Outcomes of a Multidisciplinary Team in the Management of Patients with Early-Stage Breast Cancer Undergoing Neoadjuvant Chemotherapy at a Community Cancer Center
Abstract
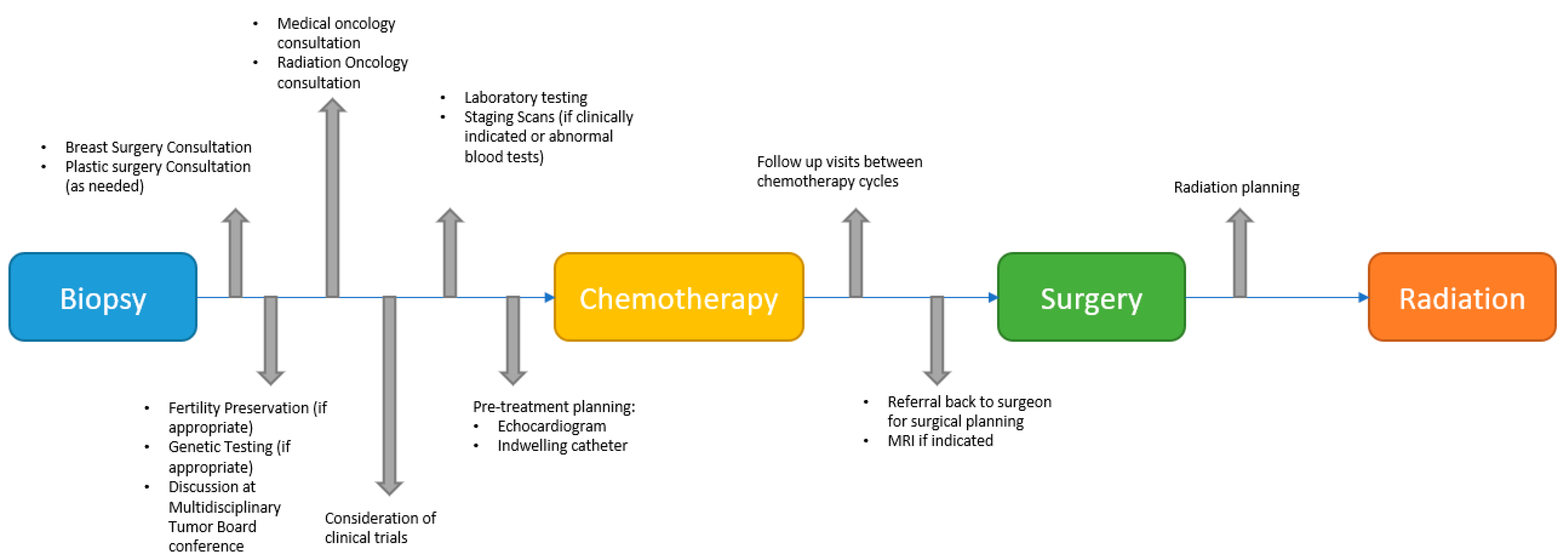
:1. Introduction
2. Methods
2.1. Outcomes
2.2. Data Collection
2.3. Analysis
2.4. MDT
3. Results
3.1. Patient and Tumor Characteristics
3.2. Pathological Complete Response
3.3. Other Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houssami, N.; Sainsbury, R. Breast Cancer: Multidisciplinary Care and Clinical Outcomes. Eur. J. Cancer Oxf. Engl. 1990 2006, 42, 2480–2491. [Google Scholar] [CrossRef] [PubMed]
- Van der Hage, J.A.; van de Velde, C.J.H.; Julien, J.-P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative Chemotherapy in Primary Operable Breast Cancer: Results From the European Organization for Research and Treatment of Cancer Trial 10902. J. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef] [PubMed]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative Chemotherapy in Patients With Operable Breast Cancer: Nine-Year Results From National Surgical Adjuvant Breast and Bowel Project B-18. JNCI Monogr. 2001, 2001, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P.A. Neoadjuvant Versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef]
- Kaufmann, M.; Hortobagyi, G.N.; Goldhirsch, A.; Scholl, S.; Makris, A.; Valagussa, P.; Blohmer, J.-U.; Eiermann, W.; Jackesz, R.; Jonat, W.; et al. Recommendations From an International Expert Panel on the Use of Neoadjuvant (Primary) Systemic Treatment of Operable Breast Cancer: An Update. J. Clin. Oncol. 2006, 24, 1940–1949. [Google Scholar] [CrossRef]
- Mamtani, A.; Barrio, A.V.; King, T.A.; Van Zee, K.J.; Plitas, G.; Pilewskie, M.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.S.; Sclafani, L.M.; et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients with Histologically Confirmed Nodal Metastases: Results of a Prospective Study. Ann. Surg. Oncol. 2016, 23, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of Preoperative Chemotherapy on Local-Regional Disease in Women with Operable Breast Cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef]
- Kelly, A.M.; Dwamena, B.; Cronin, P.; Carlos, R.C. Breast Cancer: Sentinel Node Identification and Classification after Neoadjuvant Chemotherapy—Systematic Review and Meta Analysis. Acad. Radiol. 2009, 16, 551–563. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Raab, G.; Caputo, A.; Schütte, M.; Hilfrich, J.; Blohmer, J.U.; Gerber, B.; Costa, S.D.; Merkle, E.; Eidtmann, H.; et al. Doxorubicin With Cyclophosphamide Followed by Docetaxel Every 21 Days Compared With Doxorubicin and Docetaxel Every 14 Days As Preoperative Treatment in Operable Breast Cancer: The GEPARDUO Study of the German Breast Group. J. Clin. Oncol. 