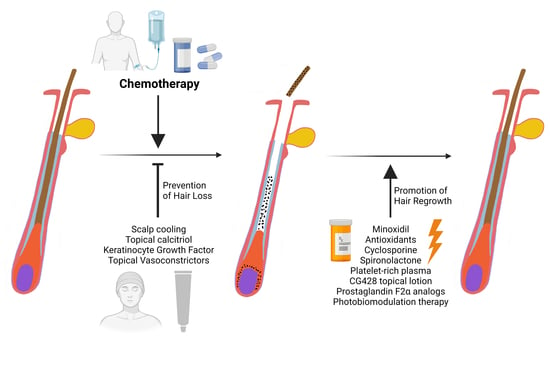
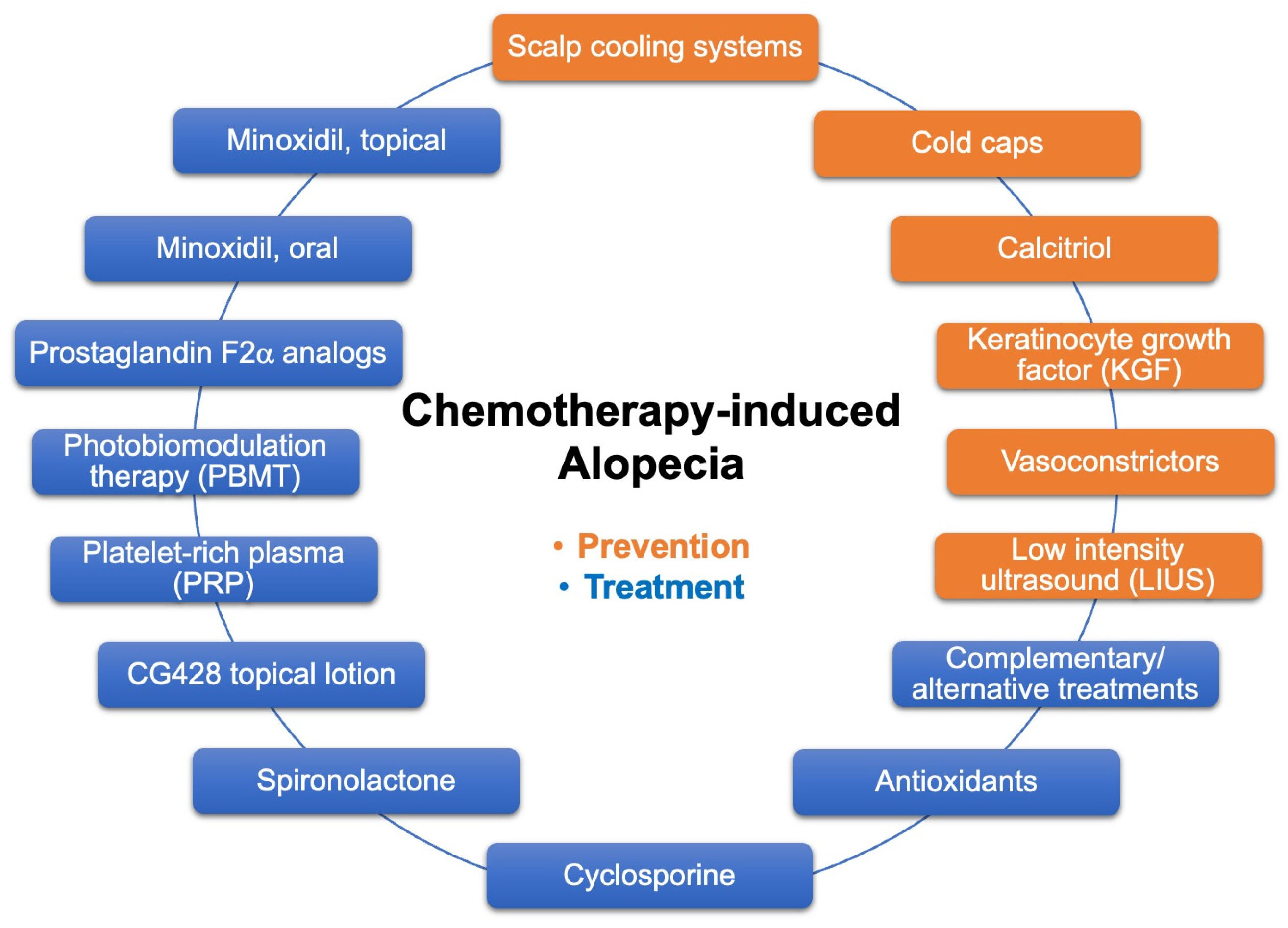
Prevention and Treatment of Chemotherapy-Induced Alopecia: What Is Available and What Is Coming?
Abstract
1. Introduction
2. CIA Prevention: Scalp Cooling and Topical Vasoconstrictors
2.1. Scalp Cooling Systems and Cold Caps
2.2. Topical Calcitriol
2.3. Keratinocyte Growth Factor (KGF)
2.4. Topical Vasoconstrictors
2.5. Low Intensity Ultrasound (LIUS)
3. CIA Treatment
3.1. Minoxidil
3.2. Bimatoprost/Prostaglandin F2α Analog
3.3. Photobiomodulation Therapy (PBMT)
3.4. Platelet-Rich Plasma (PRP)
3.5. Spironolactone
3.6. Other Agents
3.7. Complementary and Alternative Treatments
4. Supplementary Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Cancer Research Fund International. Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 14 February 2023).
