The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma
Abstract
:1. Introduction
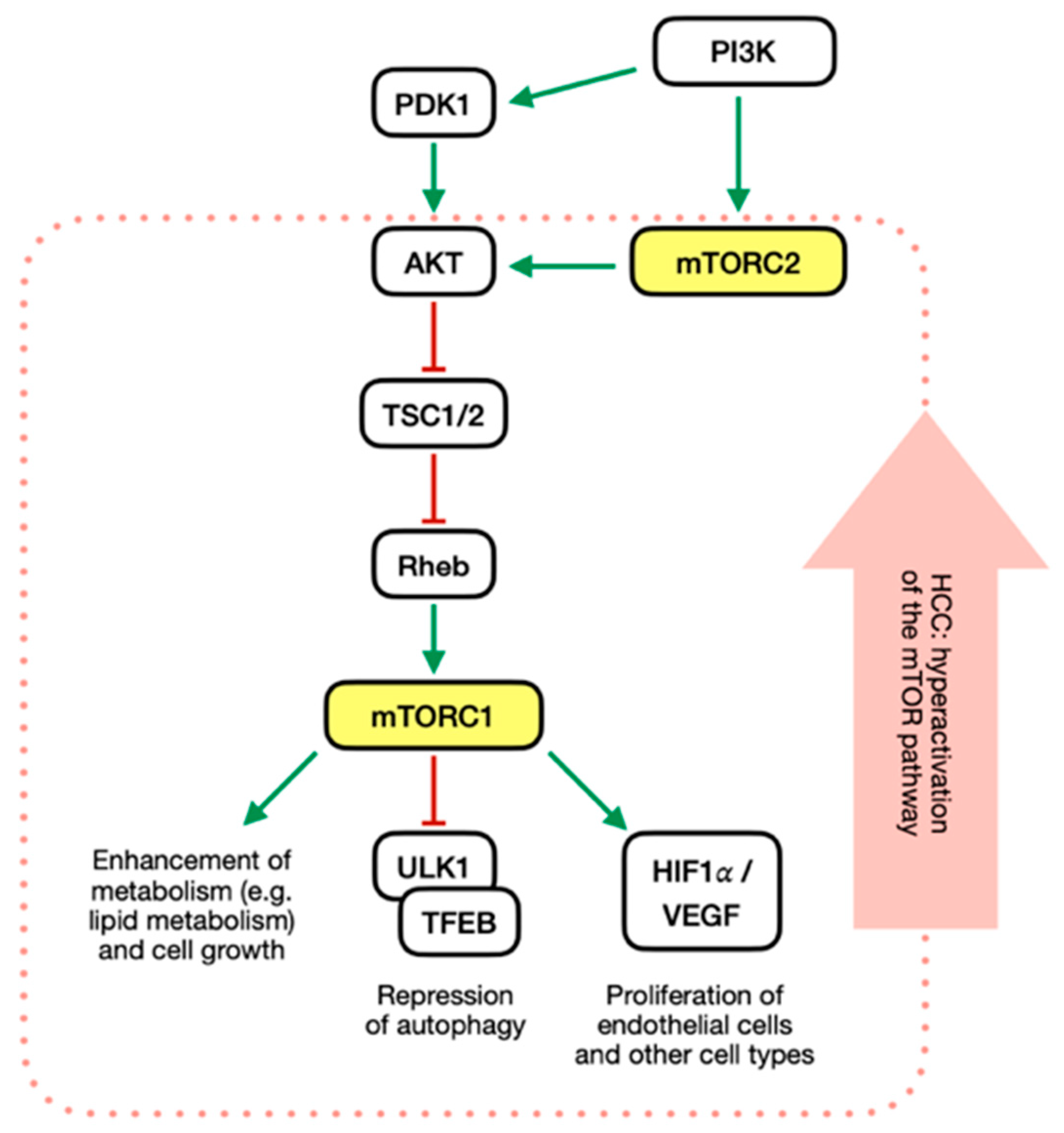
2. Akt-mTOR Signaling Pathway
PI3K/mTOR Pathway in HCC
3. Inhibitors of Akt-mTOR Signaling Pathway
3.1. Sirolimus
3.2. Everolimus
4. mTORi and HCC Recurrence
4.1. Prevention of HCC Recurrence
4.1.1. Sirolimus
4.1.2. Everolimus
4.2. Treatment of HCC Recurrence
4.3. Prevention of De Novo Malignancy
5. Impact on Overall Survival
6. mTORi in Immunosuppression after LT
6.1. Immunosuppressive Regimens
6.2. Impact on Renal Function
6.2.1. Sirolimus
6.2.2. Everolimus
6.3. Impact on Graft Rejection
7. Tolerability and AEs
7.1. Dyslipidemia
7.2. Hyperglycemia
7.3. Proteinuria
7.4. Wound Healing Complications
7.5. Hematologic Adverse Effects
7.6. Mucosal and Integumental Adverse Effects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Cristin, L.; Montini, A.; Martinino, A.; Scarano Pereira, J.P.; Giovinazzo, F.; Agnes, S. The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review. Cells 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Santopaolo, F.; Lenci, I.; Milana, M.; Manzia, T.M.; Baiocchi, L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J. Gastroenterol. 2019, 25, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Castaing, D.; Neuhaus, P.; Jamieson, N.; Salizzoni, M.; et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.R.; Lake, J.R.; Smith, J.M.; Skeans, M.A.; Schladt, D.P.; Edwards, E.B.; Harper, A.M.; Wainright, J.L.; Snyder, J.J.; Israni, A.K.; et al. OPTN/SRTR 2013 Annual Data Report: Liver. Am. J. Transplant. 2015, 15, 1–28. [Google Scholar] [CrossRef] [PubMed]
- de Villa, V.; Lo, C.M. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist 2007, 12, 1321–1331. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.-H.; Huang, C.-C.; Lin, P.-R.; Chang, H.-W.; Ger, L.-P.; Lin, Y.-W.; Changchien, C.-S.; Lee, C.-M.; Tai, M.-H. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003, 97, 1929–1940. [Google Scholar] [CrossRef]
- Ferrín, G.; Guerrero, M.; Amado, V.; Rodríguez-Perálvarez, M.; De la Mata, M. Activation of mTOR Signaling Pathway in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 1266. [Google Scholar] [CrossRef] [Green Version]
- Unni, N.; Arteaga, C.L. Is Dual mTORC1 and mTORC2 Therapeutic Blockade Clinically Feasible in Cancer? JAMA Oncol. 2019, 5, 1564–1565. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Turnquist, H.R.; Raimondi, G. Immunoregulatory Functions of mTOR Inhibition. Nat. Rev. Immunol. 2009, 9, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazal, K.; Stenard, F.; Dahlqvist, G.; Barjon, C.; Aoudjehane, L.; Scatton, O.; Conti, F. Treatment with mTOR inhibitors after liver transplantation enables a sustained increase in regulatory T-cells while preserving their suppressive capacity. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 237–244. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Wu, Z.-Q.; Qiu, S.-J.; Yu, Y.; Huang, X.-W.; Tang, Z.-Y.; Fan, J. Sirolimus-Based Immunosuppression Therapy in Liver Transplantation for Patients with Hepatocellular Carcinoma Exceeding the Milan Criteria. Transplant. Proc. 2008, 40, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.C.; Johnson, S.R.; Mandelbrot, D.A.; Pavlakis, M.; Horwedel, T.; Karp, S.J.; Egbuna, O.; Rodrigue, J.R.; Chudzinski, R.E.; Goldfarb-Rumyantzev, A.S.; et al. Timing of sirolimus conversion influences recovery of renal function in liver transplant recipients. Clin. Transplant. 2009, 23, 887–896. [Google Scholar] [CrossRef]
- Vivarelli, M.; Dazzi, A.; Cucchetti, A.; Gasbarrini, A.; Zanello, M.; Di Gioia, P.; Bianchi, G.; Tamè, M.R.; Gaudio, M.D.; Ravaioli, M.; et al. Sirolimus in Liver Transplant Recipients: A Large Single-Center Experience. Transplant. Proc. 2010, 42, 2579–2584. [Google Scholar] [CrossRef]
- Gomez-Martin, C.; Bustamante, J.; Castroagudin, J.F.; Salcedo, M.; Garralda, E.; Testillano, M.; Herrero, I.; Matilla, A.; Sangro, B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transplant. 2012, 18, 45–52. [Google Scholar] [CrossRef]
