Factors Associated with Long-Term Prostate Cancer Survival after Palliative Radiotherapy to a Bone Metastasis and Contemporary Palliative Systemic Therapy: A Retrospective, Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Considerations
4. Results
4.1. Patient Characteristics
4.2. Disease Characteristics
4.3. Treatment Characteristics
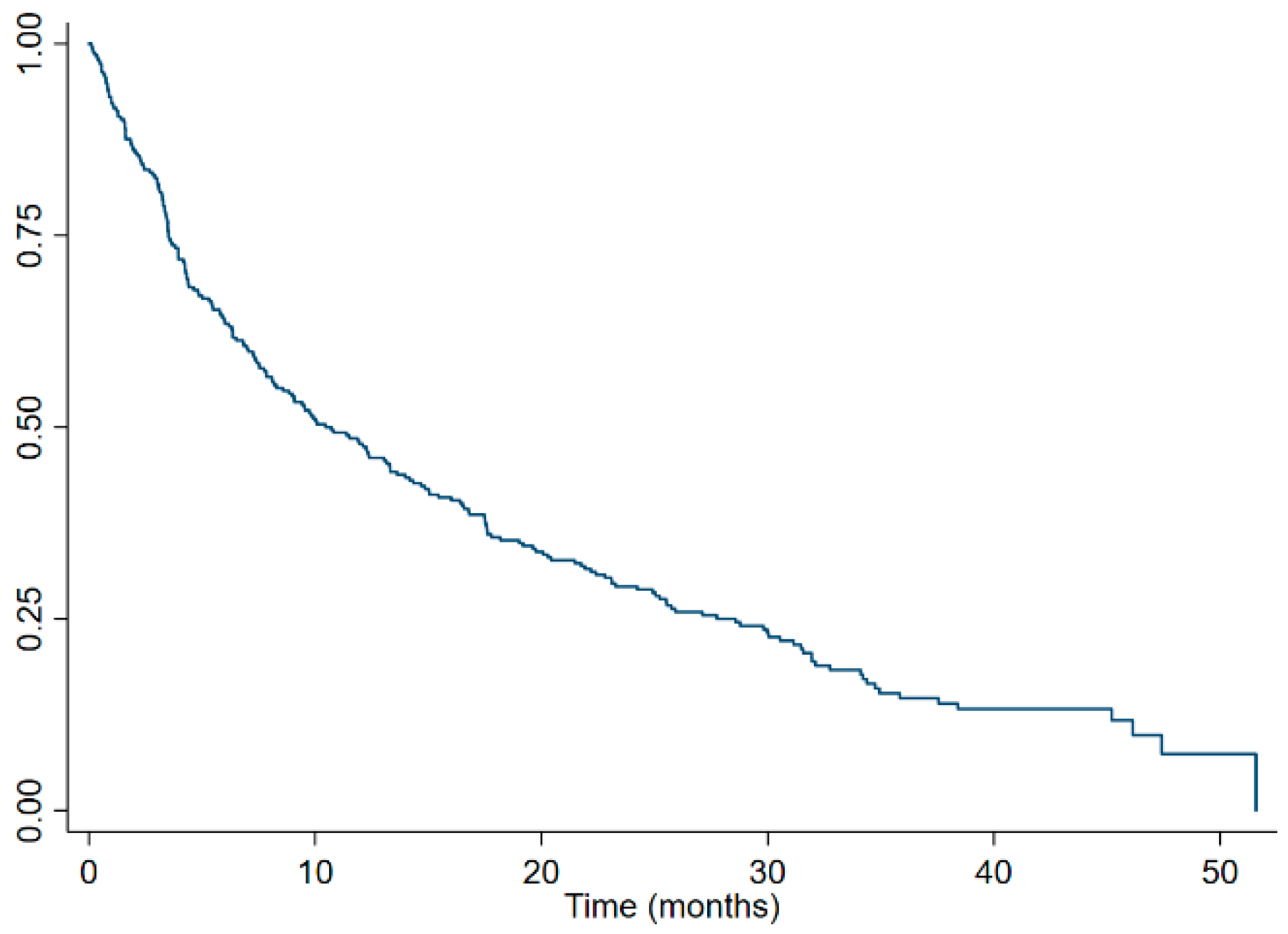
4.4. Survival Characteristics
4.5. Hazard Regression Analysis
5. Discussion
Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected Estimates of Cancer in Canada in 2022. Can. Med. Assoc. J. 2022, 194, E601–E607. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of Metastatic Sites in Patients with Prostate Cancer: A Population-Based Analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]
- Carlin, B.I.; Andriole, G.L. The Natural History, Skeletal Complications, and Management of Bone Metastases in Patients with Prostate Carcinoma. Cancer 2000, 88, 2989–2994. [Google Scholar] [CrossRef]
- Autio, K.A.; Scher, H.I.; Morris, M.J. Therapeutic Strategies for Bone Metastases and Their Clinical Sequelae in Prostate Cancer. Curr. Treat. Options Oncol. 2012, 13, 174–188. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.A.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M. Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients with Metastatic Hormone-Refractory Prostate Cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef]
- Dearnaley, D.; Hinder, V.; Hijab, A.; Horan, G.; Srihari, N.; Rich, P.; Houston, J.G.; Henry, A.M.; Gibbs, S.; Venkitaraman, R.; et al. Observation versus Screening Spinal MRI and Pre-Emptive Treatment for Spinal Cord Compression in Patients with Castration-Resistant Prostate Cancer and Spinal Metastases in the UK (PROMPTS): An Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 501–513. [Google Scholar] [CrossRef]
- Yarnold, J.R. 8 Gy Single Fraction Radiotherapy for the Treatment of Metastatic Skeletal Pain: Randomised Comparison with a Multifraction Schedule over 12 Months of Patient Follow-upOn Behalf of the Bone Pain Trial Working Party. Radiother. Oncol. 1999, 52, 111–121. [Google Scholar] [CrossRef]