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Amarillo, D.; de Boni, D.; Cuello, M. Chemotherapy, Alopecia, and Scalp Cooling Systems. Actas Dermosifiliogr. 2022, 113, 278–283. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Shapiro, J.; Goldfarb, S.; Nangia, J.; Jimenez, J.J.; Paus, R.; Lacouture, M.E. Hair disorders in patients with cancer. J. Am. Acad. Dermatol. 2019, 80, 1179–1196. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Saraswat, N.; Chopra, A.; Sood, A.; Kamboj, P.; Kumar, S. A Descriptive Study to Analyze Chemotherapy-Induced Hair Loss and its Psychosocial Impact in Adults: Our Experience from a Tertiary Care Hospital. Indian Dermatol. Online J. 2019, 10, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Trueb, R.M. Chemotherapy-induced alopecia. Semin. Cutan. Med. Surg. 2009, 28, 11–14. [Google Scholar] [CrossRef]
- Rossi, A.; Fortuna, M.C.; Caro, G.; Pranteda, G.; Garelli, V.; Pompili, U.; Carlesimo, M. Chemotherapy-induced alopecia management: Clinical experience and practical advice. J. Cosmet. Dermatol. 2017, 16, 537–541. [Google Scholar] [CrossRef]
- Rossi, A.; Caro, G.; Fortuna, M.C.; Pigliacelli, F.; D’Arino, A.; Carlesimo, M. Prevention and Treatment of Chemotherapy-Induced Alopecia. Dermatol. Pract. Concept. 2020, 10, e2020074. [Google Scholar] [CrossRef]
- Santos, T.S.; Hernandez Galvis, K.; Vano Galvan, S.; Saceda-Corralo, D. Post-chemotherapy alopecia: What the dermatologist needs to know. Int. J. Dermatol. 2021, 60, 1313–1317. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Chan, D.; Sibaud, V.; Shapiro, J.; Fabbrocini, G.; Tosti, A.; Cho, J.; Goldfarb, S.; Modi, S.; Gajria, D.; et al. Assessment of Quality of Life and Treatment Outcomes of Patients With Persistent Postchemotherapy Alopecia. JAMA Dermatol. 2019, 155, 724–728. [Google Scholar] [CrossRef]
- Kang, D.; Kim, I.R.; Choi, E.K.; Im, Y.H.; Park, Y.H.; Ahn, J.S.; Lee, J.E.; Nam, S.J.; Lee, H.K.; Park, J.H.; et al. Permanent Chemotherapy-Induced Alopecia in Patients with Breast Cancer: A 3-Year Prospective Cohort Study. Oncologist 2019, 24, 414–420. [Google Scholar] [CrossRef]
- Chan, J.; Adderley, H.; Alameddine, M.; Armstrong, A.; Arundell, D.; Fox, R.; Harries, M.; Lim, J.; Salih, Z.; Tetlow, C.; et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: A retrospective survey at two tertiary UK cancer centres. Eur. J. Cancer Care 2021, 30, e13395. [Google Scholar] [CrossRef]
- Bhoyrul, B.; Asfour, L.; Lutz, G.; Mitchell, L.; Jerjen, R.; Sinclair, R.D.; Holmes, S.; Chaudhry, I.H.; Harries, M.J. Clinicopathologic Characteristics and Response to Treatment of Persistent Chemotherapy-Induced Alopecia in Breast Cancer Survivors. JAMA Dermatol. 2021, 157, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Piccini, I.; Brunken, L.; Cheret, J.; Ghatak, S.; Ramot, Y.; Alam, M.; Purba, T.S.; Hardman, J.; Erdmann, H.; Jimenez, F.; et al. Peroxisome proliferator-activated receptor-gamma signalling protects hair follicle stem cells from chemotherapy-induced apoptosis and epithelial-mesenchymal transition. Br. J. Dermatol. 2022, 186, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Purba, T.S.; Ng’andu, K.; Brunken, L.; Smart, E.; Mitchell, E.; Hassan, N.; O’Brien, A.; Mellor, C.; Jackson, J.; Shahmalak, A.; et al. CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Mol. Med. 2019, 11, e11031. [Google Scholar] [CrossRef] [PubMed]
- Hadshiew, I.M.; Foitzik, K.; Arck, P.C.; Paus, R. Burden of hair loss: Stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J. Investig. Dermatol. 2004, 123, 455–457. [Google Scholar] [CrossRef]
- Paus, R.; Haslam, I.S.; Sharov, A.A.; Botchkarev, V.A. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013, 14, e50–e59. [Google Scholar] [CrossRef]
- Gandhi, M.; Oishi, K.; Zubal, B.; Lacouture, M.E. Unanticipated toxicities from anticancer therapies: Survivors’ perspectives. Support. Care Cancer 2010, 18, 1461–1468. [Google Scholar] [CrossRef]
- Delgado Rodriguez, J.; Ramos-Garcia, V.; Infante-Ventura, D.; Suarez-Herrera, J.C.; Rueda-Dominguez, A.; Serrano-Aguilar, P.; Del Mar Trujillo-Martin, M. Ethical, legal, organizational and social issues related to the use of scalp cooling for the prevention of chemotherapy-induced alopecia: A systematic review. Health Expect. 2022, 26, 567–578. [Google Scholar] [CrossRef]
