The Association between Metformin and the Cancer-Specific Mortality Rate in Nasopharyngeal Cancer Patients: Real-World Evidence
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Main Outcome and Covariates
2.4. Statistical Analysis
3. Results
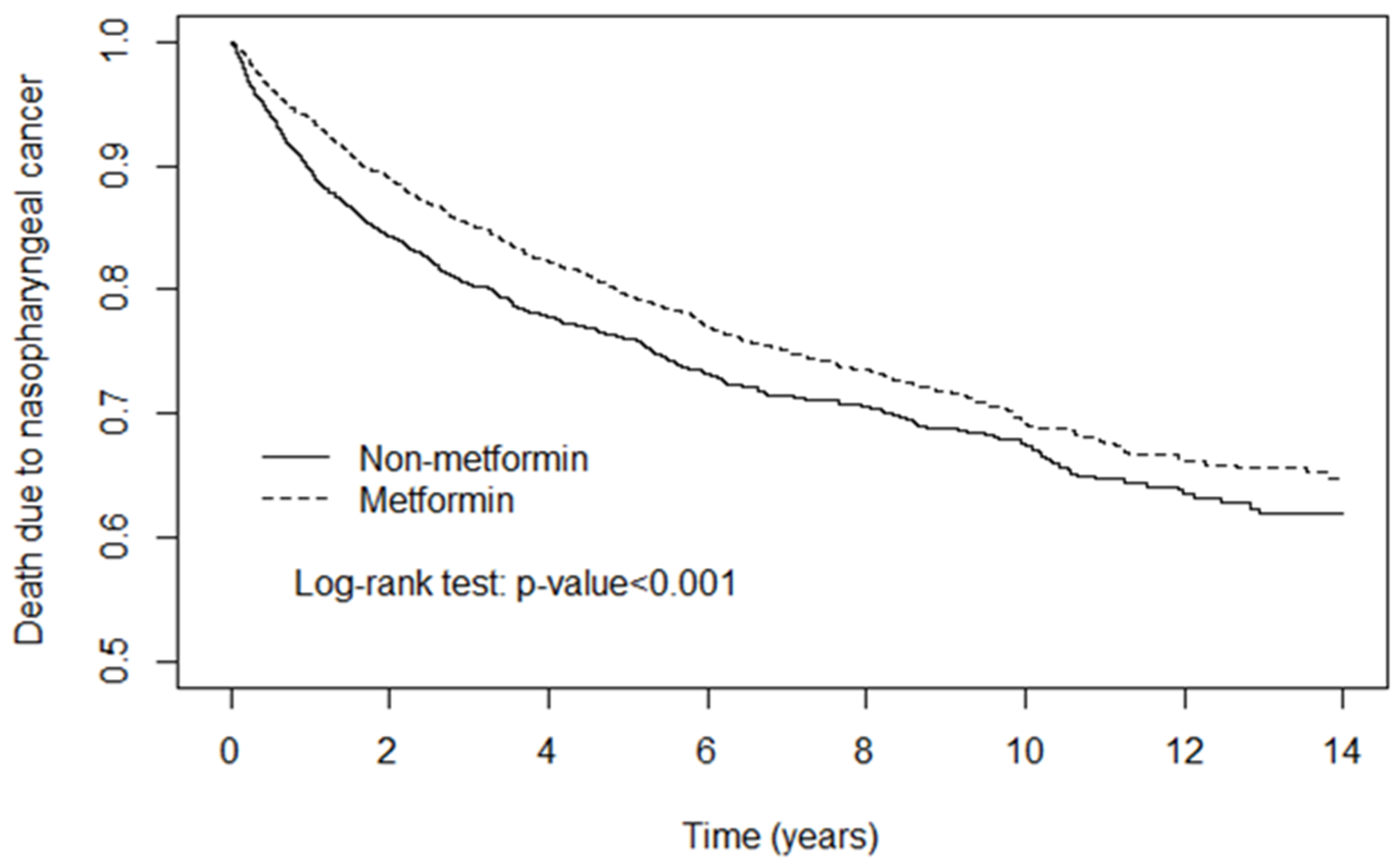
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehiniya, H.; Mohammadian, M.; Mohammadian-Hafshejani, A.; Mahdavifar, N. Nasopharyngeal cancer in the world: Epidemiology, incidence, mortality and risk factors. World Cancer Res. J. 2018, 5, e1046. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Fu, M.; Wei, S.; Chen, R. Impact of diabetes mellitus on the risk and survival of nasopharyngeal carcinoma: A meta-analysis. OncoTargets Ther. 2018, 11, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS ONE 2012, 7, e33411. [Google Scholar] [CrossRef]
- Abudawood, M. Diabetes and cancer: A comprehensive review. J. Res. Med. Sci. 2019, 24, e33411. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and risk of developing nasopharyngeal cancer in patients with type 2 diabetes mellitus. Metabolism 2018, 85, 223–226. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [Green Version]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, X.; Yu, Y.; Wang, Z.; Zuo, Y.; Li, S.; Yang, D.; Hu, S.; Xiang, M.; Xu, Z.; et al. Metformin inhibits the growth of nasopharyngeal carcinoma cells and sensitizes the cells to radiation via inhibition of the DNA damage repair pathway. Oncol. Rep. 2014, 32, 2596–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Chen, X.; Zhou, Y.; Qiu, S.; Wu, Y.; Xie, M.; Zhu, G.; Liang, S.; Li, H.; Zhou, D.; et al. Metformin reverses the drug resistance of cisplatin in irradiated CNE-1 human nasopharyngeal carcinoma cells through PECAM-1 mediated MRPs down-regulation. Int. J. Med. Sci. 2020, 17, 2416. [Google Scholar] [CrossRef]
- Shi, L.; Mei, Y.; Duan, X.; Wang, B. Effects of Cisplatin Combined with Metformin on Proliferation and Apoptosis of Nasopharyngeal Carcinoma Cells. Comput. Math. Methods. Med. 2022, 2022, 2056247. [Google Scholar] [CrossRef] [PubMed]