- Lutz, S.; Berk, L.; Chang, E.; Chow, E.; Hahn, C.; Hoskin, P.; Howell, D.; Konski, A.; Kachnic, L.; Lo, S.; et al. Palliative Radiotherapy for Bone Metastases: An ASTRO Evidence-Based Guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 965–976. [Google Scholar] [CrossRef]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative Radiation Therapy for Bone Metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef]
- Seidenfeld, J.; Samson, D.J.; Hasselblad, V.; Aronson, N.; Albertsen, P.C.; Bennett, C.L.; Wilt, T.J. Single-Therapy Androgen Suppression in Men with Advanced Prostate Cancer. Ann. Intern. Med. 2000, 132, 566–577. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and Safety of Radium-223 Dichloride in Patients with Castration-Resistant Prostate Cancer and Symptomatic Bone Metastases, with or without Previous Docetaxel Use: A Prespecified Subgroup Analysis from the Randomised, Double-Blind, Phase 3 ALSYMPCA Trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus Zoledronic Acid for Treatment of Bone Metastases in Men with Castration-Resistant Prostate Cancer: A Randomised, Double-Blind Study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B. A Randomized, Placebo-Controlled Trial of Zoledronic Acid. in Patients with Hormone-Refractory Metastatic Prostate Carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef]
- Cheon, P.M.; Wong, E.; Thavarajah, N.; Dennis, K.; Lutz, S.; Zeng, L.; Chow, E. A Definition of “Uncomplicated Bone Metastases” Based on Previous Bone Metastases Radiation Trials Comparing Single-Fraction and Multi-Fraction Radiation Therapy. J. Bone Oncol. 2015, 4, 13–17. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Soban, F.; De Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer: Updated Survival in the TAX 327 Study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the Primary Tumour for Newly Diagnosed, Metastatic Prostate Cancer (STAMPEDE): A Randomised Controlled