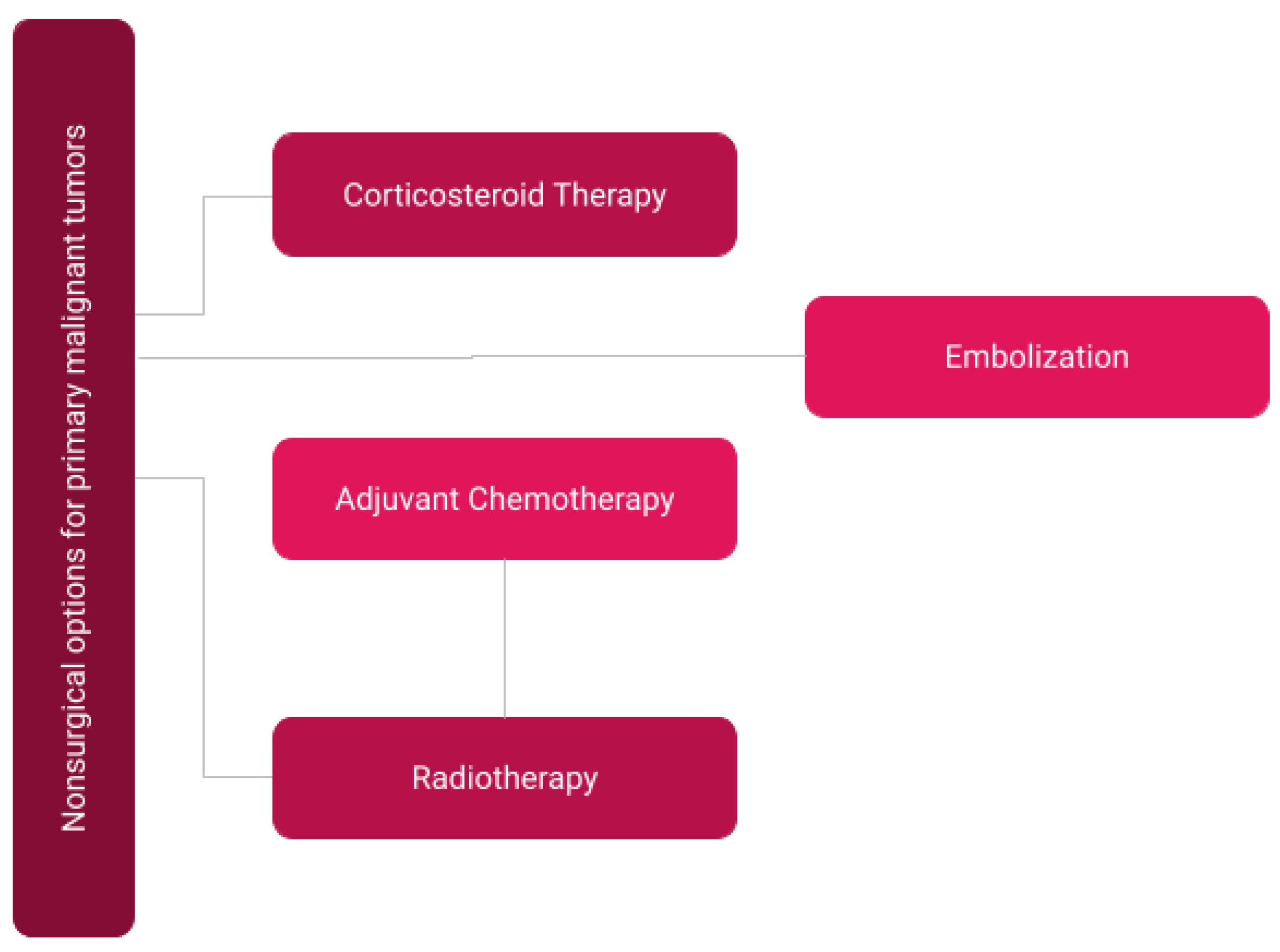
3.2. Non-Surgical Treatment for Primary Malignant Tumors
3.3. Surgical Interventions for Primary Malignant Tumors
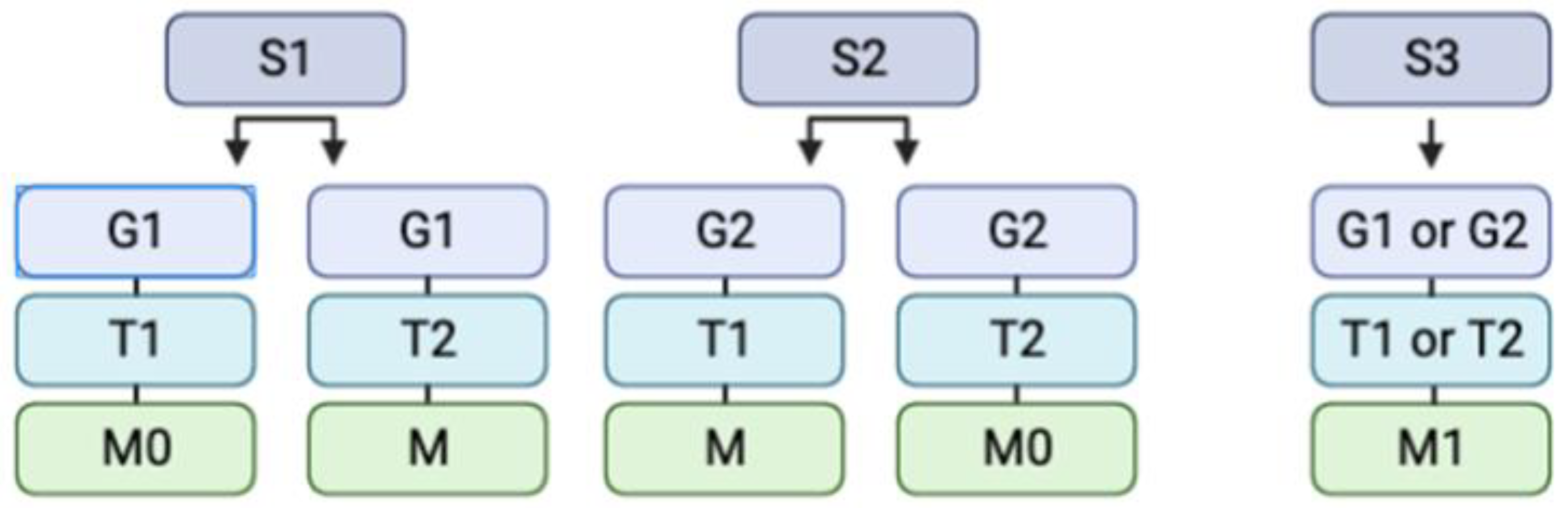
3.3.1. Surgical Determination
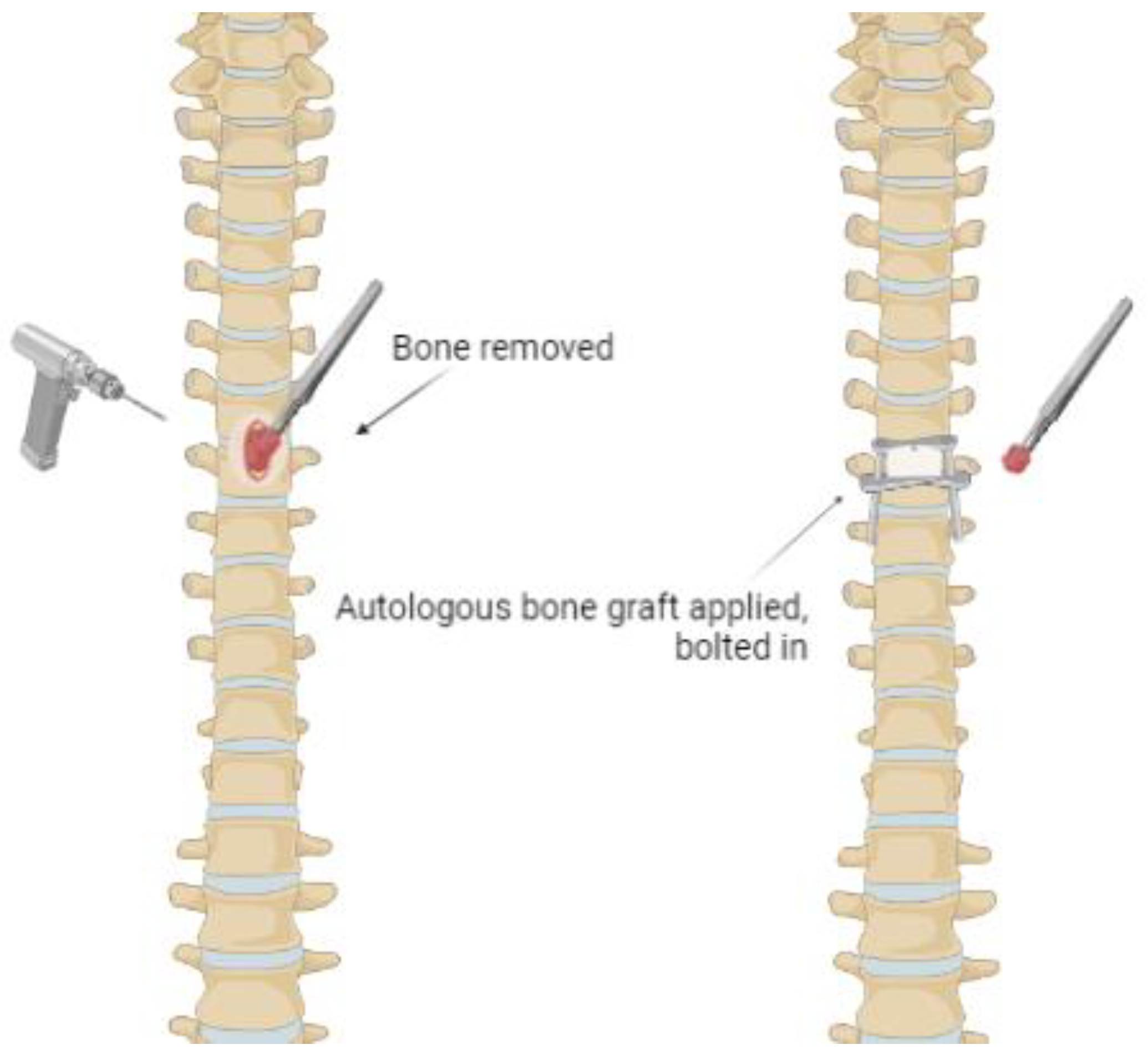
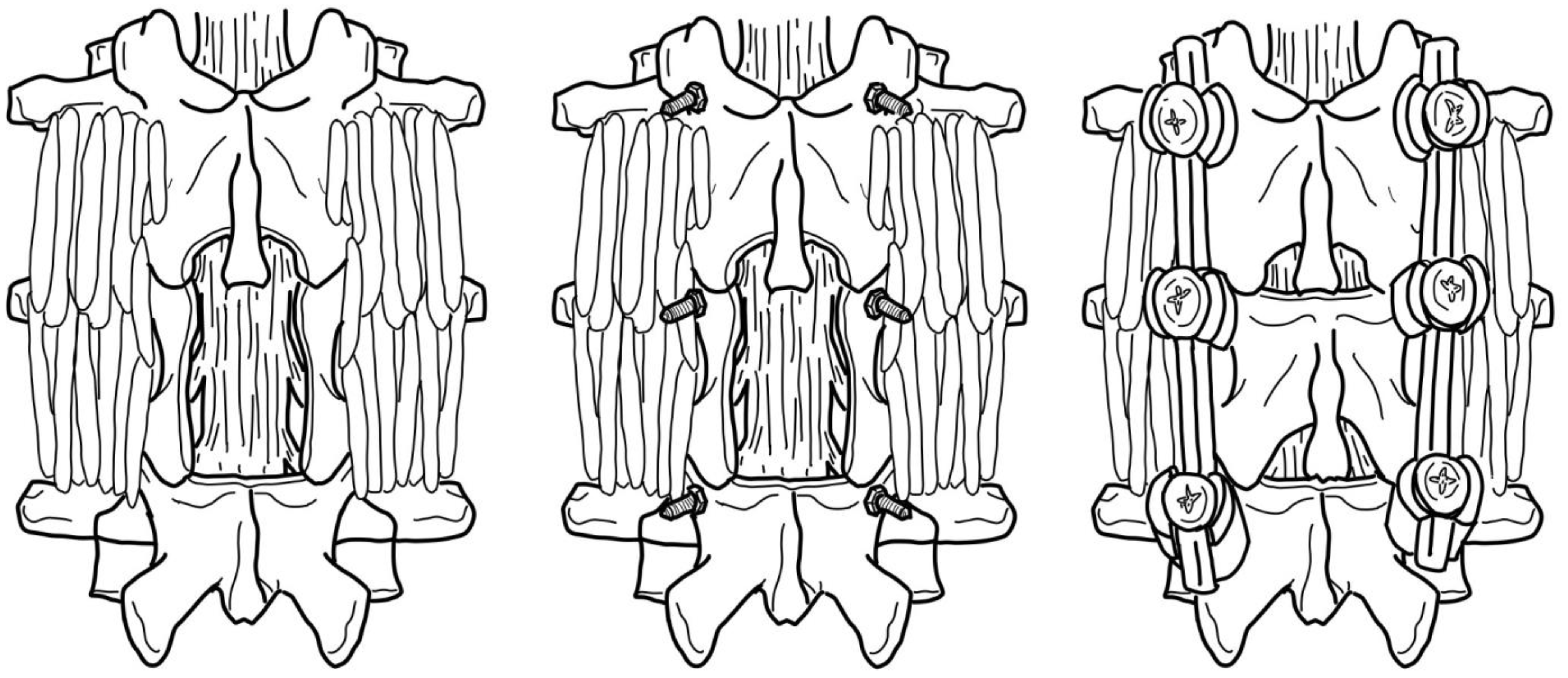
3.3.2. Direct Surgical Decompression and Stabilization
3.3.3. Minimally Invasive Surgical Alternatives
4. Emerging Interventions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almetrek, M.A.; Mahjari, A.A.; Aldharman, S.S.; Amer, K.A.; Balobaid, M.F.; Madkhali, A.; Alsayary, A.M.; Alsubaie, S.F. Surgical Intervention for Spinal Lesions Due to Multiple Myeloma: A Case Report. Cureus 2023, 15, 33505. [Google Scholar] [CrossRef]
- Coumans, J.-V.C.E.; Walcott, B.P. Incidental vertebral lesions. Neurosurg. Focus 2011, 31, E17. [Google Scholar] [CrossRef] [Green Version]
- Moscinski, C.N.; Sullivan, P.Z.; Gokaslan, Z.L. Benign primary bone tumors, long-term management into adulthood. Interdiscip. Neurosurg. 2023, 31, 101687. [Google Scholar] [CrossRef]
- Waldt, S. Primary and Secondary Bone Tumors. In Diagnostic and Interventional Radiology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1007–1032. [Google Scholar]
- Esposito, M.; Guise, T.; Kang, Y. The biology of bone metastasis. In Cold Spring Harbor Perspectives in Medicine; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2017; Volume 8, p. a031252. [Google Scholar]
- Kasch, R.; Scheele, J.; Hancock, M.; Hofer, A.; Maher, C.; Bülow, R.; Lange, J.; Lahm, A.; Napp, M.; Wassilew, G.; et al. Prevalence of benign osseous lesions of the spine and association with spinal pain in the general population in whole body MRI. PLoS ONE 2019, 14, e0219846. [Google Scholar] [CrossRef]
- Sugiyama, H.; Omonishi, K.; Yonehara, S.; Ozasa, K.; Kajihara, H.; Tsuya, T.; Takeshima, Y. Characteristics of benign and malignant bone tumors registered in the hiroshima tumor tissue registry, 1973-2012. JBJS Open Access 2018, 3, e0064. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Leckie, S.; Methot, C. Metastatic Disease of the Spine: Operative Considerations. In Surgical Procedures of the Spine for Intraoperative Neurophysiological Monitoring Providers; Springer International Publishing: Cham, Germany, 2023; pp. 67–79. [Google Scholar]
- Plant, J.; Cannon, S. Diagnostic work up and recognition of primary bone tumours: A review. EFORT Open Rev. 2016, 1, 247–253. [Google Scholar] [CrossRef]
- Mohler, D.G.; Chiu, R.; McCall, D.A.; Avedian, R.S. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin. Orthop. Relat. Res. 2010, 468, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangual-Peréz, D.; Martínez-Rivera, A.; Torres-Lugo, N.J.; Deliz-Jimenez, D.; Rivera-Rodriguez, G.; Claudio-Marcano, A.; Rivera-Colón, Y. Complete Endoscopic Resection of an Osteoid Osteoma in the Body of a Thoracic Vertebra: A Case Report. JBJS Case Connect. 2023, 13, e22. [Google Scholar] [CrossRef]
- Patnaik, S.; Jyotsnarani, Y.; Uppin, S.G.; Susarla, R. Imaging features of primary tumors of the spine: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.; Jörger, A.-K.; Ryang, Y.-M.; Liesche-Starnecker, F.; Gempt, J.; Meyer, B. Primary Bone Tumors of the Spine—Proposal for Treatment Based on a Single Centre Experience. Diagnostics 2022, 12, 2264. [Google Scholar] [CrossRef] [PubMed]