- Quesada, S.; Guichard, A.; Fiteni, F. Cancer-Related Alopecia: From Etiologies to Global Management. Cancers 2021, 13, 5556. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Alabdaljabar, M.S.; Ruddy, K.J.; Haddad, T.C.; Thompson, C.A.; Lehman, J.S.; Hashmi, S.K. Management of chemotherapy-induced alopecia (CIA): A comprehensive review and future directions. Crit. Rev. Oncol. Hematol. 2020, 156, 103093. [Google Scholar] [CrossRef] [PubMed]
- Boyle, F.M.; Shaw, J.; Young, A.; van den Hurk, C.; Rugo, H.S.; Fogarty, G.B.; Lacouture, M.E. Management of Alopecia Due to Cancer Therapies; Olver, I., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Olsen, E.A. Chemotherapy-Induced Alopecia: Overview and Methodology for Characterizing Hair Changes and Regrowth. In The MASCC Textbook of Cancer Supportive Care and Survivorship; Olver, I., Ed.; Springer: New York, NY, USA, 2011; pp. 381–386. [Google Scholar]
- Chen, L.; Xu, Y.; Ye, X. Low temperature mitigating the paclitaxel-induced damages in mouse cell and hair follicle model. Biochem. Biophys. Res. Commun. 2022, 603, 94–101. [Google Scholar] [CrossRef]
- Hillen, H.F.; Breed, W.P.; Botman, C.J. Scalp cooling by cold air for the prevention of chemotherapy-induced alopecia. Neth. J. Med. 1990, 37, 231–235. [Google Scholar] [PubMed]
- Wang, S.; Yang, T.; Shen, A.; Qiang, W.; Zhao, Z.; Zhang, F. The scalp cooling therapy for hair loss in breast cancer patients undergoing chemotherapy: A systematic review and meta-analysis. Support. Care Cancer 2021, 29, 6943–6956. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.H.; Okhovat, J.P.; Hagigeorges, D.; Manatis-Lornell, A.J.; Isakoff, S.J.; Lacouture, M.E.; Senna, M.M. The effect of scalp cooling on CIA-related quality of life in breast cancer patients: A systematic review. Breast Cancer Res. Treat. 2019, 175, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Kanat, O.; Ertas, H.; Caner, B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J. Clin. Oncol. 2017, 8, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Komen, M.M.; Smorenburg, C.H.; van den Hurk, C.J.; Nortier, J.W. Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist 2013, 18, 885–891. [Google Scholar] [CrossRef]
- Saini, K.; Mysore, V. Role of vitamin D in hair loss: A short review. J. Cosmet. Dermatol. 2021, 20, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.F.; Elgamal, E.; Fouda, I. Intralesional vitamin D3 in treatment of alopecia areata: A randomized controlled clinical trial. J. Cosmet. Dermatol. 2022, 21, 4617–4622. [Google Scholar] [CrossRef]
- Hidalgo, M.; Rinaldi, D.; Medina, G.; Griffin, T.; Turner, J.; Von Hoff, D.D. A phase I trial of topical topitriol (calcitriol, 1,25-dihydroxyvitamin D3) to prevent chemotherapy-induced alopecia. Anticancer Drugs 1999, 10, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Dion, H.; Ravipaty, S.; Jimenez, J.J.; Thapa, K.; Grund, E.M.; Benaim, E.; Tanna, N.; Luan, S.; DiTommaso, N.; et al. A phase I safety study of topical calcitriol (BPM31543) for the prevention of chemotherapy-induced alopecia. Breast Cancer Res. Treat. 2021, 186, 107–114. [Google Scholar] [CrossRef]
- Yen, T.T.; Thao, D.T.; Thuoc, T.L. An overview on keratinocyte growth factor: From the molecular properties to clinical applications. Protein Pept. Lett. 2014, 21, 306–317. [Google Scholar] [CrossRef]
- Kapoor, R.; Shome, D. Intradermal injections of a hair growth factor formulation for enhancement of human hair regrowth—Safety and efficacy evaluation in a first-in-man pilot clinical study. J. Cosmet. Laser Ther. 2018, 20, 369–379. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Bodo, E.; Tobin, D.J.; Kamenisch, Y.; Biro, T.; Berneburg, M.; Funk, W.; Paus, R. Dissecting the impact of chemotherapy on the human hair follicle: A pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am. J. Pathol. 2007, 171, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Soref, C.M.; Fahl, W.E. A new strategy to prevent chemotherapy and radiotherapy-induced alopecia using topically applied vasoconstrictor. Int. J. Cancer 2015, 136, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Amaya, C.; Smith, E.R.; Xu, X.X. Low Intensity Ultrasound as an Antidote to Taxane/Paclitaxel-induced Cytotoxicity. J. Cancer 2022, 13, 2362–2373. [Google Scholar] [CrossRef] [PubMed]
- Amaya, C.; Luo, S.; Baigorri, J.; Baucells, R.; Smith, E.R.; Xu, X.X. Exposure to low intensity ultrasound removes paclitaxel cytotoxicity in breast and ovarian cancer cells. BMC Cancer 2021, 21, 981. [Google Scholar] [CrossRef]