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; Jonas, S.; Sudan, D.; Fung, J.; Fischer, L.; et al. Everolimus with Reduced Tacrolimus Improves Renal Function in De Novo Liver Transplant Recipients: A Randomized Controlled Trial. Am. J. Transplant. 2012, 12, 3008–3020. [Google Scholar] [CrossRef] [Green Version]
- Fischer, L.; Klempnauer, J.; Beckebaum, S.; Metselaar, H.J.; Neuhaus, P.; Schemmer, P.; Settmacher, U.; Heyne, N.; Clavien, P.-A.; Muehlbacher, F.; et al. A Randomized, Controlled Study to Assess the Conversion From Calcineurin-Inhibitors to Everolimus after Liver Transplantation—PROTECT. Am. J. Transplant. 2012, 12, 1855–1865. [Google Scholar] [CrossRef]
- Ferreiro, A.O.; Vazquez-Millán, M.A.; López, F.S.; Gutiérrez, M.G.; Diaz, S.P.; Patiño, M.J.L. Everolimus-Based Immunosuppression in Patients with Hepatocellular Carcinoma at High Risk of Recurrence after Liver Transplantation: A Case Series. Transplant. Proc. 2014, 46, 3496–3501. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schnitzbauer, A.A.; Zülke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.-W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients with Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, D.-H.; Tak, E.; Hwang, S.; Song, G.-W.; Ahn, C.-S.; Kim, K.-H.; Moon, D.-B.; Ha, T.-Y.; Park, G.-C.; Ryoo, B.-Y.; et al. Antitumor effect of sorafenib and mammalian target of rapamycin inhibitor in liver transplantation recipients with hepatocellular carcinoma recurrence. Liver Transplant. 2018, 24, 932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Invernizzi, F.; Iavarone, M.; Zavaglia, C.; Mazza, S.; Maggi, U.; Cesarini, L.; Antonelli, B.; Airoldi, A.; Manini, M.A.; Sangiovanni, A.; et al. Experience with Early Sorafenib Treatment with mTOR Inhibitors in Hepatocellular Carcinoma Recurring after Liver Transplantation. Transplantation 2020, 104, 568. [Google Scholar] [CrossRef]
- Saliba, F.; Dharancy, S.; Salamé, E.; Conti, F.; Eyraud, D.; Radenne, S.; Antonini, T.; Guillaud, O.; Guguenheim, J.; Neau-Cransac, M.; et al. Time to Conversion to an Everolimus-Based Regimen: Renal Outcomes in Liver Transplant Recipients from the EVEROLIVER Registry. Liver Transplant. 2020, 26, 1465. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Filmann, N.; Adam, R.; Bachellier, P.; Bechstein, W.O.; Becker, T.; Bhoori, S.; Bilbao, I.; Brockmann, J.; Burra, P.; et al. mTOR Inhibition Is Most Beneficial after Liver Transplantation for Hepatocellular Carcinoma in Patients with Active Tumors. Ann. Surg. 2020, 272, 855. [Google Scholar] [CrossRef]
- Tejedor-Tejada, J.; Alonso-Martín, C.; Almohalla-Álvarez, C.; Valenzuela, E.F.; Muñoz, R.N.; Delgado, L.S.; Martín, C.M.; Sánchez-Martín, F.; García-Pajares, F.; Sánchez-Antolín, G. Immunosuppressive Treatment with mTOR Inhibitors for Malignancies after Liver Transplantation: Long-Term Survival Retrospective Analysis. Transplant. Proc. 2020, 52, 1507–1510. [Google Scholar] [CrossRef]
- Kadry, Z.; Stine, J.G.; Dohi, T.; Jain, A.; Robyak, K.L.; Kwon, O.; Hamilton, C.J.; Janicki, P.; Riley, T.R.I.; Butt, F.; et al. Renal Protective Effect of Everolimus in Liver Transplantation: A Prospective Randomized Open-Label Trial. Transplant. Direct 2021, 7, e709. [Google Scholar] [CrossRef]
- Nitta, H.; Younès, A.; El-domiaty, N.; Karam, V.; Sobesky, R.; Vibert, E.; Coilly, A.; Maria Antonini, T.; De Martin, E.; Cherqui, D.; et al. High trough levels of everolimus combined to sorafenib improve patients survival after hepatocellular carcinoma recurrence in liver transplant recipients. Transpl. Int. 2021, 34, 1293–1305. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Colmenero, J.; González, A.; Gastaca, M.; Curell, A.; Caballero-Marcos, A.; Sánchez-Martínez, A.; Maira, T.D.; Herrero, J.I.; Almohalla, C.; et al. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am. J. Transplant. 2022, 22, 1671–1682. [Google Scholar] [CrossRef]
- Sapisochin, G.; Lee, W.C.; Joo, D.J.; Joh, J.-W.; Hata, K.; Soin, A.S.; Veldandi, U.K.; Kaneko, S.; Meier, M.; Leclair, D.; et al. Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipients with Hepatocellular Carcinoma. Ann. Transplant. 2022, 27, e937988-1. [Google Scholar] [CrossRef]