- Ciftdemir, M.; Kaya, M.; Selcuk, E.; Yalniz, E. Tumors of the spine. World J. Orthop. 2016, 7, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.A.; Castillo, M. Imaging appearance of primary bony tumors and pseudo-tumors of the spine. J. Neuroradiol. 2010, 37, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Erlemann, R. MRI is highly sensitive for the detection of bone marrow abnormalities, cortical destruction or soft tissue tumors adjacent or infiltrating neighboring bones. Eur. J. Radiol. 2006, 58, 48–67. [Google Scholar] [CrossRef]
- Boriani, S.; Bandiera, S.; Casadei, R.; Boriani, L.; Donthineni, R.; Gasbarrini, A.; Schwab, J.H. Giant cell tumor of the mobile spine: A review of 49 cases. Spine 2012, 37, E37–E45. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Thelen, J.C.; Bhatt, A.A. Bone up on spinal osseous lesions: A case review series. Insights Into Imaging 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Cianfoni, A.; Zecchi, V.; Cortese, M.C.; Rumi, N.; Colosimo, C. Instability and impending instability in patients with vertebral metastatic disease. Skelet. Radiol. 2018, 48, 195–207. [Google Scholar] [CrossRef]
- Bilsky, M.; Smith, M. Surgical approach to epidural spinal cord compression. Hematol. Clin. N. Am. 2006, 20, 1307–1317. [Google Scholar] [CrossRef]
- Jarvik, J.G.; Deyo, R.A. Diagnostic evaluation of low back pain with emphasis on imaging. Ann. Intern. Med. 2002, 137, 586–597. [Google Scholar] [CrossRef]
- Lateef, H.; Patel, D. What is the role of imaging in acute low back pain? Curr. Rev. Musculoskelet. Med. 2009, 2, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.S.; Stewart, M.E.; Davies, B.M.; Kotter, M.R.N. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: A Systematic Review and Meta-analysis. Glob. Spine J. 2020, 11, 597–607. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Jawad, M.U.; Scully, S.P. In brief: Classifications in brief: Enneking classification: Benign and malignant tumors of the musculoskeletal system. Clin. Orthop. Relat. Res.® 2010, 468, 2000–2002. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Tomita, K. Global Spine Tumor Study Group: Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010, 19, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Rodallec, M.H.; Feydy, A.; Larousserie, F.; Anract, P.; Campagna, R.; Babinet, A.; Zins, M.; Drapé, J.-L. Diagnostic imaging of solitary tumors of the spine: What to do and say. Radiographics 2008, 28, 1019–1041. [Google Scholar] [CrossRef] [Green Version]
- Abul-Kasim, K.; Persson, E.; Levinsson, A.; Strömbeck, A.; Selariu, E.; Ohlin, A. Vertebral Hemangiomas: Prevalence, new classification and natural history. magnetic resonance imaging-based retrospective longitudinal study. Neuroradiol. J. 2022, 36, 23–30. [Google Scholar] [CrossRef]
- Kan, P.; Schmidt, M.H. Osteoid Osteoma and Osteoblastoma of the Spine. Neurosurg. Clin. N. Am. 2008, 19, 65–70. [Google Scholar] [CrossRef]
- Lee, E.H.; Shafi, M.; Hui, J.H. Osteoid Osteoma: A current review. J. Pediatr. Orthop. 2006, 26, 695–700. [Google Scholar] [CrossRef]
- Kumar, N.; Tan, W.L.B.; Wei, W.; Vellayappan, B.A. An overview of the tumors affecting the spine—Inside to out. Neuro-Oncol. Pract. 2020, 7, i10–i17. [Google Scholar] [CrossRef]