Phase 3 Trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.L.; Van Der Velden, J.M.; Wong, E.; Seravalli, E.; Sahgal, A.; Chow, E.; Verlaan, J.J.; Verkooijen, H.M.; Van Der Linden, Y.M. Systematic Review of the Role of Stereotactic Radiotherapy for Bone Metastases. J. Natl. Cancer Inst. 2019, 111, 1023–1032. [Google Scholar] [CrossRef]
- Shiota, M.; Terada, N.; Saito, T.; Yokomizo, A.; Kohei, N.; Goto, T.; Kawamura, S.; Hashimoto, Y.; Takahashi, A.; Kimura, T.; et al. Differential Prognostic Factors in Low- and High-Burden de Novo Metastatic Hormone-Sensitive Prostate Cancer Patients. Cancer Sci. 2021, 112, 1524–1533. [Google Scholar] [CrossRef]
- Halabi, S.; Lin, C.Y.; Kelly, W.K.; Fizazi, K.; Moul, J.W.; Kaplan, E.B.; Morris, M.J.; Small, E.J. Updated Prognostic Model for Predicting Overall Survival in First-Line Chemotherapy for Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2014, 32, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Eigl, B.J.; Leibowitz-Amit, R.; Lester, R.; Kollmannsberger, C.; Murray, N.; Clayton, R.; Heng, D.Y.C.; Joshua, A.M.; Chi, K.N. Outcomes with Abiraterone Acetate in Metastatic Castration-Resistant Prostate Cancer Patients Who Have Poor Performance Status. Eur. Urol. 2015, 67, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Kong, D.M.; Li, L. Prognostic Value of ECOG Performance Status and Gleason Score in the Survival of Castration-Resistant Prostate Cancer: A Systematic Review. Asian J. Androl. 2021, 23, 163–169. [Google Scholar] [CrossRef]
- Makita, K.; Hamamoto, Y.; Kanzaki, H.; Nagasaki, K.; Takata, N.; Tsuruoka, S.; Uwatsu, K.; Kido, T. Factors Affecting Survival and Local Control in Patients with Bone Metastases Treated with Radiotherapy. Med. Sci. 2023, 11, 17. [Google Scholar] [CrossRef]
- Chow, E.; Fung, K.; Panzarella, T.; Bezjak, A.; Danjoux, C.; Tannock, I. A predictive model for survival in metastatic cancer patients attending an outpatient palliative radiotherapy clinic. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1291–1302. [Google Scholar] [CrossRef]