- Mulder, M.B.; van Hoek, B.; van den Berg, A.P.; Polak, W.G.; Alwayn, I.P.J.; de Jong, K.P.; de Winter, B.C.M.; Verhey-Hart, E.; Erler, N.S.; den Hoed, C.M.; et al. Three-year results of renal function in liver transplant recipients on low-dose sirolimus and tacrolimus: A multicenter, randomized, controlled trial. Liver Transplant. 2023, 29, 184. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, L.; Tamburini, J.; Chapuis, N.; Lacombe, C.; Mayeux, P.; Bouscary, D. PI3K and mTOR signaling pathways in cancer: New data on targeted therapies. Curr. Oncol. Rep. 2012, 14, 129–138. [Google Scholar] [CrossRef]
- Davis, W.J.; Lehmann, P.Z.; Li, W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front. Cell Dev. Biol. 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Moschetta, M.; Reale, A.; Marasco, C.; Vacca, A.; Carratù, M.R. Therapeutic targeting of the mTOR-signalling pathway in cancer: Benefits and limitations. Br. J. Pharmacol. 2014, 171, 3801–3813. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef] [Green Version]
- Guri, Y.; Colombi, M.; Dazert, E.; Hindupur, S.K.; Roszik, J.; Moes, S.; Jenoe, P.; Heim, M.H.; Riezman, I.; Riezman, H.; et al. mTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer Cell 2017, 32, 807–823.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Weng, J.; Zhang, Y.; Liang, K.; Fu, G.; Li, Y.; Bai, X.; Gao, Y. mTOR direct crosstalk with STAT5 promotes de novo lipid synthesis and induces hepatocellular carcinoma. Cell Death Dis. 2019, 10, 619. [Google Scholar] [CrossRef] [Green Version]
- Menon, S.; Yecies, J.L.; Zhang, H.H.; Howell, J.J.; Nicholatos, J.; Harputlugil, E.; Bronson, R.T.; Kwiatkowski, D.J.; Manning, B.D. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci. Signal. 2012, 5, ra24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, M.; Sonenberg, N.; Gores, G. The mTOR Pathway in Hepatic Malignancies. Hepatology 2013, 58, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, A.; Chiang, D.Y.; Newell, P.; Peix, J.; Thung, S.; Alsinet, C.; Tovar, V.; Roayaie, S.; Minguez, B.; Sole, M.; et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008, 135, 1972–1983, 1983.e1–e11. [Google Scholar] [CrossRef] [Green Version]
- Matter, M.S.; Decaens, T.; Andersen, J.B.; Thorgeirsson, S.S. Targeting the mTOR pathway in hepatocellular carcinoma: Current state and future trends. J. Hepatol. 2014, 60, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Huang, Y.; Li, J.; Wang, Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med. Oncol. Northwood Lond. Engl. 2010, 27, 255–261. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. WJG 2007, 13, 4974–4978. [Google Scholar] [CrossRef]
- Menk, M.; Graw, J.A.; Poyraz, D.; Möbius, N.; Spies, C.D.; von Haefen, C. Chronic Alcohol Consumption Inhibits Autophagy and Promotes Apoptosis in the Liver. Int. J. Med. Sci. 2018, 15, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, M.; Ferrín, G.; Rodríguez-Perálvarez, M.; González-Rubio, S.; Sánchez-Frías, M.; Amado, V.; Pozo, J.C.; Poyato, A.; Ciria, R.; Ayllón, M.D.; et al. mTOR Expression in Liver Transplant Candidates with Hepatocellular Carcinoma: Impact on Histological Features and Tumour Recurrence. Int. J. Mol. Sci. 2019, 20, 336. [Google Scholar] [CrossRef] [Green Version]
- Calne, R.Y.; Lim, S.; Samaan, A.; Collier, D.S.T.J.; Pollard, S.G.; White, D.J.G.; Thiru, S. Rapamycin for Immunosuppression in Organ Allografting. Lancet 1989, 334, 227. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L. Sirolimus approved with renal transplant indication. Am. J. Health Syst. Pharm. 1999, 56, 2177–2178. [Google Scholar] [CrossRef]
- MacKeigan, J.P.; Krueger, D.A. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro-Oncol. 2015, 17, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalati, K.; Kahan, B.D. Clinical Pharmacokinetics of Sirolimus. Clin. Pharmacokinet. 2001, 40, 573–585. [Google Scholar] [CrossRef]
- De&rsquo, N.; Angelis, F.L. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J. Gastroenterol. 2015, 21, 11185–11198. [Google Scholar] [CrossRef]
- Hoffman, D.; Mehta, N. Recurrence of hepatocellular carcinoma following liver transplantation. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 91–102. [Google Scholar] [CrossRef]