- Atif, M.; Hasan, O.H.A.; Ashraf, U.; Mustafa, M.; Umer, M. Benign tumours and tumour like lesions of bone. J. Pak. Med. Assoc. 2018, 68, 1502–1507. [Google Scholar]
- Schaser, K.D.; Bail, H.J.; Haas, N.P.; Melcher, I. Treatment concepts of benign bone tumors and tumor-like bone lesions. Der 2022, 73, 1181–1190. [Google Scholar]
- Garcia, R.A.; Inwards, C.Y.; Unni, K.K. Benign bone tumors—Recent developments. In Seminars in Di-Agnostic Pathology; WB Saunders: Philadelphia, PA, USA, 2011; Volume 28, pp. 73–85. [Google Scholar]
- Fritzsche, H.; Schaser, K.D.; Hofbauer, C. Benign tumours and tumour-like lesions of the bone: General treatment principles. Der Orthopäde 2017, 46, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.S. Common benign lesions of bone in children and adolescents. J. Pediatr. Orthop. 2002, 22, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, C.; Couch, C.; Emory, C.L.; Nicholas, R. Giant Cell Tumor of Bone: Review of current literature, evaluation, and treatment options. J. Knee Surg. 2018, 32, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.S.; Akram, R.; Zehra, U.; Aziz, A. Management of Giant Cell Tumor of Talus with Extended Intralesional Curettage and Reconstruction Using Polymethylmethacrylate Cement. Foot Ankle Spec. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Knochentumoren, A. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. JBJS 2008, 90, 1060–1067. [Google Scholar] [CrossRef]
- Cummings, J.E.; Smith, R.A.; Heck, R.K. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: A preliminary study. Clin. Orthop. Relat. Res.® 2010, 468, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Omlor, G.W.; Lange, J.; Streit, M.; Gantz, S.; Merle, C.; Germann, T.; Lehner, B. Retrospective analysis of 51 intralesionally treated cases with progressed giant cell tumor of the bone: Local adjuvant use of hydrogen peroxide reduces the risk for tumor recurrence. World J. Surg. Oncol. 2019, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Ebeid, W.A.; Hasan, B.Z.; Badr, I.T.; Mesregah, M.K. Functional and Oncological Outcome After Treatment of Chondroblastoma With Intralesional Curettage. J. Pediatr. Orthop. 2019, 39, e312–e317. [Google Scholar] [CrossRef]
- Veth, R.; Schreuder, B.; van Beem, H.; Pruszczynski, M.; de Rooy, J. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005, 6, 25–34. [Google Scholar] [CrossRef]
- Dabak, N.; Tomak, Y.; Piskin, A.; Gulman, B.; Ozcan, H. Early results of a modified technique of cryosurgery. Int. Orthop. 2003, 27, 249–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deventer, N.; Gosheger, G.; de Vaal, M.; Budny, T.; Laufer, A.; Heitkoetter, B.; Luebben, T. Chondroblastoma: Is intralesional curettage with the use of adjuvants a sufficient way of therapy? J. Bone Oncol. 2020, 26, 100342. [Google Scholar] [CrossRef]
- Aro, H.T.; Välimäki, V.-V.; Strandberg, N.; Lankinen, P.; Löyttyniemi, E.; Saunavaara, V.; Seppänen, M. Bioactive glass granules versus standard autologous and allogeneic bone grafts: A randomized trial of 49 adult bone tumor patients with a 10-year follow-up. Acta Orthop. 2022, 93, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.E.R.; Ogink, P.T.; Chu, K.F.; Patel, A.; Rosenthal, B.; Shin, J.H.; Lee, S.-G.; Hornicek, F.J.; Schwab, J.H. The use of autologous free vascularized fibula grafts in reconstruction of the mobile spine following tumor resection: Surgical technique and outcomes. J. Neurosurg. Spine 2020, 34, 283–292. [Google Scholar] [CrossRef]