- Cho, C.K.J.; Sunderland, K.; Pickles, T.; Bachand, F.; Chi, K.N.; Tyldesley, S. A Population-Based Study of Palliative Radiation Therapy for Bone Metastases in Patients Dying of Prostate Cancer. Pract. Radiat. Oncol. 2019, 9, e274–e282. [Google Scholar] [CrossRef] [PubMed]

| Variable | Whole Cohort (n = 274) | Short Term Survivors (n = 137) | Long Term Survivors (n = 137) | p-Value |
|---|---|---|---|---|
| Age at time of RT (median, range) | 76 (69–83) | 76 (69–83) | 76 (70–82) | 0.99 |
| Year of RT 2018 | 137 (50%) | 69 (50.4%) | 68 (49.6%) | 0.904 |
| 2019 | 137 (50%) | 68 (49.6%) | 69 (50.4%) | |
| Site of bone metastasis RT Skull/Spine | 114 (41.6%) | 57 (41.6%) | 57 (41.6%) | 0.720 |
| Chest and upper extremity | 30 (10.9%) | 17 (12.4%) | 13 (9.5%) | |
| Pelvis and lower extremity | 130 (47.4%) | 63 (46.0%) | 67 (48.9%) | |
| Multifraction Radiotherapy Yes | 64 (23.4%) | 29 (21.17%0) | 35 (25.55%) | 0.392 |
| No | 210 (76.6%) | 108 (78.8%) | 102 (74.5%) | |
| Charlson Index 0–1 | 185 (67.5%) | 91 (66.4%) | 94 (68.6%) | 0.523 |
| 2–3 | 68 (24.8%) | 33 (24.1%) | 35 (25.6%) | |
| ≥4 | 21 (7.7%) | 13 (9.5%) | 8 (5.8%) | |
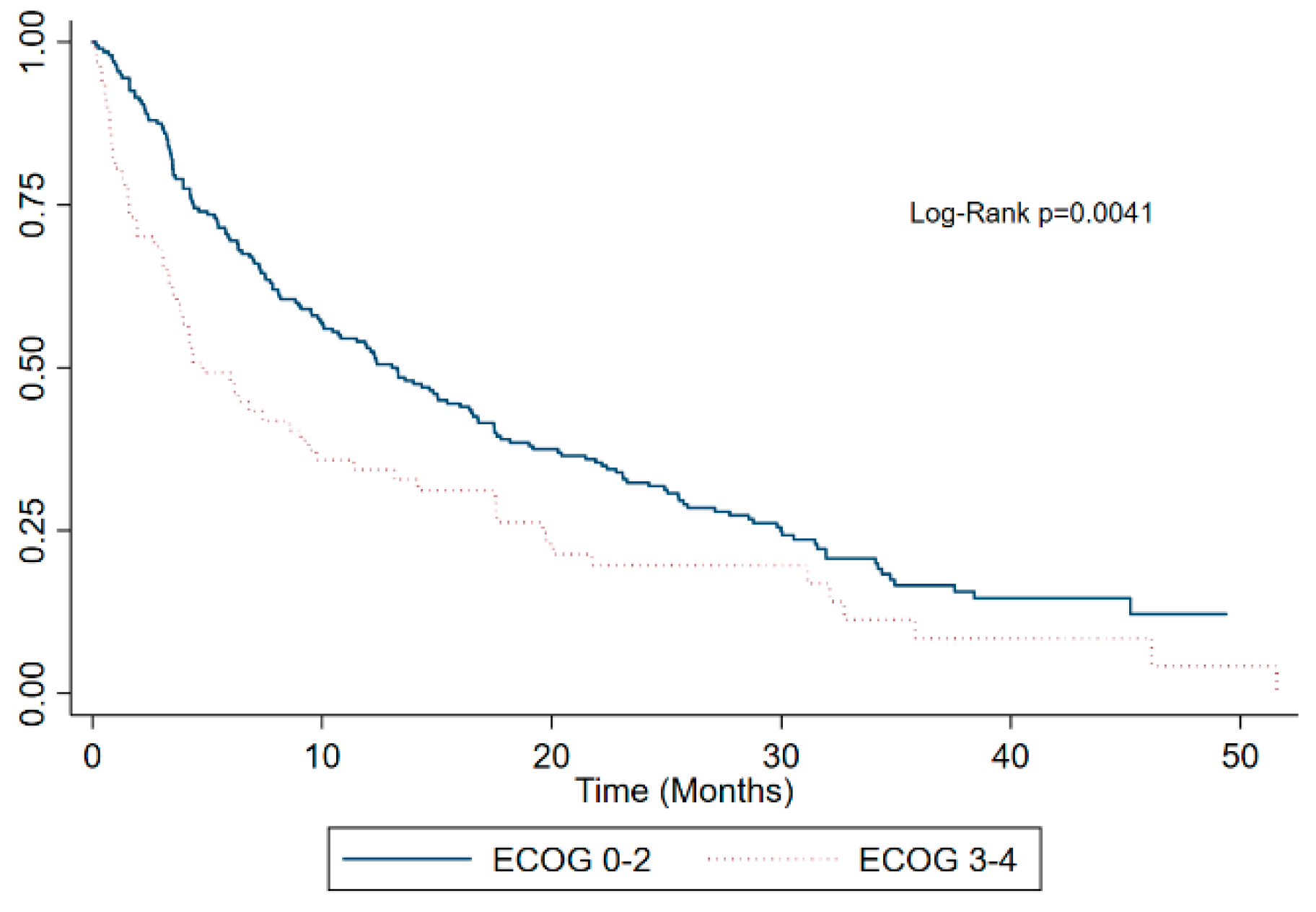
| ECOG Performance Status 0–2 | 200 (73.0%) | 89 (67.4%) | 111 (82.2%) | 0.005 |
| 3–4 | 67 (24.5%) | 43 (32.6%) | 24 (17.8%) | |
| Complicated bone metastases Yes | 98 (35.8%) | 50 (36.5%) | 48 (35.0%) | 0.801 |
| No | 176 (64.2%) | 87 (63.5%) | 89 (64.2%) | |
| Visceral metastases at RT Yes | 60 (21.9%) | 35 (28.2%) | 25 (19.8%) | 0.121 |
| No | 190 (69.3%) | 89 (71.8%) | 101 (80.2%) | |
| Number of bone metastases 1–3 | 43 (15.7%) | 15 (11.0%) | 28 (20.4%) | 0.136 |
| ≥4 | 231 (84.3%) | 122 (89.0%) | 109 (79.6%) | |
| CHAARTED Disease Burden Low | 35 (12.8%) | 13 (9.5%) | 22 (16.1%) | 0.103 |
| High | 239 (87.2%) | 124 (90.5%) | 115 (83.9%) | |
| Castration resistance with 2nd line systemic therapy Yes | 151 (55.1%) | 69 (50.4%) | 82 (59.9%) | 0.160 |
| No | 28 (10.2%) | 18 (13.1%) | 10 (7.3%) |