- Duvoux, C.; Toso, C. mTOR inhibitor therapy: Does it prevent HCC recurrence after liver transplantation? Transplant. Rev. 2015, 29, 168–174. [Google Scholar] [CrossRef]
- Khorsandi, S.E.; Heaton, N. Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence. Transl. Gastroenterol. Hepatol. 2016, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Thomas Decaens, F.; oise Roudot-Thoraval, S.B.-H.; bastien Dharancy, O.C.; Res, D.C. Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: A multicenter study of 412 patients. World J. Gastroenterol. 2006, 12, 7319–7325. [Google Scholar] [CrossRef]
- Yokoyama, I.; Carr, B.; Saitsu, H.; Iwatsuki, S.; Starzl, T.E. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer 1991, 68, 2095–2100. [Google Scholar] [CrossRef]
- Maluccio, M.; Sharma, V.; Lagman, M.; Vyas, S.; Yang, H.; Li, B.; Suthanthiran, M. Tacrolimus enhances transforming growth factor-β1 expression and promotes tumor progression. Transplantation 2003, 76, 597. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Mamou, C.; Rodríguez-Castro, K.I.; Burra, P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: A systematic review. Transpl. Int. 2014, 27, 1039–1049. [Google Scholar] [CrossRef]
- Cholongitas, E.; Burra, P.; Vourli, G.; Papatheodoridis, G.V. Safety and efficacy of everolimus initiation from the first month after liver transplantation: A systematic review and meta-analysis. Clin. Transplant. 2023, 37, e14957. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.-H.; Zhang, C.-Z.; Gao, F.-Q.; Wei, X.-Y.; Ling, S.-B.; Wang, K.; Wang, J.-G.; Zheng, S.-S.; Nikfarjam, M.; Xu, X. A mixed blessing for liver transplantation patients—Rapamycin. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 14–21. [Google Scholar] [CrossRef]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Magistri, P.; Ballarin, R.; Di Francia, R.; Berretta, M.; Di Benedetto, F. Oncological Impact of M-Tor Inhibitor Immunosuppressive Therapy after Liver Transplantation for Hepatocellular Carcinoma: Review of the Literature. Front. Pharmacol. 2016, 7, 387. [Google Scholar] [CrossRef]
- Cholongitas, E.; Antoniadis, N.; Goulis, I.; Theocharidou, E.; Ιmvrios, G.; Giouleme, O.; Filis, D.; Mouloudi, E.; Akriviadis, E.; Fouzas, I. Trough Levels of Everolimus Are Associated with Recurrence Rates of Hepatocellular Carcinoma after Liver Transplantation. Transplant. Proc. 2019, 51, 450–453. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma after Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann. Surg. 2017, 266, 118. [Google Scholar] [CrossRef]
- Foerster, F.; Hoppe-Lotichius, M.; Vollmar, J.; Marquardt, J.U.; Weinmann, A.; Wörns, M.-A.; Otto, G.; Zimmermann, T.; Galle, P.R. Long-term observation of hepatocellular carcinoma recurrence after liver transplantation at a European transplantation centre. United Eur. Gastroenterol. J. 2019, 7, 838–849. [Google Scholar] [CrossRef]
- Chagas, A.L.; Felga, G.E.G.; Diniz, M.A.; Silva, R.F.; Mattos, A.A.; Silva, R.C.M.A.; Boin, I.F.S.F.; Garcia, J.H.P.; Lima, A.S.; Coelho, J.C.U.; et al. Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: Clinical profile and prognostic factors of survival. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1148. [Google Scholar] [CrossRef]
- Sapisochin, G.; Goldaracena, N.; Astete, S.; Laurence, J.M.; Davidson, D.; Rafael, E.; Castells, L.; Sandroussi, C.; Bilbao, I.; Dopazo, C.; et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann. Surg. Oncol. 2015, 22, 2286–2294. [Google Scholar] [CrossRef]
- Rajendran, L.; Ivanics, T.; Claasen, M.P.; Muaddi, H.; Sapisochin, G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 28, 1–16. [Google Scholar] [CrossRef]
- Adnane, L.; Trail, P.A.; Taylor, I.; Wilhelm, S.M. Sorafenib (BAY 43-9006, Nexavar®), a Dual-Action Inhibitor That Targets RAF/MEK/ERK Pathway in Tumor Cells and Tyrosine Kinases VEGFR/PDGFR in Tumor Vasculature. In Methods in Enzymology; Regulators and Effectors of Small GTPases: Ras Family; Academic Press: Cambridge, MA, USA, 2006; Volume 407, pp. 597–612. Available online: https://www.sciencedirect.com/science/article/pii/S0076687905070473 (accessed on 5 April 2023).