- Stokes, G.S.; Oates, H.F.; MacCarthy, E.P. Antihypertensive therapy: New pharmacological approaches. Am. Heart J. 1980, 100, 741–752. [Google Scholar] [CrossRef]
- Rossi, A.; Anzalone, A.; Fortuna, M.C.; Caro, G.; Garelli, V.; Pranteda, G.; Carlesimo, M. Multi-therapies in androgenetic alopecia: Review and clinical experiences. Dermatol. Ther. 2016, 29, 424–432. [Google Scholar] [CrossRef]
- Rodriguez, R.; Machiavelli, M.; Leone, B.; Romero, A.; Cuevas, M.A.; Langhi, M.; Romero Acuna, L.; Romero Acuna, J.; Amato, S.; Barbieri, M.; et al. Minoxidil (Mx) as a prophylaxis of doxorubicin--induced alopecia. Ann. Oncol. 1994, 5, 769–770. [Google Scholar] [CrossRef]
- Stoehr, J.R.; Choi, J.N.; Colavincenzo, M.; Vanderweil, S. Off-Label Use of Topical Minoxidil in Alopecia: A Review. Am. J. Clin. Dermatol. 2019, 20, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef]
- Duvic, M.; Lemak, N.A.; Valero, V.; Hymes, S.R.; Farmer, K.L.; Hortobagyi, G.N.; Trancik, R.J.; Bandstra, B.A.; Compton, L.D. A randomized trial of minoxidil in chemotherapy-induced alopecia. J. Am. Acad. Dermatol. 1996, 35, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovitsky, A.; Segal, O.; Maly, A.; Zlotogorski, A.; Barzilai, A. Permanent chemotherapy-induced alopecia after hematopoietic stem cell transplantation treated with low-dose oral minoxidil. JAAD Case Rep. 2022, 22, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Chen, L.H.; Yeh, Y.M.; Wu, P.Y.; Chen, Y.F.; Chang, L.Y.; Chang, J.Y.; Shen, M.R. Minoxidil is a potential neuroprotective drug for paclitaxel-induced peripheral neuropathy. Sci. Rep. 2017, 7, 45366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhan, W.; Wei, X. Clinical pharmacology and pharmacogenetics of prostaglandin analogues in glaucoma. Front. Pharmacol. 2022, 13, 1015338. [Google Scholar] [CrossRef]
- Mohan, N.; Chakrabarti, A.; Nazm, N.; Mehta, R.; Edward, D.P. Newer advances in medical management of glaucoma. Indian J. Ophthalmol. 2022, 70, 1920–1930. [Google Scholar] [CrossRef]
- Herane, M.I.; Urbina, F. Acquired trichomegaly of the eyelashes and hypertrichosis induced by bimatoprost. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Shafranov, G. Hypertrichosis of vellus hairs of the malar region after unilateral treatment with bimatoprost. Am. J. Ophthalmol. 2004, 137, 756–757. [Google Scholar] [CrossRef]
- Law, S.K. Bimatoprost in the treatment of eyelash hypotrichosis. Clin. Ophthalmol. 2010, 4, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Barron-Hernandez, Y.L.; Tosti, A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin. Investig. Drugs 2017, 26, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Harii, K.; Arase, S.; Tsuboi, R.; Weng, E.; Daniels, S.; VanDenburgh, A. Bimatoprost for eyelash growth in Japanese subjects: Two multicenter controlled studies. Aesthetic Plast. Surg. 2014, 38, 451–460. [Google Scholar] [CrossRef]
- Glaser, D.A.; Hossain, P.; Perkins, W.; Griffiths, T.; Ahluwalia, G.; Weng, E.; Beddingfield, F.C. Long-term safety and efficacy of bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: A randomized controlled trial. Br. J. Dermatol. 2015, 172, 1384–1394. [Google Scholar] [CrossRef]
- Coondoo, A.; Sengupta, S. Serendipity and its role in dermatology. Indian J. Dermatol. 2015, 60, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Lonnfors, S.; Hillmann, K.; Garcia Bartels, N. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012, 66, 794–800. [Google Scholar] [CrossRef]
- Levy, L.L.; Emer, J.J. Female pattern alopecia: Current perspectives. Int. J. Womens Health 2013, 5, 541–556. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for the management of alopecia: Mechanisms of action, patient selection and perspectives. Clin. Cosmet. Investig. Dermatol. 2019, 12, 669–678. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Lesniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Cell biology: Power games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef]
- da Silveira, S.P.; Moita, S.R.U.; da Silva, S.V.; Rodrigues, M.; da Silva, D.F.T.; Pavani, C. The role of photobiomodulation when associated with microneedling in female pattern hair loss: A randomized, double blind, parallel group, three arm, clinical study protocol. Medicine (Baltimore) 2019, 98, e14938. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Wikramanayake, T.C.; Bergfeld, W.; Hordinsky, M.; Hickman, J.G.; Hamblin, M.R.; Schachner, L.A. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: A multicenter, randomized, sham device-controlled, double-blind study. Am. J. Clin. Dermatol. 2014, 15, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Buscone, S.; Mardaryev, A.N.; Raafs, B.; Bikker, J.W.; Sticht, C.; Gretz, N.; Farjo, N.; Uzunbajakava, N.E.; Botchkareva, N.V. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg. Med. 2017, 49, 705–718. [Google Scholar] [CrossRef]