- Drumond, J.M.N. Benign Bone Tumors And Tumor-Like Bone Lesions: Treatment Update and New Trends. Rev. Bras. Ortop. 2009, 44, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.-N.; Chen, Y.-J.; Huang, T.-J.; Hsu, K.-Y.; Hsu, R.W.-W. Semistructural allografting in bone defects after curettage. J. Surg. Oncol. 1998, 68, 159–165. [Google Scholar] [CrossRef]
- Rougraff, B.T. Bone graft alternatives in the treatment of benign bone tumors. Instr. Course Lect. 2005, 54, 505–512. [Google Scholar]
- Gitelis, S.; Virkus, W.; Anderson, D.; Piasecki, P.; Yao, T.K. Functional outcomes of bone graft substitutes for benign bone tumors. Orthopedics 2004, 27, S141–S144. [Google Scholar] [CrossRef]
- Heyd, R.; Seegenschmiedt, M.H.; Rades, D.; Winkler, C.; Eich, H.T.; Bruns, F.; Gosheger, G.; Willich, N.; Micke, O. Radiotherapy for symptomatic vertebral hemangiomas: Results of a multicenter study and literature REVIEW. Int. J. Radiat. Oncol. 2010, 77, 217–225. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Simmons, E.D.; Zheng, Y. Vertebral tumors: Surgical versus nonsurgical treatment. Clin. Orthop. Relat. Res.® 2006, 443, 233–247. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Mansfield, F.L.; Borges, L.F. Spinal radiation before surgical decompression adversely affects out-comes of surgery for symptomatic metastatic spinal cord compression. Spine 2001, 26, 818–824. [Google Scholar] [CrossRef]
- Barbieri, E.; Chiaulon, G.; Bunkeila, F.; Putti, C.; Frezza, G.; Neri, S.; Babini, L. Radiotherapy in vertebral tumors. Indications and limits: A report on 28 cases of Ewing’s sarcoma of the spine. Chir. Degli Organi Mov. 1998, 83, 105–111. [Google Scholar]
- Lecouvet, F.; Richard, F.O.; Berg, B.V.; Malghem, J.; Maldague, B.; Jamart, J.; Michaux, J.L. Long-term effects of localized spinal radiation therapy on vertebral fractures and focal lesions appearance in patients with multiple my-eloma. Br. J. Haematol. 1997, 96, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Schuck, A.; Ahrens, S.; von Schorlemer, I.; Kuhlen, M.; Paulussen, M.; Hunold, A.; Jürgens, H. Radiotherapy in Ewing tumors of the vertebrae: Treatment results and local relapse analysis of the CESS 81/86 and EICESS 92 trials. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Heyd, R.; Strassmann, G.; Filipowicz, I.; Borowsky, K.; Martin, T.; Zamboglou, N. Radiotherapy in vertebral he-mangioma. Rontgenpraxis. Z. Radiol. Tech. 2001, 53, 208–220. [Google Scholar]
- Rosen, G.; Caparros, B.; Nirenberg, A.; Marcove, R.C.; Huvos, A.G.; Kosloff, C.; Murphy, M.L. Ewing’s sarcoma: Ten-year experience with adjuvant chemotherapy. Cancer 1981, 47, 2204–2213. [Google Scholar] [CrossRef]
- Facchini, G.; Parmeggiani, A.; Peta, G.; Martella, C.; Gasbarrini, A.; Evangelisti, G.; Rossi, G. The role of percuta-neous transarterial embolization in the management of spinal bone tumors: A literature review. Eur. Spine J. 2021, 30, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Cooke, D.; Ghodke, B.; Mirza, S. Retrospective analysis of preoperative embolization of spinal tumors. Am. J. Neuroradiol. 2009, 31, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Finstein, J.L.; Chin, K.R.; Alvandi, F.; Lackman, R.D. Case report: Postembolization paralysis in a man with a thoracolumbar giant cell tumor. Clin. Orthop. Relat. Res. 2006, 453, 335–340. [Google Scholar] [CrossRef]