- Sposito, C.; Mariani, L.; Germini, A.; Reyes, M.F.; Bongini, M.; Grossi, G.; Bhoori, S.; Mazzaferro, V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: A case-control study. J. Hepatol. 2013, 59, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Mazzola, A.; Cabibbo, G.; Perricone, G.; Enea, M.; Galvano, A.; Zavaglia, C.; Belli, L.; Cammà, C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: A systematic review and meta-analysis. Dig. Liver Dis. 2015, 47, 324–330. [Google Scholar] [CrossRef]
- Colmenero, J.; Tabrizian, P.; Bhangui, P.; Pinato, D.J.; Rodríguez-Perálvarez, M.L.; Sapisochin, G.; Bhoori, S.; Pascual, S.; Senzolo, M.; Al-Adra, D.; et al. De Novo Malignancy after Liver Transplantation: Risk Assessment, Prevention, and Management—Guidelines from the ILTS-SETH Consensus Conference. Transplantation 2022, 106, e30. [Google Scholar] [CrossRef]
- Altieri, M.; Sérée, O.; Lobbedez, T.; Segol, P.; Abergel, A.; Blaizot, X.; Boillot, O.; Boudjema, K.; Coilly, A.; Conti, F.; et al. Risk factors of de novo malignancies after liver transplantation: A French national study on 11,004 adult patients. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101514. [Google Scholar] [CrossRef]
- De Simone, P.; Fagiuoli, S.; Cescon, M.; De Carlis, L.; Tisone, G.; Volpes, R.; Cillo, U.; Panel, C. Use of Everolimus in Liver Transplantation: Recommendations From a Working Group. Transplantation 2017, 101, 239. [Google Scholar] [CrossRef] [Green Version]
- Bzeizi, K.I.; Abdullah, M.; Vidyasagar, K.; Alqahthani, S.A.; Broering, D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers 2022, 14, 5114. [Google Scholar] [CrossRef]
- Rana, A.; Ackah, R.L.; Webb, G.J.; Halazun, K.J.; Vierling, J.M.; Liu, H.; Wu, M.-F.; Yoeli, D.; Kueht, M.; Mindikoglu, A.L.; et al. No Gains in Long-term Survival after Liver Transplantation Over the Past Three Decades. Ann. Surg. 2019, 269, 20. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic Renal Failure after Transplantation of a Nonrenal Organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; García-Caparrós, C.; O’Beirne, J.; Poyato-González, A.; Ferrín-Sánchez, G.; Montero-Álvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, S.; Yang, Y.; Lu, Z.; Li, F.; Jiang, L.; Jiang, Y.; Liu, J. Sirolimus or Everolimus Improves Survival after Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Transplant. 2022, 28, 1063. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Iwatsuki, S.; Shaw, B.W.; Gordon, R.D.; Esquivel, C. Liver Transplantation in the Ciclosporin Era. Prog. Allergy 1986, 38, 366–394. [Google Scholar] [PubMed] [Green Version]
- Komolmit, P.; Davies, M.H. Tacrolimus in liver transplantation. Expert Opin. Investig. Drugs 1999, 8, 1239–1254. [Google Scholar] [CrossRef]
- Mihatsch, M.J.; Kyo, M.; Morozumi, K.; Yamaguchi, Y.; Nickeleit, V.; Ryffel, B. The side-effects of ciclosporine-A and tacrolimus. Clin. Nephrol. 1998, 49, 356–363. [Google Scholar]
- Schuler, W.; Sedrani, R.; Cottens, S.; Häberlin, B.; Schulz, M.; Schuurman, H.-J.; Zenke, G.; Zerwes, H.-G.; Schreier, M.H. SDZ RAD, A NEW RAPAMYCIN DERIVATIVE: Pharmacological Properties In Vitro and In Vivo. Transplantation 1997, 64, 36. [Google Scholar] [CrossRef]
- Cillo, U.; De Carlis, L.; Del Gaudio, M.; De Simone, P.; Fagiuoli, S.; Lupo, F.; Tisone, G.; Volpes, R. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: Consensus recommendations from an Italian Working Group. Hepatol. Int. 2020, 14, 930–943. [Google Scholar] [CrossRef]
- Naesens, M.; Kuypers, D.R.J.; Sarwal, M. Calcineurin Inhibitor Nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481. [Google Scholar] [CrossRef] [Green Version]
- Trinh, E.; Alam, A.; Tchervenkov, J.; Cantarovich, M. Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin. Transplant. 2017, 31, e12863. [Google Scholar] [CrossRef]
- Tsai, K.-F.; Li, L.-C.; Hsu, C.-N.; Lin, C.-C.; Lin, Y.-H.; Cheng, Y.-F.; Wang, C.-C.; Chen, C.-L. Effects of Conversion from Calcineurin Inhibitors to Sirolimus or Everolimus on Renal Function and Possible Mechanisms in Liver Transplant Recipients. J. Clin. Pharmacol. 2019, 59, 326–334. [Google Scholar] [CrossRef]
- Asrani, S.K.; Leise, M.D.; West, C.P.; Murad, M.H.; Pedersen, R.A.; Erwin, P.J.; Tian, J.; Wiesner, R.H.; Kim, W.R. Use of Sirolimus in Liver Transplant Recipients with Renal Insufficiency: Systematic Review and Meta-Analysis. Hepatology 2010, 52, 1360–1370. [Google Scholar] [CrossRef] [Green Version]