- Lanzafame, R.J.; Blanche, R.R.; Bodian, A.B.; Chiacchierini, R.P.; Fernandez-Obregon, A.; Kazmirek, E.R. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg. Med. 2013, 45, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, R.J.; Blanche, R.R.; Chiacchierini, R.P.; Kazmirek, E.R.; Sklar, J.A. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg. Med. 2014, 46, 601–607. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014, 46, 144–151. [Google Scholar] [CrossRef]
- Luck, M.; Mathes, T.; Bruun, S.; Fudim, R.; Hagedorn, R.; Tran Nguyen, T.M.; Kateriya, S.; Kennis, J.T.; Hildebrandt, P.; Hegemann, P. A photochromic histidine kinase rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light. J. Biol. Chem. 2012, 287, 40083–40090. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Villasante, A.C.; Mauro, L.M.; Nouri, K.; Schachner, L.A.; Perez, C.I.; Jimenez, J.J. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA). Lasers Med. Sci. 2013, 28, 701–706. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Mercuri, S.R.; Paolino, G.; Di Nicola, M.R.; Vollono, L. Investigating the Safety and Efficacy of Platelet-Rich Plasma (PRP) Treatment for Female Androgenetic Alopecia: Review of the Literature. Medicina (Kaunas) 2021, 57, 311. [Google Scholar] [CrossRef]
- Verma, R.; Kumar, S.; Garg, P.; Verma, Y.K. Platelet-rich plasma: A comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. 2022, 1–22. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Alimohamadi, Y.; Janani, M.; Hejazi, P.; Kamali, M.; Goodarzi, A. Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2022, 16, 875–899. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Ni Riordain, R. Autologous platelet concentrates in oral surgery: Protocols, properties, and clinical applications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryc, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryc, R.; Dolegowska, B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022, 64, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, S.R.; Di Nicola, M.R.; Brianti, P.; Bianchi, V.G.; Paolino, G. Pilot Study on the Use of the “Monocyte-Rich” Platelet-Rich Plasma in Combination with 1927 nm Fractional and 308 nm Excimer Lasers for the Treatment of Vitiligo. Medicina (Kaunas) 2021, 57, 904. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, A.; Hosseini, H.; Gholami, J.; Mirminachi, B.; Firooz, F.; Firooz, A. Platelet rich plasma for treatment of non-scarring hair loss: Systematic review of literature. J. Dermatolog. Treat 2017, 28, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Rudnicka, L. Chemotherapy-induced alopecia—The urgent need for treatment options. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e69–e70. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Dev. Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527–539. [Google Scholar] [CrossRef]
- Cruciani, M.; Masiello, F.; Pati, I.; Marano, G.; Pupella, S.; De Angelis, V. Platelet-rich plasma for the treatment of alopecia: A systematic review and meta-analysis. Blood Transfus. 2021, 21, 24–36. [Google Scholar] [CrossRef]
- Stamatiou, C.A.; Lens, A.; Perez, C.I.; Daunert, S.; Jimenez, J.J. The Role of Platelet-Rich Plasma in the Prevention of Chemotherapy-Induced Alopecia. Ski. Appendage Disord. 2020, 6, 58–60. [Google Scholar] [CrossRef]
- Searle, T.N.; Al-Niaimi, F.; Ali, F.R. Spironolactone in dermatology: Uses in acne and beyond. Clin. Exp. Dermatol. 2020, 45, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Medina, D.A.; Cazarin, J.; Magana, M. Spironolactone in dermatology. Dermatol. Ther. 2022, 35, e15321. [Google Scholar] [CrossRef]