| Variable | Whole Cohort (n = 274) | Short Term Survivors (n = 137) | Long Term Survivors (n = 137) | p-Value |
|---|---|---|---|---|
| Palliative RT to bone metastases other than index lesion Yes | 150 (54.7%) | 62 (45.3%) | 88 (64.2%) | 0.002 |
| No | 124 (45.3%) | 75 (54.7%) | 49 (35.8%) | |
| Repeat Palliative RT to index lesion Yes | 22 (8.0%) | 15 (11.0%) | 7 (5.1%) | 0.075 |
| No | 252 (92.0%) | 122 (89.1%) | 130 (94.9%) | |
| Prior Management of prostate primary None | 175 (63.9%) | 89 (65.0%) | 86 (62.8%) | 0.278 |
| Prostatectomy | 27 (9.9%) | 13 (9.5%) | 14 (10.2%) | |
| Radical radiation therapy | 32 (11.7%) | 21 (15.3%) | 18 (13.1%) | |
| Transuretheral resection of prostate only | 7 (2.6%) | 2 (1.5%) | 5 (3.7%) | |
| Primary brachytherapy | 8 (2.9%) | 6 (4.4%) | 2 (1.5%) | |
| Prostatectomy followed by salvage RT | 17 (6.2%) | 5 (3.7%) | 12 (8.8%) | |
| High frequency ultrasound | 1 (0.4%) | 1 (0.7%) | 0 (0%) | |
| Bisphosphonate use No | 192 (70.1%) | 103 (75.2%) | 89 (65.0%) | 0.128 |
| Yes | 81 (29.6%) | 34 (24.8%) | 47 (34.3%) | |
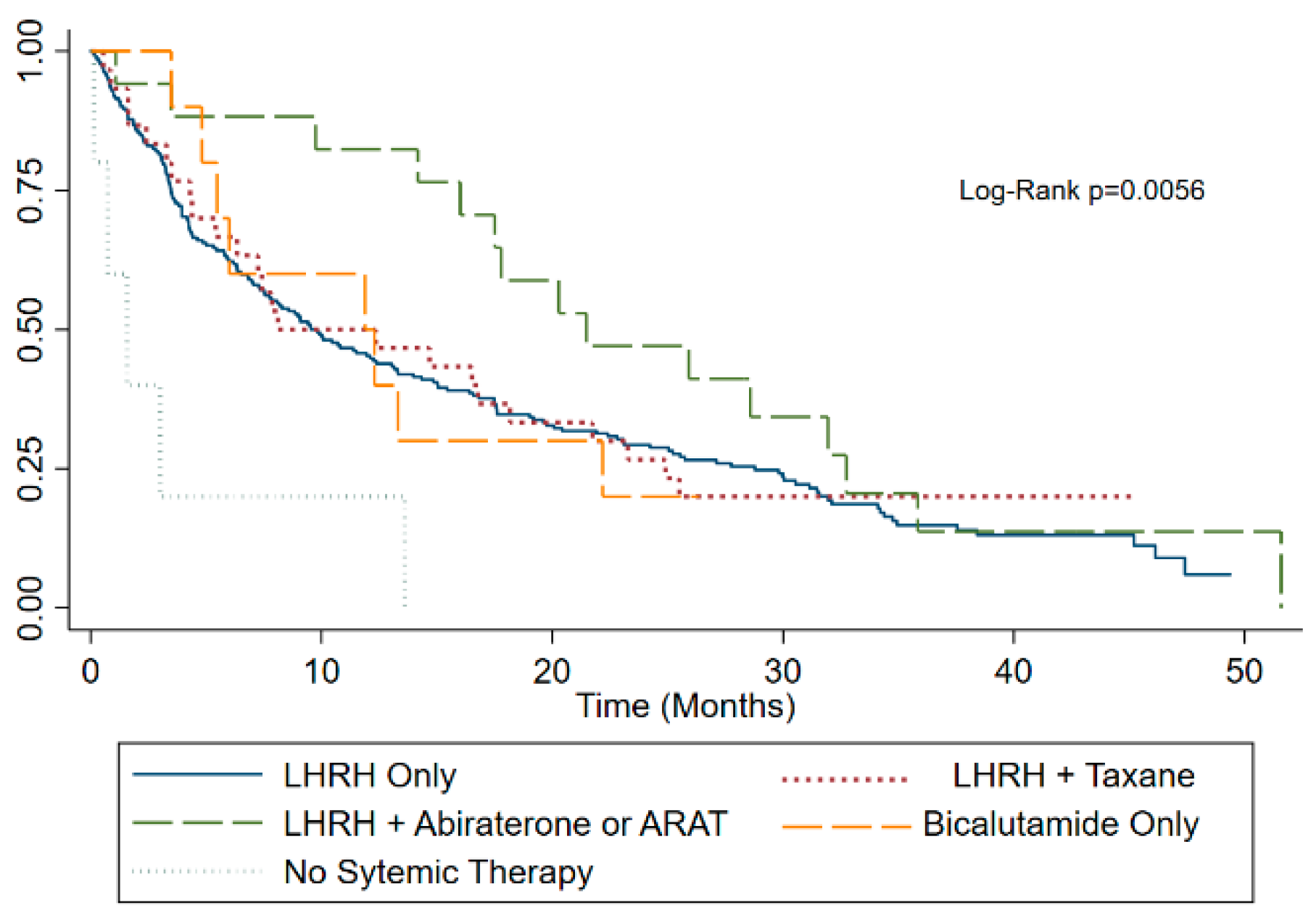
| First line systemic therapy LHRH agonist alone | 212 (77.4%) | 111 (81.0%) | 101 (73.7%) | 0.044 |
| LHRH and Taxane | 30 (10.9%) | 15 (11.0%) | 15 (11.0%) | |
| LHRH and abiraterone or ARAT | 17 (6.2%) | 3 (2.2%) | 14 (10.2%) | |
| Bicalutamide alone | 10 (3.6%) | 4 (2.9%) | 6 (4.4%) | |
| No systemic therapy | 5 (1.8%) | 4 (2.9%) | 1 (0.7%) | |
| Second line systemic therapy LHRH and Taxane | 20 (7.3%) | 10 (7.3%) | 10 (7.3%) | 0.83 |
| LHRH and Abiraterone or ARAT | 112 (40.9%) | 54 (39.4%) | 58 (42.3%) | |
| LHRH and bicalutamide | 43 (15.7%) | 22 (16.1%) | 21 (15.3%) | |
| LHRH and Radium-223 | 4 (1.5%) | 1 (0.7%) | 3 (2.2) | |
| No second line systemic therapy | 95 (34.7%) | 50 (36.5%) | 45 (32.9%) | |
| Third line systemic therapy LHRH and Taxane | 72 (26.3%) | 35 (25.6%) | 37 (27.0%) | 0.774 |
| LHRH and Abiraterone or ARAT | 17 (6.2%) | 10 (7.3%) | 7 (5.1%) | |
| LHRH and Radium-223 | 5 (1.8%) | 3 (2.2%) | 2 (1.5%) | |
| LHRH and Rupcaparib | 1 (0.4%) | 0 (0%) | 1 (0.7%) | |