- Tedesco-Silva, H.; Saliba, F.; Barten, M.J.; De Simone, P.; Potena, L.; Gottlieb, J.; Gawai, A.; Bernhardt, P.; Pascual, J. An overview of the efficacy and safety of everolimus in adult solid organ transplant recipients. Transplant. Rev. 2022, 36, 100655. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Saigal, S.; Bansal, R.K.; Saraf, N.; Gautam, D.; Soin, A.S. Acute and Chronic Rejection after Liver Transplantation: What A Clinician Needs to Know. J. Clin. Exp. Hepatol. 2017, 7, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Jeng, L.-B.; Lee, S.G.; Soin, A.S.; Lee, W.-C.; Suh, K.-S.; Joo, D.J.; Uemoto, S.; Joh, J.; Yoshizumi, T.; Yang, H.-R.; et al. Efficacy and safety of everolimus with reduced tacrolimus in living-donor liver transplant recipients: 12-month results of a randomized multicenter study. Am. J. Transplant. 2018, 18, 1435–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, M.; Stabilini, A.; Roncarolo, M.-G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005, 105, 4743–4748. [Google Scholar] [CrossRef] [Green Version]
- De Simone, P.; Metselaar, H.J.; Fischer, L.; Dumortier, J.; Boudjema, K.; Hardwigsen, J.; Rostaing, L.; De Carlis, L.; Saliba, F.; Nevens, F. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: A prospective, randomized, multicenter trial. Liver Transplant. 2009, 15, 1262–1269. [Google Scholar] [CrossRef]
- Mártinez, J.M.A.; Pulido, L.B.; Bellido, C.B.; Usero, D.D.; Aguilar, L.T.; Moreno, J.L.G.; Artacho, G.S.; Díez-Canedo, J.S.; Gómez, L.M.M.; Bravo, M.Á.G. Rescue Immunosuppression with Mammalian Target of Rapamycin Inhibitor Drugs in Liver Transplantation. Transplant. Proc. 2010, 42, 641–643. [Google Scholar] [CrossRef]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of Immune Responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [Green Version]
- Dunkelberg, J.C.; Trotter, J.F.; Wachs, M.; Bak, T.; Kugelmas, M.; Steinberg, T.; Everson, G.T.; Kam, I. Sirolimus as primary immunosuppression in liver transplantation is not associated with hepatic artery or wound complications. Liver Transplant. 2003, 9, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Petrulionis, M.; Lin, S.; Gao, C.; Galli, U.; Richter, S.; Winkler, S.; Houben, P.; Schultze, D.; Hatano, E.; et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma—Systematic review and meta-analysis. Cancer Med. 2013, 2, 862–871. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Vautier, M.; Allenbach, Y.; Zahr, N.; Benveniste, O.; Funck-Brentano, C.; Salem, J.-E. Sirolimus and mTOR Inhibitors: A Review of Side Effects and Specific Management in Solid Organ Transplantation. Drug Saf. 2019, 42, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, M.; Neff, G.W.; Yamashiki, N.; Meyer, D.; Bettiol, M.; Slapak-Green, G.; Ruiz, P.; Manten, E.; Safdar, K.; O’Brien, C.; et al. A Retrospective Review of Liver Transplant Patients Treated with Sirolimus from a Single Center: An Analysis of Sirolimus-Related Complications. Transplantation 2004, 78, 264. [Google Scholar] [CrossRef]
- Plotogea, O.; Ilie, M.; Sandru, V.; Chiotoroiu, A.; Bratu, O.; Diaconu, C. Cardiovascular and Metabolic Consequences of Liver Transplantation: A Review. Medicina 2019, 55, 489. [Google Scholar] [CrossRef] [Green Version]
- Klintmalm, G.B.; Nashan, B. The Role of mTOR Inhibitors in Liver Transplantation: Reviewing the Evidence. J. Transplant. 2014, 2014, 845438. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Watt, K.D. Long-term Medical Management of the Liver Transplant Recipient: What the Primary Care Physician Needs to Know. Mayo Clin. Proc. 2012, 87, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhao, Y.; Zhao, L.; Yuan, J.; Chen, Y.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; Ruan, X.Z. Paradoxical effect of rapamycin on inflammatory stress-induced insulin resistance in vitro and in vivo. Sci. Rep. 2015, 5, 14959. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.-C.; Amri, E.-Z.; Hutchinson, D.S.; Bengtsson, T. Glucose uptake in brown fat cells is dependent on mTOR complex 2–promoted GLUT1 translocation. J. Cell Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Wallace, M.; Sanchez-Gurmaches, J.; Hsiao, W.-Y.; Li, H.; Lee, P.L.; Vernia, S.; Metallo, C.M.; Guertin, D.A. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat. Commun. 2016, 7, 11365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peláez-Jaramillo, M.J.; Cárdenas-Mojica, A.A.; Gaete, P.V.; Mendivil, C.O. Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment. Diabetes Ther. 2018, 9, 521–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashan, B.; Citterio, F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: A critical review of the literature. Transplantation 2012, 94, 547–561. [Google Scholar] [CrossRef]