- Ropper, A.E.; Cahill, K.S.; Hanna, J.W.; McCarthy, E.F.; Gokaslan, Z.L.; Chi, J.H. Primary vertebral tumors: A review of epidemiologic, histological, and imaging findings, Part I: Benign tumors. Neurosurgery 2011, 69, 1171–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zileli, M.; Çagli, S.; Basdemir, G.; Ersahin, Y. Osteoid osteomas and osteoblastomas of the spine. Neurosurg. Focus 2003, 15, 1–7. [Google Scholar] [CrossRef]
- Skeoch, G.D.; Tobin, M.K.; Khan, S.; Linninger, A.A.; Mehta, A.I. Corticosteroid treatment for metastatic spinal cord compression: A review. Glob. Spine J. 2017, 7, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Mok, S.; Park, S.C.; Kim, H.; Chang, B.-S. Treatment strategy for metastatic spinal tumors: A narrative review. Asian Spine J. 2020, 14, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Aoude, A.; Amiot, L.-P. A comparison of the modified Tokuhashi and Tomita scores in determining prognosis for patients afflicted with spinal metastasis. Can. J. Surg. 2014, 57, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Eap, C.; Tardieux, E.; Goasgen, O.; Bennis, S.; Mireau, E.; Delalande, B.; Cvitkovik, F.; Baussart, B.; Aldea, S.; Jovenin, N.; et al. Tokuhashi score and other prognostic factors in 260 patients with surgery for vertebral metastases. Orthop. Traumatol. Surg. Res. 2015, 101, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costăchescu, B.; Niculescu, A.-G.; Iliescu, B.F.; Dabija, M.G.; Grumezescu, A.M.; Rotariu, D. Current and Emerging Approaches for Spine Tumor Treatment. Int. J. Mol. Sci. 2022, 23, 15680. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakar, D.; Tanenbaum, J.E.; Phan, K.; Alentado, V.J.; Steinmetz, M.P.; Benzel, E.C.; Mroz, T.E. Decompression surgery for spinal metastases: A systematic review. Neurosurg. Focus 2016, 41, E2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodarzi, A.; Clouse, J.; Capizzano, T.; Kim, K.D.; Panchal, R. The Optimal Surgical Approach to Intradural Spinal Tumors: Laminectomy or Hemilaminectomy? Cureus 2020, 12, e7084. [Google Scholar] [CrossRef] [Green Version]
- Delank, K.S.; Wendtner, C.; Eich, H.T.; Eysel, P. The treatment of spinal metastases. Dtsch. Aerzteblatt Int. 2011, 108, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gokaslan, Z.L.; York, J.E.; Walsh, G.L.; McCutcheon, I.E.; Lang, F.F.; Putnam, J.B.; Wildrick, D.M.; Swisher, S.G.; Abi-Said, D.; Sawaya, R. Transthoracic vertebrectomy for metastatic spinal tumors. J. Neurosurg. 1998, 89, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzschneider, S.; Schmidt, H.; Weber, P.; Schmidt, G.P.; Jansson, V.; Dürr, H.R. Surgical therapy of skeletal com-plications in multiple myeloma. Int. Orthop. 2011, 35, 1209–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dürr, H.R.; Wegener, B.; Krödel, A.; Müller, P.E.; Jansson, V.; Refior, H.J. Multiple myeloma: Surgery of the spine: Retrospective analysis of 27 patients. Spine 2002, 27, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, R.; Donnarumma, P.; Nigro, L.; Delfini, R. Surgery in extensive vertebral hemangioma: Case report, literature review and a new algorithm proposal. Neurosurg. Rev. 2015, 38, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, M.; Koob, S.; Kehrer, A.; Wirtz, D.C.; Schmolders, J. Multiple Myeloma—Current Standards in Surgical Treatment. Z. Orthopädie Unf. 2018, 157, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.R. Surgical treatment of spinal tumors. Clin. Orthop. Relat. Res.® 1997, 335, 54–63. [Google Scholar] [CrossRef]