2005, 23, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.E.; Smith, I.E.; Ashley, S.; Fulford, L.G.; Lakhani, S.R. Oestrogen Receptor Status, Pathological Complete Response and Prognosis in Patients Receiving Neoadjuvant Chemotherapy for Early Breast Cancer. Br. J. Cancer 2004, 91, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Huober, J.; von Minckwitz, G.; Denkert, C.; Tesch, H.; Weiss, E.; Zahm, D.M.; Belau, A.; Khandan, F.; Hauschild, M.; Thomssen, C.; et al. Effect of Neoadjuvant Anthracycline–Taxane-Based Chemotherapy in Different Biological Breast Cancer Phenotypes: Overall Results from the GeparTrio Study. Breast Cancer Res. Treat. 2010, 124, 133–140. [Google Scholar] [CrossRef]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; André, F.; Baselga, J.; et al. Tailoring Therapies—Improving the Management of Early Breast Cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2015, 26, v8–v30. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Sutton, T.L.; Schlitt, A.; Gardiner, S.K.; Johnson, N.; Garreau, J.R. Time to Surgery Following Neoadjuvant Chemotherapy for Breast Cancer Impacts Residual Cancer Burden, Recurrence, and Survival. J. Surg. Oncol. 2020, 122, 1761–1769. [Google Scholar] [CrossRef]
- Sanford, R.A.; Lei, X.; Barcenas, C.H.; Mittendorf, E.A.; Caudle, A.S.; Valero, V.; Tripathy, D.; Giordano, S.H.; Chavez-MacGregor, M. Impact of Time from Completion of Neoadjuvant Chemotherapy to Surgery on Survival Outcomes in Breast Cancer Patients. Ann. Surg. Oncol. 2016, 23, 1515–1521. [Google Scholar] [CrossRef]
- Yoo, T.-K.; Moon, H.-G.; Han, W.; Noh, D.-Y. Time Interval of Neoadjuvant Chemotherapy to Surgery in Breast Cancer: How Long Is Acceptable? Gland Surg. 2017, 6, 1–3. [Google Scholar] [CrossRef]
- Cullinane, C.; Shrestha, A.; Al Maksoud, A.; Rothwell, J.; Evoy, D.; Geraghty, J.; McCartan, D.; McDermott, E.W.; Prichard, R.S. Optimal Timing of Surgery Following Breast Cancer Neoadjuvant Chemotherapy: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 1507–1513. [Google Scholar] [CrossRef]
- Silva, S.B.; Pereira, A.A.L.; Marta, G.N.; de Barros Lima, K.M.L.; de Freitas, T.B.; Matutino, A.R.B.; de Azevedo Souza, M.C.L.; de Azevedo, R.G.M.V.; de Viveiros, P.A.H.; da Silva Lima, J.M.; et al. Clinical Impact of Adjuvant Radiation Therapy Delay after Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Breast 2018, 38, 39–44. [Google Scholar] [CrossRef]