- Butt, A.K.; Patel, J.; Shirwany, H.; Mirza, Q.; Hoover, J.; Khouzam, R.N. Beneficial Extracardiac Effects of Cardiovascular Medications. Curr. Cardiol. Rev. 2022, 18, e151021197270. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.J.; De Souza, B.; Flynn, E.; Hagigeorges, D.; Senna, M.M. Spironolactone for treatment of female pattern hair loss. J. Am. Acad. Dermatol. 2020, 83, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Cantelli, M.; Masara, A.; Annunziata, M.C.; Marasca, C.; Cacciapuoti, S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. Int. J. Womens Dermatol. 2018, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- James, J.F.; Jamerson, T.A.; Aguh, C. Efficacy and safety profile of oral spironolactone use for androgenic alopecia: A systematic review. J. Am. Acad. Dermatol. 2022, 86, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.D. Female pattern hair loss: A pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int. J. Dermatol. 2018, 57, 104–109. [Google Scholar] [CrossRef]
- Vargas-Mora, P.; Morgado-Carrasco, D. Spironolactone in Dermatology: Uses in Acne, Hidradenitis Suppurativa, Female Pattern Baldness, and Hirsutism. Actas Dermosifiliogr. (Engl. Ed.) 2020, 111, 639–649. [Google Scholar] [CrossRef]
- Rozner, R.N.; Freites-Martinez, A.; Shapiro, J.; Geer, E.B.; Goldfarb, S.; Lacouture, M.E. Safety of 5alpha-reductase inhibitors and spironolactone in breast cancer patients receiving endocrine therapies. Breast Cancer Res. Treat 2019, 174, 15–26. [Google Scholar] [CrossRef]
- Funder, J.W. Spironolactone in cardiovascular disease: An expanding universe? F1000Research 2017, 6, 1738. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, K.; Hamade, H.; Lopez-Olivo, M.A.; Wehner, M.; Tosh, T.; Barbieri, J.S. Association of Spironolactone Use With Risk of Cancer: A Systematic Review and Meta-analysis. JAMA Dermatol. 2022, 158, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.S.; Morant, S.V.; Wei, L.; Thompson, A.M.; MacDonald, T.M. Spironolactone use and risk of incident cancers: A retrospective, matched cohort study. Br. J. Clin. Pharmacol. 2017, 83, 653–663. [Google Scholar] [CrossRef]
- Hiebert, B.M.; Janzen, B.W.; Sanjanwala, R.M.; Ong, A.D.; Feldman, R.D.; Kim, J.O. Impact of spironolactone exposure on prostate cancer incidence amongst men with heart failure: A Pharmacoepidemiological study. Br. J. Clin. Pharmacol. 2021, 87, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.; Eini, L.; Nissim-Rafinia, M.; Viner, R.; Ezer, S.; Erez, K.; Aqaqe, N.; Hanania, R.; Milyavsky, M.; Meshorer, E.; et al. Spironolactone inhibits the growth of cancer stem cells by impairing DNA damage response. Oncogene 2019, 38, 3103–3118. [Google Scholar] [CrossRef]
- Beckmann, K.; Garmo, H.; Lindahl, B.; Holmberg, L.; Stattin, P.; Adolfsson, J.; Cruickshank, J.K.; Van Hemelrijck, M. Spironolactone use is associated with lower prostate cancer risk: A population-wide case-control study. Prostate Cancer Prostatic Dis. 2020, 23, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Dmytriw, A.A.; Morzycki, W.; Green, P.J. Prevention of alopecia in medical and interventional chemotherapy patients. J. Cutan. Med. Surg. 2015, 19, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hawkshaw, N.J.; Hardman, J.A.; Haslam, I.S.; Shahmalak, A.; Gilhar, A.; Lim, X.; Paus, R. Identifying novel strategies for treating human hair loss disorders: Cyclosporine A suppresses the Wnt inhibitor, SFRP1, in the dermal papilla of human scalp hair follicles. PLoS Biol. 2018, 16, e2003705. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.R.; Toussi, A.; Awasthi, S.; Kiuru, M. Treatment of pediatric alopecia areata: A systematic review. J. Am. Acad. Dermatol. 2022, 86, 1318–1334. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Letule, V.; Laniauskaite, I.; Reinholz, M.; Tietze, J.K.; Wolff, H.; Ruzicka, T.; Sattler, E.C. Frontal Fibrosing Alopecia: A Retrospective Analysis of 72 Patients from a German Academic Center. Facial Plast. Surg. 2018, 34, 88–94. [Google Scholar] [CrossRef]
- Gamret, A.C.; Potluri, V.S.; Krishnamurthy, K.; Fertig, R.M. Frontal fibrosing alopecia: Efficacy of treatment modalities. Int. J. Womens Health 2019, 11, 273–285. [Google Scholar] [CrossRef]
- De Tran, Q.H.; Guay, E.; Chartier, S.; Tousignant, J. Tacrolimus in dermatology. J. Cutan. Med. Surg. 2001, 5, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Fujii, M.; Imaoka, A.; Kobayashi, R.; Hayashi, R.; Yoshida, Y.; Kohno, T.; Tsuji, T. Preventive effect of edaravone ointment on cyclophosphamide-chemotherapy induced alopecia. Support. Care Cancer 2021, 29, 6127–6134. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Lim, Y.C.; Kim, H.; Lim, S.M.; Kim, J.S. Genetic analysis of a novel antioxidant multi-target iron chelator, M30 protecting against chemotherapy-induced alopecia in mice. BMC Cancer 2019, 19, 149. [Google Scholar] [CrossRef]