- Binh, N.T.; Hoa, T.Q.; Linh, L.T.; My, T.T.T.; Anh, P.Q.; Duc, N.M. Preoperative embolization of hypervascular spinal tumors: Two case reports. J. Clin. Imaging Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Mossa-Basha, M.; Gerszten, P.C.; Myrehaug, S.; Mayr, N.A.; Yuh, W.T.; Maralani, P.J.; Sahgal, A.; Lo, S.S. Spinal metastasis: Diagnosis, management and follow-up. Br. J. Radiol. 2019, 92, 20190211. [Google Scholar] [CrossRef]
- Alpantaki, K.; Ioannidis, A.; Raptis, K.; Spartalis, E.; Koutserimpas, C. Surgery for spinal metastatic tumors: Prognostication systems in clinical practice (Review). Mol. Clin. Oncol. 2020, 12, 399–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, O.L.; Reinas, R.; Kitumba, D.; Pereira, L. Minimally invasive surgery for spinal fractures due to multiple myeloma. J. Craniovertebral Junction Spine 2021, 12, 117–122. [Google Scholar] [CrossRef]
- Hosono, N.; Yonenobu, K.; Fuji, T.; Ebara, S.; Yamashita, K.; Ono, K. Vertebral body replacement with a ceramic prosthesis for metastatic spinal tumors. Spine 1995, 20, 2454–2462. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Trang, J.; Kos, J.; Sears, W. Experience with Recombinant Human Bone Morphogenetic Protein-2 in Posterior Lumbar Interbody Fusion: A Retrospective Review of Procedures. Int. J. Spine Surg. 2023, 17, 86–94. [Google Scholar] [CrossRef]
- Choi, H.Y.; Hyun, S.J.; Lee, C.H.; Youn, J.H.; Ryu, M.Y.; Kim, K.J. Safety and efficacy of recombinant human bone morphogenetic protein-2 in multilevel posterolateral lumbar fusion in a prospective, randomized, controlled trial. Neurospine 2022, 19, 838–846. [Google Scholar] [CrossRef]
- Chen, F.; Xia, Y.H.; Cao, W.Z.; Shan, W.; Gao, Y.; Feng, B.; Wang, D. Percutaneous kyphoplasty for the treatment of spinal metastases. Oncol. Lett. 2016, 11, 1799–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.-K.; Kim, Y.-H. Percutaneous vertebroplasty for painful spinal metastasis: A good option for better quality of life. Korean J. Anesthesiol. 2013, 64, 201–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsui, C.E.; Stafford, R.J.; Li, J.; Sellin, J.N.; Amini, B.; Rao, G.; Suki, D.; Ghia, A.J.; Brown, P.; Lee, S.-H.; et al. Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. J. Neurosurg. Spine 2015, 23, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Bate, B.G.; Khan, N.R.; Kimball, B.Y.; Gabrick, K.; Weaver, J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J. Neurosurg. Spine 2015, 22, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Chang, U.-K.; Lee, D.H.; Kim, M.-S. Stereotactic radiosurgery for primary malignant spinal tumors. Neurol. Res. 2014, 36, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Elibe, E.; Boyce-Fappiano, D.; Ryu, S.; Siddiqui, M.S.; Lee, I.; Rock, J.; Siddiqui, F. Stereotactic radiosurgery for primary tumors of the spine and spinal. J. Radiosurgery SBRT 2018, 5, 107–113. [Google Scholar]