- Saliba, F.; Duvoux, C.; Gugenheim, J.; Kamar, N.; Dharancy, S.; Salamé, E.; Neau-Cransac, M.; Durand, F.; Houssel-Debry, P.; Vanlemmens, C.; et al. Efficacy and Safety of Everolimus and Mycophenolic Acid with Early Tacrolimus Withdrawal after Liver Transplantation: A Multicenter Randomized Trial. Am. J. Transplant. 2017, 17, 1843–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.A.M.; Khalil, M.A.U.; Khan, T.F.T.; Tan, J. Drug-Induced Hematological Cytopenia in Kidney Transplantation and the Challenges It Poses for Kidney Transplant Physicians. J. Transplant. 2018, 2018, 9429265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffellner, S.; Jakoby, E.; Kniepeiss, D.; Stadlbauer, V.; Duller, D.; Iberer, F.; Tscheliessnigg, K.H. Center experience in liver transplantation (LTX): Management of dermal side effects caused by sirolimus. Int. Immunopharmacol. 2005, 5, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, A.P.; Hohos, M.B.; Polson, K.M.O.; Huftalen, T.M.; Treister, N. Managing stomatitis in patients treated with Mammalian target of rapamycin inhibitors. Clin. J. Oncol. Nurs. 2011, 15, E83–E89. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Qazi, Y.; Wellen, J.R. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant. Rev. 2014, 28, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Campistol, J.M.; de Fijter, J.W.; Flechner, S.M.; Langone, A.; Morelon, E.; Stockfleth, E. mTOR inhibitor-associated dermatologic and mucosal problems. Clin. Transplant. 2010, 24, 149–156. [Google Scholar] [CrossRef]
| Study, Year | Study Design | Country | Populations Studied | Results |
|---|---|---|---|---|
| Zhou et al., 2008 [14] | R | China | 73 consecutive patients who underwent LT for HCC exceeding the Milan criteria, treated with an SRL-based regimen (n = 27) or an FK506-based regimen (n = 46) | Benefit in terms of 1-year survival (594 ± 35 days vs. 480 ± 42 days, p = 0.011) and RFS (519 ± 43 days vs. 477 ± 48 days, p = 0.234) in the SRL group |
| Rogers et al., 2009 [15] | R | USA | 72 LT recipients converted to SRL | Significantly higher eGFR mean values at all time points when the conversion was early (within 3 months) |
| Vivarelli et al., 2010 [16] | R | Italy | 78 LT recipients treated with SRL in a CNI-sparing regimen (n = 38) or in combination with CNIs (n = 40) | eGFR increased from 30 mL/min to 57 mL/min in patients that started SRL within 3 months from LT |
| Gomez-Martin et al., 2011 [17] | RUC | Spain | 31 patients who suffered from HCC recurrence after LT converted to mTORi-based immunosuppression plus systemic SOR | Stabilization of the disease and a median overall survival of 19.3 months are achieved using combination therapy with mTORi |
| De Simone et al., 2012 [18] | RCT | Multicenter worldwide | 242 de novo LT patients randomized to EVR with TAC elimination (n = 231), EVR + reduced TAC (n = 245), or standard TAC (n = 243) | Significant difference of 8.5 mL/min/1.73 m2 (p < 0.001) in eGFR values at any point from week 6 post-LT in patients treated with EVR + low TAC Higher rejection rate at 1 year after LT when TAC is eliminated (19.9%), compared to EVR + reduced TAC (3.7%) and even TAC controls (10.7%) |
| Fischer et al., 2012 [19] | RCT | Multicenter worldwide | 203 LT recipients initially treated with basiliximab/CNIs randomized to an EVR-based regimen (n= 101) or CNI continuation (n = 102) | Statistically significant increase in GFR values in the EVR group |
| Ferreiro et al., 2014 [20] | CS | Spain | 52 LT recipients with a high risk of post-transplant recurrence receiving EVR (n = 21) or CNIs (n = 31) after a first course of CNIs-based immunosuppression | Higher survival rate at 5 years (60.2% vs. 32.3%, p = 0.05) in the EVR group |
| Geissler et al., 2016 [21] | RCT | Multicenter worldwide | 525 LT recipients with HCC initially receiving mTORi–free immunosuppression randomized to mTORi–free regimen (n = 264) or an SRL-based regimen (n = 261) | Higher RFS rate in the SRL group (70.2% vs. 64.5%, p = 0.28), with a statistically significant difference only at 1 and 3 years after LT (92.5% vs. 85.2%, p < 0.0125; 80.6% vs. 72.3%, p < 0.0499) Average gain of RFS of 6.4 months in the SRL group In the SRL group: 13% to 15% higher RFS when SRL was administered in monotherapy; higher RFS rate at 4 years in low-risk patients; more significant advantage for younger recipients (≤60 years) |
| Jung et al., 2018 [22] | R | Korea | 232 patients who suffered from HCC recurrence after LT treated with SOR (n = 54), mTORi (n = 16), SOR + mTORi (n = 23), or none of them (n = 139) | Survival rates are not affected by SOR administration (p = 0.17) but improve following the administration of mTORi (p < 0.001) or SOR + mTORi (p = 0.011) No difference in the post-recurrence OS period between combination or monotherapy in mTORi-based regimens (p = 0.26) |
| Invernizzi et al., 2020 [23] | R | Italy | 50 patients with HCC-recurrence after LT treated with SOR | Impact on 1-year OS is more significant with a SOR + mTORi regimen (p = 0.03) |