| No third line systemic therapy | 179 (65.3%) | 89 (64.96%) | 90 (65.7%) |
| Variable | Hazard Ratio | p Value |
|---|---|---|
| Age at the time of RT <76 years | Ref | Ref |
| ≥76 years | 1.23 | 0.122 |
| Year of RT 2018 | Ref | Ref |
| 2019 | 1.05 | 0.699 |
| Site of RT Skull | Ref | Ref |
| Upper extremity | 5.09 | 0.112 |
| Chest | 5.51 | 0.114 |
| Spine | 5.28 | 0.098 |
| Pelvis | 4.56 | 0.131 |
| Lower extremity | 3.52 | 0.219 |
| Receipt of Multi-fraction RT | 0.81 | 0.215 |
| Charlson Index 0–1 | Ref | Ref |
| 2–3 | 1.2 | 0.221 |
| ≥4 | 1.8 | 0.014 |
| ECOG Performance status 0–2 | Ref | Ref |
| 3–4 | 1.54 | 0.004 |
| Complicated bone metastases No | Ref | Ref |
| Yes | 1.18 | 0.211 |
| Visceral metastases No | Ref | Ref |
| Yes | 1.30 | 0.102 |
| Number of bone metastases 1–3 | Ref | Ref |
| ≥4 | 1.81 | 0.031 |
| CHAARTED Disease Burden Low | Ref | Ref |
| High | 1.62 | 0.029 |
| Bisphosphonate use No | Ref | Ref |
| Yes | 0.80 | 0.128 |
| First Line Systemic Therapy | ||
| LHRH agonist alone | Ref | Ref |
| LHRH and taxane | 0.93 | 0.773 |
| LHRH with abiraterone or ARAT | 0.65 | 0.126 |
| Bicalutamide alone | 1.03 | 0.915 |
| No systemic therapy | 4.16 | 0.002 |
| Factor | Hazard Ratio | p Value |
|---|---|---|
| Age at the time of RT <76 years | Ref | Ref |
| ≥76 years | 1.01 | 0.146 |
| Charlson Index 0–1 | Ref | Ref |
| 2–3 | 1.18 | 0.303 |
| ≥4 | 1.46 | 0.125 |
| ECOG Performance Status 0–2 | Ref | Ref |
| 3–4 | 1.45 | 0.02 |
| CHAARTED Disease Burden Low | Ref | Ref |
| High | 1.71 | 0.023 |
| First Line Systemic Therapy | ||
| LHRH agonist alone | Ref | Ref |
| LHRH and taxane | 1.06 | 0.777 |
| LHRH with abiraterone or ARAT | 0.63 | 0.111 |
| Bicalutamide alone | 0.907 | 0.8 |
| No systemic therapy | 3.61 | 0.006 |
| Variable | Median Post-RT Survival in Months (IQR) |
|---|---|
| ECOG Performance Status | |
| 0 | 25.5 (15.4–34.3) |
| 1 | 12.1 (4.6–24.3) |
| 2 | 10.4 (3.4–25.9) |
| 3 | 6.1 (1.9–19.6) |
| 4 | 2.2 (0.6–8.7) |
| CHAARTED Disease Burden | |
| Low | 16.8 (3.5–29.1) |