- Huo, M.; Sahgal, A.; Pryor, D.; Redmond, K.; Lo, S.; Foote, M. Stereotactic spine radiosurgery: Review of safety and efficacy with respect to dose and fractionation. Surg. Neurol. Int. 2017, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Devaux, B.C.; Roux, F.X. Experimental and clinical standards, and evolution of lasers in neurosurgery. Acta Neurochir. 1996, 138, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Boggan, J.E. Argon laser surgery of pediatric neural neoplasms. Pediatr. Neurosurg. 1984, 11, 171–175. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Chiba, K.; Heller, J.G.; Patel, T.C.; Thalgott, J.S.; Truumees, E.; Wang, J.C. Bone grafting alter-natives in spinal surgery. Spine J. 2002, 2, 206–215. [Google Scholar] [CrossRef]
- Charest-Morin, R.; Fisher, C.G.; Sahgal, A.; Boriani, S.; Gokaslan, Z.L.; Lazary, A.; Reynolds, J.; Bettegowda, C.; Rhines, L.D.; Dea, N. Primary bone tumor of the spine—An evolving field: What a general spine surgeon should know. Glob. Spine J. 2019, 9, 108S–116S. [Google Scholar] [CrossRef]
- Orenstein, L.A.; Nguyen, T.V.; Damiani, G.; Sayed, C.; Jemec, G.B.; Hamzavi, I. Medical and surgical management of hidradenitis suppurativa: A review of international treatment guidelines and implementation in general dermatology practice. Dermatology 2020, 236, 393–412. [Google Scholar] [CrossRef]
- Saiz, P.; Virkus, W.; Piasecki, P.; Templeton, A.; Shott, S.; Gitelis, S. Results of giant cell tumor of bone treated with intralesional excision. Clin. Orthop. Relat. Res. 2004, 424, 221–226. [Google Scholar] [CrossRef]
- Suit, H.; Spiro, I. Radiation treatment of benign mesenchymal disease. Semin. Radiat. Oncol. 1999, 9, 171–178. [Google Scholar] [CrossRef]
- Lo, S.S.; Fakiris, A.J.; Chang, E.L.; Mayr, N.A.; Wang, J.Z.; Papiez, L.; Timmerman, R.D. Stereotactic body radiation therapy. A novel treatment modality. Nat. Rev. Clin. Oncol. 2010, 7, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Hoskin, P. Stereotactic body radiotherapy for spinal and bone metastases. Clin. Oncol. 2015, 27, 298–306. [Google Scholar] [CrossRef]
- Bydon, M.; De la Garza-Ramos, R.; Bettagowda, C.; Gokaslan, Z.L.; Sciubba, D.M. The use of stereotactic radio-surgery for the treatment of spinal axis tumors: A review. Clin. Neurol. Neurosurg. 2014, 125, 166–172. [Google Scholar] [CrossRef]
- Zhang, H.R.; Li, J.K.; Yang, X.G.; Qiao, R.Q.; Hu, Y.C. Conventional radiotherapy and stereotactic radiosurgery in the management of metastatic spine disease. Technol. Cancer Res. Treat. 2020, 19, 1533033820945798. [Google Scholar] [CrossRef]
- Guckenberger, M.; Mantel, F.; Gerszten, P.C.; Flickinger, J.C.; Sahgal, A.; Létourneau, D.; Grills, I.S.; Jawad, M.; Fahim, D.K.; Shin, J.H.; et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: A multi-institutional analysis. Radiat. Oncol. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callstrom, M.R.; Atwell, T.D.; Charboneau, J.W.; Farrell, M.A.; Goetz, M.P.; Rubin, J.; Sloan, J.A.; Novotny, P.J.; Welch, T.J.; Maus, T.P.; et al. Painful metastases involving bone: Percutaneous image-guided cryoablation—Prospective trial Interim analysis. Radiology 2006, 241, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, N.S.; Haider, A.S.; Ozair, A.; Vannabouathong, C.; Rahman, M.; Haider, M.; Vira, S. Percutaneous im-age-guided cryoablation of spinal metastases: A systematic review. J. Clin. Neurosci. 2022, 96, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Moses, Z.B.; Lee, T.C.; Huang, K.T.; Guenette, J.P.; Chi, J.H. MRI-guided cryoablation for metastatic spine disease: Intermediate-term clinical outcomes in 14 consecutive patients. J. Neurosurg. Spine 2020, 32, 676–681. [Google Scholar] [CrossRef]