- Schütt, M.; Zimmermann, A.; Hood, R.; Hummel, M.; Seufert, J.; Siegel, E.; Tytko, A.; Holl, R.W.; DPV Initiative; German BMBF Competence Network Diabetes Mellitus. Gender-specific effects of treatment with lifestyle, Metformin or sulfonylurea on glycemic control and body weight: A German multicenter analysis on 9 108 patients. Exp. Clin. Endocrinol. Diabetes 2015, 123, 622–626. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Piskunova, T.S.; Popovich, I.G.; Zabezhinski, M.A.; Tyndyk, M.L.; Egormin, P.A.; Yurova, M.V.; Rosenfeld, S.V.; Semenchenko, A.V.; Kovalenko, I.G.; et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging 2010, 2, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, Y.; Lou, H.; Shan, L. Alpha-glucosidase inhibitors and risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Oncotarget 2017, 8, 81027. [Google Scholar] [CrossRef] [Green Version]
- Orlandella, R.M.; Turbitt, W.J.; Gibson, J.T.; Boi, S.K.; Li, P.; Smith, D.L.; Norian, L.A. The antidiabetic agent acarbose improves anti-PD-1 and rapamycin efficacy in preclinical renal cancer. Cancers 2020, 12, 2872. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Hong, Y.-R.; Bishnoi, R.; Ali, A.; Skelton, I.V.W.P.; Dang, L.H.; Huo, J.; Dang, N.H. Impact of DPP4 inhibitors in survival of patients with prostate, pancreas, and breast cancer. Front. Oncol. 2020, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Kamada, S.; Namekawa, T.; Ikeda, K.; Suzuki, T.; Kagawa, M.; Takeshita, H.; Yano, A.; Okamoto, K.; Ichikawa, T.; Horie-Inoue, K.; et al. Functional inhibition of cancer stemness-related protein DPP4 rescues tyrosine kinase inhibitor resistance in renal cell carcinoma. Oncogene 2021, 40, 3899–3913. [Google Scholar] [CrossRef]
- Vigneri, R.; Sciacca, L.; Vigneri, P. Rethinking the relationship between insulin and cancer. Trends Endocrinol. Metab. 2020, 31, 551–560. [Google Scholar] [CrossRef]
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef]
- Dąbrowski, M. Diabetes Antidiabetic Medications and Cancer Risk in Type 2 Diabetes: Focus on SGLT-2 Inhibitors. Int. J. Mol. Sci. 2021, 22, 1680. [Google Scholar] [CrossRef]
- NCCN. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2023. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 28 March 2023).