- Marta, G.N.; AlBeesh, R.; Pereira, A.A.L.; Oliveira, L.J.; Mano, M.S.; Hijal, T. The Impact on Clinical Outcomes of Post-Operative Radiation Therapy Delay after Neoadjuvant Chemotherapy in Patients with Breast Cancer: A Multicentric International Study. Breast 2020, 54, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Livingston-Rosanoff, D.; Hanlon, B.; Marka, N.; Vande Walle, K.; Stankowski-Drengler, T.; Schumacher, J.; Greenberg, C.C.; Neuman, H.; Wilke, L.G. Time to Initiation of Neo-Adjuvant Chemotherapy for Breast Cancer Treatment Does Not Influence Patient Survival: A Study of US Breast Cancer Patients. Breast J. 2020, 26, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, J.; Yin, S.; Geng, C.; Xu, B. An Appropriate Treatment Interval Does Not Affect the Prognosis of Patients with Breast Cancer. Holist. Integr. Oncol. 2022, 1, 8. [Google Scholar] [CrossRef]
- De Melo Gagliato, D.; Lei, X.; Giordano, S.H.; Valero, V.; Barcenas, C.H.; Hortobagyi, G.N.; Chavez-MacGregor, M. Impact of Delayed Neoadjuvant Systemic Chemotherapy on Overall Survival Among Patients with Breast Cancer. Oncologist 2020, 25, 749–757. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Naikan, J.; Rozenblit, M.; Mandeli, J.; Bleiweiss, I.; Tiersten, A. Changes in Pathological Complete Response Rates after Neoadjuvant Chemotherapy for Breast Carcinoma over Five Years. J. Oncol. 2016, 2016, e4324863. [Google Scholar] [CrossRef]
- Kovac, A.; Cankar, K.; Dobovisek, L.; Cavka, L.; Godina, E.; Horvat, V.J.; Rajer, M.; Starman, T.; Matos, E.; Borstnar, S. 207P 10-Year Real-World Outcomes with Neoadjuvant Systemic Therapy in Non-Inflammatory Breast Cancer. Ann. Oncol. 2020, 31, S324–S325. [Google Scholar] [CrossRef]
- De Paula, B.H.R.; Kumar, S.; Morosini, F.M.; Cardoso, D.E.M.C.; de Sousa, C.A.M.; Crocamo, S. Real-World Assessment of the Effect of Impact of Tumor Size on Pathological Complete Response Rates in Triple Negative Breast Cancer after Neoadjuvant Chemotherapy. Chin. Clin. Oncol. 2020, 9, 78. [Google Scholar] [CrossRef]
- Michel, E.; Vincent, L.; Beltjens, F.; Arnould, L.; Ladoire, S.; Coutant, C.; Jankowski, C. Axillary Pathologic Response after Neoadjuvant Chemotherapy and Surgery According to Breast Cancers Subtypes and Survival Impact. J. Clin. Oncol. 2022, 40, e12595. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, P.; Liu, Y.; Liang, Y.; Wang, Y. Prediction of Axillary Response after Neoadjuvant Chemotherapy in Clinical Node Positive Breast Cancer. Transl. Cancer Res. 2021, 10, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Kim, H.J.; Park, H.Y.; Park, J.Y.; Chae, Y.S.; Lee, S.M.; Cho, S.H.; Shin, K.M.; Lee, S.Y. Axillary Pathologic Complete Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Breast Cancer Patients: A Predictive Model Integrating the Imaging Characteristics of Ultrasound Restaging with Known Clinicopathologic Characteristics. Ultrasound Med. Biol. 2019, 45, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Doe, S.; Petersen, S.; Buekers, T.; Swain, M. Does a Multidisciplinary Approach to Invasive Breast Cancer Care Improve Time to Treatment and Patient Compliance? J. Natl. Med. Assoc. 