| Saliba et al., 2020 [24] | OS | France | LT recipients receiving EVR | Better improvement of renal function at 36 months in LT recipients with eGFR <60 mL/min/1.73 m2 undergoing CNIs conversion within 12 months (55% if within 3 months, 39.4% if at 4–12 months, 20.9% if after 12 months) |
| Schnitzbauer et al., 2020 [25] | R | Multicenter worldwide | 508 patients of the intention-to-treat analysis from the SiLVER study [21] | Later tumour redevelopment and more prolonged survival after recurrence in the SRL group Prolonged SRL exposure after LT (≥3 months), higher AFP levels before LT (≥10 ng/mL, HR: 1.84; 95% CI: 1.36–2.48; p < 0.001), and inclusion within Milan criteria are predictors of higher OS and reduced danger of death |
| Tejedor-Tejada et al., 2020 [26] | R | Spain | 111 LT recipients treated with a mTORi-based immunosuppression | Higher survival rates at 1 year when EVR is initiated immediately after LT (89%) compared to switch within 3 months (83%) or later (67%). No significant difference was found when EVR was used alone or in combination with CNIs or MPA |
| Kadry et al., 2021 [27] | RCT | USA | 24 LT recipients randomized to a EVR + MPA-based regimen (n = 12) or CNI + MPA-based regimen (n = 12) | Improved renal function at 12 (88.01 vs. 60.63 mL/min/1.73 m2, p = 0.020) and 24 (87.37 vs. 53.29 mL/min/1.73 m2, p = 0.013) months after LT in the EVR + MPA group |
| Nitta et al., 2021 [28] | RCS | France | 308 consecutive patients who underwent LT for HCC | Longer mean survival time when EVR ≥5 ng/mL in patients treated with EVR alone (19.9 months vs. 10.7 months; p = 0.021) or in combination with SOR (22.5 months vs. 10.7 months, p = 0.030) |
| Rodríguez-Perálvarez et al., 2022 [29] | qC | Spain | 425 patients who developed malignancy after LT and 425 matching controls, selected among an eligible cohort population comprising 2495 LT patients who received TAC-based immunosuppression | Increased risk of DNM has been demonstrated for prolonged exposure to CNI-based regimens but not for mTOR inhibitors |
| Sapisochin et al., 2022 [30] | P, R | Multicenter worldwide | 86 LT recipients treated with EVR + reduced TAC (n = 41) or TAC (n = 45) | Lower rate of HCC recurrence at 5 years after LT when an EVR-facilitated TAC reduction is initiated early (3.6% vs. 11.5%, p = 0.136) Lower recurrence rates when mean trough levels of EVR >6 ng/mL |
| Mulder et al., 2023 [31] | RCT | Multicenter worldwide | 196 LT recipients randomized to SRL + low TAC (n = 98) or TAC (n = 98) | Significantly fewer patients had a CKD grade ≥3 at 6 months in the low SRL + low TAC group |
| Adverse Effects (AEs) | Rate of AEs in Patients Treated with mTORi | Proposed Strategies to Prevent AEs |
|---|---|---|
| Dyslipidemia: hypercholesterolemia, hypertriglyceridemia | 45%, 50% [64] | Reduction of mTORi exposure, administration of statins and other dyslipidemia drugs |
| Hyperglycemia | 17% [101] | Lifestyle modifications, pharmacological management of diabetes mellitus |
| Proteinuria | 3% [78] | Constant monitoring of proteinuria when >800 mg/d, administration of ACEi or ARBs |
| Wound healing complications | 11% [18,21] | Delayed introduction of mTORi at 4–6 weeks after LT, correction of the risk factors for WHC, administration of the minimum effective doses |
| Hematologic side effects: anemia, neutropenia, thrombocytopenia | 14%, 11%, 9% [102] | Adjustment of mTORi exposure |
| Mucosal and integumental adverse effects: oral ulcers, dermatitis, leg edema | 24%, 25%, 57% [103] | Oral hygiene, avoidance of irritant food and beverages, topical treatment of oral ulcers, adjustment of mTORi exposure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todeschini, L.; Cristin, L.; Martinino, A.; Mattia, A.; Agnes, S.; Giovinazzo, F. The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma. Curr. Oncol. 2023, 30, 5574-5592. https://doi.org/10.3390/curroncol30060421
Todeschini L, Cristin L, Martinino A, Mattia A, Agnes S, Giovinazzo F. The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma. Current Oncology. 2023; 30(6):5574-5592. https://doi.org/10.3390/curroncol30060421
Chicago/Turabian StyleTodeschini, Letizia, Luca Cristin, Alessandro Martinino, Amelia Mattia, Salvatore Agnes, and Francesco Giovinazzo. 2023. "The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma" Current Oncology 30, no. 6: 5574-5592. https://doi.org/10.3390/curroncol30060421
APA StyleTodeschini, L., Cristin, L., Martinino, A., Mattia, A., Agnes, S., & Giovinazzo, F. (2023). The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma. Current Oncology, 30(6), 5574-5592. https://doi.org/10.3390/curroncol30060421