- Hong, R.L.; Hsiao, C.F.; Ting, L.L.; Ko, J.Y.; Wang, C.W.; Chang, J.T.C.; Lou, P.J.; Wang, H.M.; Tsai, M.H.; Lai, S.C.; et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann. Oncol. 2018, 29, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.L.; Guo, R.; Zhang, N.; Deng, B.; Chen, L.; Cheng, Z.B.; Huang, J.; Hu, W.H.; Huang, S.H.; Luo, W.J.; et al. Effect of Radiotherapy Alone vs Radiotherapy with Concurrent Chemoradiotherapy on Survival without Disease Relapse in Patients with Low-risk Nasopharyngeal Carcinoma: A Randomized Clinical Trial. JAMA 2022, 328, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.C.; Lee, C.H.; Chuang, H.C.; Huang, T.L.; Chien, C.Y.; Tsai, W.L.; Fang, F.M. Acute radiation dermatitis among patients with nasopharyngeal carcinoma treated with proton beam therapy: Prognostic factors and treatment outcomes. Int. Wound J. 2023, 20, 499–507. [Google Scholar] [CrossRef] [PubMed]
| Metformin | |||||
|---|---|---|---|---|---|
| No n = 3039 | Yes n = 3039 | ||||
| Variable | n | (%) | n | (%) | p-Value |
| Sex | 0.56 | ||||
| Female | 783 | 25.8 | 763 | 25.1 | |
| Male | 2256 | 74.2 | 2276 | 74.9 | |
| Age group (years) | 0.34 | ||||
| 20–49 | 444 | 14.6 | 485 | 16.0 | |
| 50–65 | 1549 | 51.0 | 1524 | 50.2 | |
| >65 | 1046 | 34.4 | 1030 | 33.9 | |
| Age (years), mean ± standard deviation | 60.4 ± 10.4 | 59.9 ± 10.5 | 0.07 | ||
| Medications | |||||
| Sulphonylurea | 1347 | 44.3 | 2341 | 77.0 | <0.001 |
| Thiazolidinediones | 314 | 10.3 | 501 | 16.5 | <0.001 |
| AGI | 402 | 13.2 | 688 | 22.6 | <0.001 |
| Insulin | 1842 | 60.6 | 2324 | 76.5 | <0.001 |
| DPP4 inhibitors | 708 | 23.3 | 1039 | 34.2 | <0.001 |
| Meglitinides | 425 | 14.0 | 711 | 23.4 | <0.001 |
| Comorbidities | |||||
| Hypertension | 2077 | 68.3 | 2006 | 66.0 | 0.05 |
| Hyperlipidemia | 1954 | 64.3 | 1676 | 55.2 | <0.001 |
| Chronic obstructive pulmonary disease | 747 | 24.6 | 721 | 23.7 | 0.44 |
| Chronic kidney disease | 163 | 5.36 | 67 | 2.20 | 0.001 |
| Heart failure | 159 | 5.23 | 126 | 4.15 | 0.045 |
| Treatment | |||||
| Radiation therapy | 2608 | 85.8 | 2333 | 76.8 | <0.001 |
| Chemotherapy | 2176 | 71.6 | 1927 | 63.4 | <0.001 |
| Metformin No | Metformin Yes | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Event | Person- Years | Incidence Rate | Event | Person- Years | Incidence Rate | Crude HR (95% CI) | Adjusted HR (95% CI) † |
| All | 662 | 12,417 | 5.33 | 661 | 16,484 | 4.01 | 0.81 (0.73, 0.91) *** | 0.80 (0.71, 0.90) *** |
| Sex | ||||||||
| Female | 153 | 3781 | 4.05 | 133 | 4459 | 2.98 | 0.78 (0.62, 0.99) * | 0.80 (0.62, 1.04) |
| Male | 509 | 8636 | 5.89 | 528 | 12,025 | 4.39 | 0.81 (0.72, 0.92) *** | 0.79 (0.69, 0.91) *** |