2020, 112, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.N.; Hernandez, A.; Yadegarynia, S.; Ryon, E.; Franceschi, D.; Avisar, E.; Kobetz, E.N.; Merchant, N.; Kesmodel, S.; Goel, N. Overcoming Disparities: Multidisciplinary Breast Cancer Care at a Public Safety Net Hospital. Breast Cancer Res. Treat. 2021, 187, 197–206. [Google Scholar] [CrossRef]
- Kesson, E.M.; Allardice, G.M.; George, W.D.; Burns, H.J.G.; Morrison, D.S. Effects of Multidisciplinary Team Working on Breast Cancer Survival: Retrospective, Comparative, Interventional Cohort Study of 13 722 Women. BMJ 2012, 344, e2718. [Google Scholar] [CrossRef]

| Patient Characteristics | Overall | Pathological Complete Response |
|---|---|---|
| N (%) | 94 (100) | 23 (24.5) |
| Age (mean) | 56.5 (12.8) | 54.4 (12.1) |
| Race | ||
| White | 79 (84.0) | 19 (82.6) |
| Black | 12 (12.8) | 2 (8.7) |
| Asian | 3 (3.2) | 2 (8.7) |
| Ethnicity | ||
| Hispanic | 11 (11.7) | 1 (4.3) |
| Non-Hispanic | 82 (88.3) | 21 (95.7) |
| ECOG Performance Status | ||
| 0 | 78 (83.0) | 20 (87.0) |
| 1 | 12 (12.8) | 3 (13.0) |
| 2 | 1 (1.1) | 0 (0.0) |
| Not documented | 3 (3.2) | 0 (0.0) |
| Prior Breast Cancer (DCIS or invasive) | 11 (12.0) | 2 (8.7) |
| Clinical Stage | ||
| I | 5 (5.3) | 0 (0.0) |
| II | 66 (70.2) | 15 (65.2) |
| III | 21 (22.3) | 8 (34.8) |
| Clinical Tumor Stage | ||
| TI | 10 (10.6) | 1 (4.3) |
| T2 | 60 (63.8) | 12 (52.2) |
| T3 | 19 (20.2) | 9 (39.1) |
| T4 | 3 (3.2) | 1 (4.3) |
| Tx | 2 (2.1) | 0 (0.0) |
| Clinical Lymph Node Stage | ||
| N0 | 51 (54.3) | 12 (52.1) |
| N1 | 38 (40.4) | 9 (39.1) |
| N2 | 4 (4.3) | 1 (4.3) |
| N3 | 1 (1.1) | 1 (4.3) |
| ER Receptor Status | ||
| Positive | 44 (46.8) | 4 (17.4) |
| Negative | 50 (53.2) | 19 (82.6) |
| PR Receptor Status | ||
| Positive | 36 (38.3) | 2 (8.7) |
| Negative | 58 (61.7) | 19 (82.6) |
| HER-2 Neu Receptor Status | ||
| Positive | 28 (29.8) | 10 (43.5) |
| Negative | 66 (70.2) | 13 (56.5) |
| Chemotherapy Regimen | ||
| DDAC/T | 32 (34.0) | 4 (17.4) |
| DDAC/TC | 15 (16.0) | 6 (26.1) |
| TC | 9 (9.6) | 0 (0.0) |
| TCHP | 22 (23.4) | 10 (43.5) |
| THP | 3 (3.2) | 1 (4.3) |
| Time from diagnosis (1st breast biopsy) to NAC (in days)—median (min, max) | 37.5 (3, 150) * | 41.0 (21, 98) |
| Indication for NAC | ||
| Less Extensive Surgery | 6 (6.4) | 3 (13.0) |
| HER2 tailoring of treatment | 24 (25.5) | 9 (39.1) |
| Inoperable to Operable | 12 (12.8) | 2 (8.7) |
| Operable Mastectomy to BCS | 12 (12.8) | 1 (4.3) |
| Time for genetics | 21 (22.3) | 6 (26.1) |
| Time for Surgical Planning | 12 (12.8) | 3 (13.0) |