- Hagiwara, S.; Uchida, T.; Koga, H.; Inomata, M.; Yoshizumi, F.; Moriyama, M.; Kitano, S.; Noguchi, T. The alpha-lipoic acid derivative sodium zinc dihydrolipoylhistidinate reduces chemotherapy-induced alopecia in a rat model: A pilot study. Surg. Today 2011, 41, 693–697. [Google Scholar] [CrossRef]
- Aiba, T.; Kono, Y.; Etoh, T.; Kawano, Y.; Oshima, Y.; Inomata, M. Efficacy of cooling therapy and alpha-lipoic acid derivative against chemotherapy-induced alopecia in an animal model. Cancer Sci. 2023, 114, 1007–1014. [Google Scholar] [CrossRef]
- Tkachenko, E.; Okhovat, J.P.; Manjaly, P.; Huang, K.P.; Senna, M.M.; Mostaghimi, A. Complementary and alternative medicine for alopecia areata: A systematic review. J. Am. Acad. Dermatol. 2023, 88, 131–143. [Google Scholar] [CrossRef]
- Hosking, A.M.; Juhasz, M.; Atanaskova Mesinkovska, N. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Ski. Appendage Disord 2019, 5, 72–89. [Google Scholar] [CrossRef] [PubMed]
- D’Agostini, F.; Fiallo, P.; Ghio, M.; De Flora, S. Chemoprevention of doxorubicin-induced alopecia in mice by dietary administration of L-cystine and vitamin B6. Arch. Dermatol. Res. 2013, 305, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, I.R.; Park, Y.H.; Im, Y.H.; Zhao, D.; Guallar, E.; Ahn, J.S.; Cho, J. Impact of a topical lotion, CG428, on permanent chemotherapy-induced alopecia in breast cancer survivors: A pilot randomized double-blind controlled clinical trial (VOLUME RCT). Support. Care Cancer 2020, 28, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Frith, H.; Harcourt, D.; Fussell, A. Anticipating an altered appearance: Women undergoing chemotherapy treatment for breast cancer. Eur. J. Oncol. Nurs. 2007, 11, 385–391. [Google Scholar] [CrossRef]
- Boland, V.; Brady, A.M.; Drury, A. The physical, psychological and social experiences of alopecia among women receiving chemotherapy: An integrative literature review. Eur. J. Oncol. Nurs. 2020, 49, 101840. [Google Scholar] [CrossRef]
- Browall, M.; Gaston-Johansson, F.; Danielson, E. Postmenopausal women with breast cancer: Their experiences of the chemotherapy treatment period. Cancer Nurs. 2006, 29, 34–42. [Google Scholar] [CrossRef]
- Freedman, T.G. Social and cultural dimensions of hair loss in women treated for breast cancer. Cancer Nurs. 1994, 17, 334–341. [Google Scholar] [CrossRef]
- Carelle, N.; Piotto, E.; Bellanger, A.; Germanaud, J.; Thuillier, A.; Khayat, D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 2002, 95, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Shapiro, J.; van den Hurk, C.; Goldfarb, S.; Jimenez, J.J.; Rossi, A.M.; Paus, R.; Lacouture, M.E. Hair disorders in cancer survivors. J. Am. Acad. Dermatol. 2019, 80, 1199–1213. [Google Scholar] [CrossRef]
- Masser, M.R.; Di Meo, L.; Hobby, J.A. Tattooing in reconstruction of the nipple and areola: A new method. Plast. Reconstr. Surg. 1989, 84, 677–681. [Google Scholar] [CrossRef]
- Uhlmann, N.R.; Martins, M.M.; Piato, S. 3D areola dermopigmentation (nipple-areola complex). Breast J. 2019, 25, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022–2024; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
| Device | Date of FDA Clearance | Device Description | Temperature Control by Computer | Advantages | Adverse Effects | Operator | Manufacturer | Costs |
|---|---|---|---|---|---|---|---|---|
| DigniCap C3 Scalp Cooling System | 2015 (for breast cancer) * | mobile cooling unit that uses circulating cold coolant | X | • The wrap is fitted at the beginning of treatment and remains on until completion • Starting from room temperature: comfortable | feeling of coldness, headache, scalp pain and/or light-headedness, dizziness | health-care providers administered | Dignitana | ~USD 1500–2000 |
| DigniCap Delta Scalp Cooling System | 06/27/19 | Digitana | ~USD 1500–2000 | |||||
| Paxman Scalp Cooling System | 04/19/17, later expanded to solid tumors | Paxman Coolers Limited | capped at USD 2200 | |||||
| Amma Cooler Heads cold cap | 12/08/21 | Cooler Heads | ~USD 2000 rental costs | |||||
| Penguin Cold Caps | not yet ** | 3-cap system: Crylon Gel in the caps | no | • Portable • Caps chill fast | self-administered | Penguin Cold Caps | USD 419/month rental costs | |
| Chemo Cold Caps | not yet | 6-cap system: caps filled with coolant gel | Arctic Cold Caps LLC | USD 379/month rental costs | ||||
| Arctic Cold Caps | not yet | 8-cap system: caps filled with glycerin-based hydr0-gel that refreezes in 2 h | Arctic Cold Caps LLC | USD 379/month rental costs |