| Age group (years) | ||||||||
| 20–49 | 76 | 2732 | 2.78 | 107 | 3288 | 3.25 | 1.19 (0.89, 1.61) | 0.99 (0.70, 1.41) |
| 50–65 | 328 | 6581 | 4.98 | 320 | 8927 | 3.58 | 0.77 (0.66, 0.90) ** | 0.72 (0.61, 0.86) *** |
| >65 | 258 | 3105 | 8.31 | 234 | 4269 | 5.48 | 0.74 (0.62, 0.89) ** | 0.80 (0.66, 0.96) * |
| Medications | ||||||||
| Sulphonylurea | ||||||||
| No | 316 | 8109 | 3.90 | 163 | 3024 | 5.39 | 1.31 (1.08, 1.58) ** | 1.13 (0.92, 1.38) |
| Yes | 346 | 4308 | 8.03 | 498 | 13,460 | 3.70 | 0.55 (0.47, 0.63) *** | 0.63 (0.54, 0.72) *** |
| Thiazolidinediones | ||||||||
| No | 575 | 11,223 | 5.12 | 556 | 12,625 | 4.40 | 0.90 (0.80, 1.01) | 0.82 (0.72, 0.94) ** |
| Yes | 87 | 1195 | 7.28 | 105 | 3858 | 2.72 | 0.45 (0.33, 0.60) *** | 0.51 (0.38, 0.70) *** |
| AGI | ||||||||
| No | 562 | 10,938 | 5.14 | 524 | 11,558 | 4.53 | 0.92 (0.81, 1.03) | 0.83 (0.73, 0.95) ** |
| Yes | 100 | 1479 | 6.76 | 137 | 4926 | 2.78 | 0.51 (0.39, 0.66) *** | 0.62 (0.47, 0.83) ** |
| Insulin | ||||||||
| No | 152 | 4871 | 3.12 | 97 | 3809 | 2.55 | 0.89 (0.69, 1.14) | 0.97 (0.73, 1.29) |
| Yes | 510 | 7546 | 6.76 | 564 | 12,675 | 4.45 | 0.71 (0.63, 0.80) *** | 0.78 (0.68, 0.89) *** |
| DPP4 inhibitors | ||||||||
| No | 500 | 10,061 | 4.97 | 482 | 9112 | 5.29 | 1.06 (0.94, 1.20) | 0.88 (0.76, 1.01) |
| Yes | 162 | 2356 | 6.88 | 179 | 7372 | 2.43 | 0.45 (0.37, 0.57)*** | 0.54 (0.43, 0.68) *** |
| Meglitinides | ||||||||
| No | 540 | 11,089 | 4.87 | 502 | 11,868 | 4.23 | 0.91 (0.81, 1.03) | 0.83 (0.73, 0.95) ** |
| Yes | 122 | 1328 | 9.19 | 159 | 4615 | 3.45 | 0.46 (0.36, 0.58) *** | 0.60 (0.47, 0.78) *** |
| Treatment | ||||||||
| Radiation therapy | ||||||||
| No | 84 | 3239 | 2.59 | 104 | 4335 | 2.40 | 0.86 (0.64, 1.14) | 0.68 (0.47, 1.00) |
| Yes | 578 | 9179 | 6.30 | 557 | 12,149 | 4.58 | 0.82 (0.73, 0.92) *** | 0.80 (0.70, 0.91) *** |
| Chemotherapy | ||||||||
| No | 155 | 4949 | 3.13 | 156 | 6775 | 2.30 | 0.74 (0.59, 0.93) ** | 0.79 (0.60, 1.03) |
| Yes | 507 | 7468 | 6.79 | 505 | 9709 | 5.20 | 0.86 (0.76, 0.97) * | 0.79 (0.69, 0.91) *** |
| Comorbidity | ||||||||
| No | 82 | 3246 | 2.53 | 109 | 3044 | 3.58 | 1.37 (1.02, 1.82) * | 1.21 (0.83, 1.75) |
| Yes | 580 | 9171 | 6.32 | 552 | 13,440 | 4.11 | 0.74 (0.66, 0.83) *** | 0.73 (0.64, 0.83) *** |
| Crude | Adjusted † | |||||
|---|---|---|---|---|---|---|
| Variable | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value |
| Sex (male vs. female) | 1.37 | (1.20, 1.56) | <0.001 | 1.45 | (1.27, 1.65) | <0.001 |
| Age (every year) | 1.03 | (1.02, 1.03) | <0.001 | 1.03 | (1.02, 1.03) | <0.001 |
| Metformin use (nonuse as a control) | 0.81 | (0.73, 0.91) | <0.001 | 0.80 | (0.71, 0.90) | <0.001 |