| High | 9.8 (3.5–25) |
| First line systemic therapy Received | |
| LHRH agonist alone | 9.6 (3.5–24.9) |
| LHRH and taxane | 10.2 (4.3–25) |
| LHRH and abiraterone or ARAT | 21.5 (16–32) |
| Bicalutamide alone | 12.1 (5.5–22.2) |
| No systemic therapy | 1.6 (0.7–3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopal, B.; Shahhat, S.; Beck, J.; Hanumanthappa, N.; Ong, A.D.; Dubey, A.; Koul, R.; Bashir, B.; Chowdhury, A.; Sivananthan, G.; et al. Factors Associated with Long-Term Prostate Cancer Survival after Palliative Radiotherapy to a Bone Metastasis and Contemporary Palliative Systemic Therapy: A Retrospective, Population-Based Study. Curr. Oncol. 2023, 30, 5560-5573. https://doi.org/10.3390/curroncol30060420
Venugopal B, Shahhat S, Beck J, Hanumanthappa N, Ong AD, Dubey A, Koul R, Bashir B, Chowdhury A, Sivananthan G, et al. Factors Associated with Long-Term Prostate Cancer Survival after Palliative Radiotherapy to a Bone Metastasis and Contemporary Palliative Systemic Therapy: A Retrospective, Population-Based Study. Current Oncology. 2023; 30(6):5560-5573. https://doi.org/10.3390/curroncol30060420
Chicago/Turabian StyleVenugopal, Bindu, Shaheer Shahhat, James Beck, Nikesh Hanumanthappa, Aldrich D. Ong, Arbind Dubey, Rashmi Koul, Bashir Bashir, Amitava Chowdhury, Gokulan Sivananthan, and et al. 2023. "Factors Associated with Long-Term Prostate Cancer Survival after Palliative Radiotherapy to a Bone Metastasis and Contemporary Palliative Systemic Therapy: A Retrospective, Population-Based Study" Current Oncology 30, no. 6: 5560-5573. https://doi.org/10.3390/curroncol30060420
APA StyleVenugopal, B., Shahhat, S., Beck, J., Hanumanthappa, N., Ong, A. D., Dubey, A., Koul, R., Bashir, B., Chowdhury, A., Sivananthan, G., & Kim, J. O. (2023). Factors Associated with Long-Term Prostate Cancer Survival after Palliative Radiotherapy to a Bone Metastasis and Contemporary Palliative Systemic Therapy: A Retrospective, Population-Based Study. Current Oncology, 30(6), 5560-5573. https://doi.org/10.3390/curroncol30060420