- Callstrom, M.R.; Kurup, A.N. Percutaneous ablation for bone and soft tissue metastases—Why cryoablation? Skelet. Radiol. 2009, 38, 835–839. [Google Scholar] [CrossRef] [Green Version]
- Santiago, E.; Pauly, V.; Brun, G.; Guenoun, D.; Champsaur, P.; Le Corroller, T. Percutaneous cryoablation for the treatment of osteoid osteoma in the adult population. Eur. Radiol. 2018, 28, 2336–2344. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.W. Is percutaneous bone cryoablation safe? Radiology 2019, 291, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Tomasian, A.; Wallace, A.; Northrup, B.; Hillen, T.; Jennings, J. Spine cryoablation: Pain palliation and local tumor control for vertebral metastases. Am. J. Neuroradiol. 2015, 37, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auloge, P.; Cazzato, R.L.; Rousseau, C.; Caudrelier, J.; Koch, G.; Rao, P.; Chiang, J.B.; Garnon, J.; Gangi, A. Complications of percutaneous bone tumor cryoablation: A 10-year experience. Radiology 2019, 291, 521–528. [Google Scholar] [CrossRef]


| Primary Vertebral Lesion | Benign or Malignant | Radiographic Findings (MRI) |
|---|---|---|
| Spinal Meningioma | Benign | Well-circumscribed; Dural tail sign; broad-based Dural attachment |
| Osteoid Osteomas | Benign | Intracortical nidus; reactive sclerosis |
| Spinal Ependymomas | Malignant | Well-circumscribed; widened spinal cord; cystic change |
| Spinal Hemangioma | Benign | Variable enhancement; “salt and pepper” appearance |
| Chordomas | Malignant | Irregular calcification; honeycomb appearance |
| Ewing Sarcoma | Malignant | Bright with T2; not well-demarcated; non-homogenous enhancement of contrast |
| Nerve Sheath Tumors | Benign | Very well-circumscribed and difficult to identify on radiograph; hyperintense T2 |
| Osteosarcoma | Malignant | Edema around tumor; cortical destruction; considerable contrast enhancement with T1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalamgari, A.; Valle, D.; Palau Villarreal, X.; Foreman, M.; Liu, A.; Patel, A.; Dave, A.; Lucke-Wold, B. Vertebral Primary Bone Lesions: Review of Management Options. Curr. Oncol. 2023, 30, 3064-3078. https://doi.org/10.3390/curroncol30030232
Chalamgari A, Valle D, Palau Villarreal X, Foreman M, Liu A, Patel A, Dave A, Lucke-Wold B. Vertebral Primary Bone Lesions: Review of Management Options. Current Oncology. 2023; 30(3):3064-3078. https://doi.org/10.3390/curroncol30030232
Chicago/Turabian StyleChalamgari, Anjalika, Daisy Valle, Xuban Palau Villarreal, Marco Foreman, Annika Liu, Aashay Patel, Akanksha Dave, and Brandon Lucke-Wold. 2023. "Vertebral Primary Bone Lesions: Review of Management Options" Current Oncology 30, no. 3: 3064-3078. https://doi.org/10.3390/curroncol30030232
APA StyleChalamgari, A., Valle, D., Palau Villarreal, X., Foreman, M., Liu, A., Patel, A., Dave, A., & Lucke-Wold, B. (2023). Vertebral Primary Bone Lesions: Review of Management Options. Current Oncology, 30(3), 3064-3078. https://doi.org/10.3390/curroncol30030232