| Category | NCT Number | Interventions | Completed | Recruiting | Not Yet Recruiting | Device | RCT, Parallel Assignment | Single Group Assignment | Open Label | Gender | Target Sample Size/Enrollment | Prevention | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scalp cooling | NCT01831024 | Dignicap System | * | X | Non-R CT | X | Female | 110 | X | ||||
| NCT03712696 | Device: DIGNICAP™ | X | X | X | X | Female | 139 | X | |||||
| NCT04630080 | Scalp cooling | X | X | X | X | Female | 100 | X | |||||
| NCT05213936 | Scalp cooling with hairstyle using conditioner and water emulsion | X | X | Non-R CT | Single blind (Investigator) | All | 30 | X | |||||
| NCT03248193 | Concomitant limb cryocompression and scalp cooling | * | X | Non-R CT | X | All | 50 | X | |||||
| NCT04986579 | Paxman Scalp Cooling System | X | X | Non-R CT | X | All | 120 | X | |||||
| NCT01008774 | Paxman Cooling Machine; Cold Caps | X | X | Non-R CT | X | All | 239 | X | |||||
| NCT04180579 | PAXMAN Scalp Cooler | ** | X | X | X | Female | 34 | X | |||||
| NCT04764357 | Paxman Scalp Cooling System | X | X | X | X | All | 40 | X | |||||
| NCT04117815 | Paxman Scalp Cooling System | X | X | *** | Female | 128 | X | ||||||
| NCT04626895 | Paxman Scalp Cooling Device | X | X | X | X | Female | 15 | X | |||||
| NCT05533320 | Paxman Scalp Cooling System | X | X | X | X | Female | 30 | X | |||||
| NCT04678544 | Paxman Scalp Cooling System 2 | X | X | X | Single blind (Outcomes Assessor) | Female | 170 | X | |||||
| NCT04168242 | Scalp cooling Paxman Orbis II system | ** | X | X | X | Female | 80 | X | |||||
| NCT01986140 | PAXMAN Orbis Scalp Cooler Treatment with Orbis scalp cooling cap | ** | X | X | Single blind (Care Provider) | Female | 236 | X | |||||
| NCT03289364 | Penguin Cold Caps | * | X | X | X | All | 9 | ||||||
| NCT05484973 | AMMA Portable Scalp Cooling System | X | X | X | X | Female | 125 | X | |||||
| NCT05365243 | AMMA Portable Scalp Cooling System | X | X | X | X | Female | 12 | X | |||||
| NCT03711877 | Scalp cooling system; chemical cold cap | X | X | X | X | Female | 256 | X | |||||
| PBMT | NCT05177289 | Theradome® LH80 pro combined with scalp cooling | X | X | X | Single blind (Outcomes Assessor) | Female | 72 | X | X | |||
| NCT04036994 | Photobiomodulation therapy | X | X | X | Single blind (Participant) | Female | 30 | X | |||||
| NCT05397457 | Low-level light therapy | X | X | X | X | Female | 88 | X | |||||
| Topical | NCT02919735 | Topical CG 428 herbal medicinal solution | X | X | Double blind (Participant, Investigator) | Female | 40 | X | X | ||||
| NCT02986412 | Topical CG 428 herbal lotion | X | X | X | Female | 19 | X | ||||||
| NCT02605629 | Topical CG 428 herbal lotion | X | X | Double blind (Participant, Investigator) | Female | 32 | X | ||||||
| NCT04554732 | Topical Keratinocyte growth factor | X | X | X | Female | 28 | X | ||||||
| NCT01588522 | Topical compound 31, 543 Calcitriol | X | X | X | All | 30 | X | ||||||
| Oral | NCT03831334 | Drug: oral minoxidil | X | X | X | All | 25 | X | |||||
| Injection | NCT04459650 | Platelet Rich Plasma | ** | X | X | All | 30 | X | |||||
| - | NCT02530177 | Clinical Assessment of alopecia and pCIA, skin aging and nail changes: Observational | ** | **** | Female | 546 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikramanayake, T.C.; Haberland, N.I.; Akhundlu, A.; Laboy Nieves, A.; Miteva, M. Prevention and Treatment of Chemotherapy-Induced Alopecia: What Is Available and What Is Coming? Curr. Oncol. 2023, 30, 3609-3626. https://doi.org/10.3390/curroncol30040275
Wikramanayake TC, Haberland NI, Akhundlu A, Laboy Nieves A, Miteva M. Prevention and Treatment of Chemotherapy-Induced Alopecia: What Is Available and What Is Coming? Current Oncology. 2023; 30(4):3609-3626. https://doi.org/10.3390/curroncol30040275
Chicago/Turabian StyleWikramanayake, Tongyu C., Nicole I. Haberland, Aysun Akhundlu, Andrea Laboy Nieves, and Mariya Miteva. 2023. "Prevention and Treatment of Chemotherapy-Induced Alopecia: What Is Available and What Is Coming?" Current Oncology 30, no. 4: 3609-3626. https://doi.org/10.3390/curroncol30040275
APA StyleWikramanayake, T. C., Haberland, N. I., Akhundlu, A., Laboy Nieves, A., & Miteva, M. (2023). Prevention and Treatment of Chemotherapy-Induced Alopecia: What Is Available and What Is Coming? Current Oncology, 30(4), 3609-3626. https://doi.org/10.3390/curroncol30040275