| Lymph Node positive to negative | 40 (42.6) | 12 (52.2) |
| Time from completion of NAC to surgery (in days)—median (min, max) | 29.0 (9, 118) * | 30.0 (13, 48) |
| Tumor Type | Total | RCB 0 [pCR] | RCB I | RCB II | RCB III |
|---|---|---|---|---|---|
| N (%) | 91 (100.0) | 23 (25.3) | 19 (20.9) | 38 (41.8) | 11 (12.1) |
| ER + HER2 + | 18 (19.8) | 3 (16.7) | 1 (5.6) | 12 (66.7) | 2 (11.1) |
| ER + HER2 − | 24 (26.4) | 1 (4.2) | 2 (8.3) | 14 (58.3) | 7 (29.2) |
| ER-HER2 + | 10 (11.0) | 7 (70.0) | 3 (30.0) | 0 (0.0) | 0 (0.0) |
| ER-HER2 − | 39 (42.9) | 12 (30.8) | 13 (33.3) | 12 (30.8) | 2 (51.3) |
| Overall | Clinical Node Positive | Clinical Node Negative | |
|---|---|---|---|
| N (%) | 94 (100.0) | 43 (45.7) | 51 (54.3) |
| Follow up with surgeon prior to completion of NAC—Yes | 92 (98.9) | 42 (100.0) | 50 (98.0) |
| Follow up with radiation oncology prior to completion of NAC—Yes | 48 (51.1) | 29 (67.4) | 19 (37.3) |
| Enrollment in clinical trial—Yes | 5 (21.7) | 3 (21.4) | 2 (22.2) |
| Time from surgery to RT (in days)—median (min, max) | 49.5 (9, 173) N = 78 | 55 (9, 173) N = 43 | 48 (25, 140) N = 35 |
| Time to complete RT (in days)—median (min, max) | 33.0 (12, 73) | 39.0 (12, 73) | 29.0 (21, 52) |
| Duration of RT for more than 2 weeks beyond expected time—Yes | 5 (6.4) | 4 (9.3) | 1 (2.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, P.V.; Mason, H.; Kaufman, S.A.; Visintainer, P.; Makari-Judson, G. Outcomes of a Multidisciplinary Team in the Management of Patients with Early-Stage Breast Cancer Undergoing Neoadjuvant Chemotherapy at a Community Cancer Center. Curr. Oncol. 2023, 30, 4861-4870. https://doi.org/10.3390/curroncol30050366
Bhardwaj PV, Mason H, Kaufman SA, Visintainer P, Makari-Judson G. Outcomes of a Multidisciplinary Team in the Management of Patients with Early-Stage Breast Cancer Undergoing Neoadjuvant Chemotherapy at a Community Cancer Center. Current Oncology. 2023; 30(5):4861-4870. https://doi.org/10.3390/curroncol30050366
Chicago/Turabian StyleBhardwaj, Prarthna V., Holly Mason, Seth A. Kaufman, Paul Visintainer, and Grace Makari-Judson. 2023. "Outcomes of a Multidisciplinary Team in the Management of Patients with Early-Stage Breast Cancer Undergoing Neoadjuvant Chemotherapy at a Community Cancer Center" Current Oncology 30, no. 5: 4861-4870. https://doi.org/10.3390/curroncol30050366
APA StyleBhardwaj, P. V., Mason, H., Kaufman, S. A., Visintainer, P., & Makari-Judson, G. (2023). Outcomes of a Multidisciplinary Team in the Management of Patients with Early-Stage Breast Cancer Undergoing Neoadjuvant Chemotherapy at a Community Cancer Center. Current Oncology, 30(5), 4861-4870. https://doi.org/10.3390/curroncol30050366