| Medications | ||||||
| Sulphonylurea | 1.12 | (1.00, 1.25) | 0.049 | 1.10 | (0.96, 1.25) | 0.16 |
| Thiazolidinediones | 0.88 | (0.75, 1.03) | 0.10 | 0.88 | (0.75, 1.04) | 0.13 |
| AGI | 0.83 | (0.72, 0.96) | 0.01 | 0.83 | (0.71, 0.97) | 0.02 |
| Insulin | 1.95 | (1.70, 2.24) | <0.001 | 2.16 | (1.87, 2.50) | <0.001 |
| DPP4 inhibitors | 0.72 | (0.64, 0.82) | <0.001 | 0.64 | (0.56, 0.74) | <0.001 |
| Meglitinides | 1.09 | (0.95, 1.24) | 0.23 | 1.07 | (0.92, 1.23) | 0.39 |
| Treatment | ||||||
| Radiation therapy | 1.82 | (1.56, 2.13) | <0.001 | 1.20 | (0.99, 1.45) | 0.07 |
| Chemotherapy | 1.95 | (1.71, 2.21) | <0.001 | 2.03 | (1.73, 2.38) | <0.001 |
| Comorbidity | ||||||
| Hypertension | 1.39 | (1.24, 1.57) | <0.001 | 1.16 | (1.02, 1.31) | 0.03 |
| Hyperlipidemia | 1.22 | (1.09, 1.36) | <0.001 | 1.12 | (0.99, 1.26) | 0.07 |
| Chronic obstructive pulmonary disease | 1.18 | (1.04, 1.33) | 0.01 | 0.96 | (0.84, 1.09) | 0.51 |
| Chronic kidney disease | 2.23 | (1.74, 2.86) | <0.001 | 1.58 | (1.22, 2.03) | <0.001 |
| Heart failure | 1.93 | (1.53, 2.43) | <0.001 | 1.68 | (1.32, 2.13) | <0.001 |
| Variable | Event | Person- Years | Incidence Rate | Crude HR (95% CI) | Adjusted HR (95% CI) † | |
|---|---|---|---|---|---|---|
| Metformin | AGI | |||||
| No | No | 562 | 10,938 | 5.14 | 1 (Reference) | 1 (Reference) |
| No | Yes | 100 | 1479 | 6.76 | 1.25 (1.01, 1.55) * | 1.05 (0.83, 1.31) |
| Yes | No | 524 | 11,558 | 4.53 | 0.92 (0.81, 1.03) | 0.86 (0.75, 0.98) * |
| Yes | Yes | 137 | 4926 | 2.78 | 0.63 (0.52,0.76) *** | 0.62 (0.50,0.76) *** |
| Metformin | DPP4 | |||||
| No | No | 500 | 10,061 | 4.97 | 1 (Reference) | 1 (Reference) |
| No | Yes | 162 | 2356 | 6.88 | 1.23 (1.03, 1.47) * | 0.88 (0.73, 1.07) |
| Yes | No | 482 | 9112 | 5.29 | 1.06 (0.94, 1.21) | 0.93 (0.81, 1.07) |
| Yes | Yes | 179 | 7372 | 2.43 | 0.55(0.46, 0.65) *** | 0.48 (0.40, 1.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.; Hsu, C.Y.; Kao, Y.-S. The Association between Metformin and the Cancer-Specific Mortality Rate in Nasopharyngeal Cancer Patients: Real-World Evidence. Curr. Oncol. 2023, 30, 3940-3950. https://doi.org/10.3390/curroncol30040298
Hsu Y, Hsu CY, Kao Y-S. The Association between Metformin and the Cancer-Specific Mortality Rate in Nasopharyngeal Cancer Patients: Real-World Evidence. Current Oncology. 2023; 30(4):3940-3950. https://doi.org/10.3390/curroncol30040298
Chicago/Turabian StyleHsu, Yen, Chung Y. Hsu, and Yung-Shuo Kao. 2023. "The Association between Metformin and the Cancer-Specific Mortality Rate in Nasopharyngeal Cancer Patients: Real-World Evidence" Current Oncology 30, no. 4: 3940-3950. https://doi.org/10.3390/curroncol30040298





