The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef]
- Nozoe, T.; Saeki, H.; Sugimachi, K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am. J. Surg. 2001, 182, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Sattar, N. Score based on hypoalbuminemia and elevated C-reactive protein predicts survival in patients with advanced gastrointestinal cancer. Nutr. Cancer 2004, 48, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Asanuma, K.; Matsubara, T.; Sudo, A. The role of C-reactive protein in predicting post-metastatic survival of patients with metastatic bone and soft tissue sarcoma. Tumour Biol. 2015, 36, 7515–7520. [Google Scholar] [CrossRef]
- Aggerholm-Pedersen, N.; Maretty-Kongstad, K.; Keller, J.; Baerentzen, S.; Safwat, A. The Prognostic Value of Serum Biomarkers in Localized Bone Sarcoma. Transl. Oncol. 2016, 9, 322–328. [Google Scholar] [CrossRef]
- Vasquez, L.; León, E.; Beltran, B.; Maza, I.; Oscanoa, M.; Geronimo, J. Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas. J. Pediatr. Hematol. Oncol. 2017, 39, 538–546. [Google Scholar] [CrossRef]
- Aggerholm-Pedersen, N.; Maretty-Kongstad, K.; Keller, J.; Safwat, A. Serum Biomarkers as Prognostic Factors for Metastatic Sarcoma. Clin. Oncol. R. Coll. Radiol. 2019, 31, 242–249. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Matsubara, T.; Asanuma, K.; Uchida, A.; Sudo, A. The combined use of the neutrophil-lymphocyte ratio and C-reactive protein level as prognostic predictors in adult patients with soft tissue sarcoma. J. Surg. Oncol. 2013, 108, 481–485. [Google Scholar] [CrossRef]
- Nakamura, T.; Grimer, R.J.; Gaston, C.L.; Watanuki, M.; Sudo, A.; Jeys, L. The prognostic value of the serum level of C-reactive protein for the survival of patients with a primary sarcoma of bone. Bone Jt. J. 2013, 95-B, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Jettoo, P.; Tan, G.; Gerrand, C.H.; Rankin, K.S. Role of routine blood tests for predicting clinical outcomes in osteosarcoma patients. J. Orthop. Surg. 2019, 27, 2309499019838293. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.-K.; Liu, Z.-L.; Shen, D.; Lin, Q.F.; Su, J.; Mao, W.D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef]
- Li, Y.-J.; Yao, K.; Lu, M.-X.; Zhang, W.-B.; Xuai, C.; Tu, C.-Q. Prognostic value of the C-reactive protein to albumin ratio: A novel inflammation-based prognostic indicator in osteosarcoma. Onco Targets Ther. 2017, 10, 5255–5261. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Liang, W.; Luo, K.; Qin, Y.; Li, F.; Xie, T.; Qin, H.; He, J.; Wei, Q. A new model of preoperative systemic inflammatory markers predicting overall survival of osteosarcoma: A multicenter retrospective study. BMC Cancer 2022, 22, 1370. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Longhi, A.; Versari, M.; Mercuri, M.; Briccoli, A.; Picci, P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006, 106, 1154–1161. [Google Scholar] [CrossRef]
- Hao, H.; Chen, L.; Huang, D.; Ge, J.; Qiu, Y.; Hao, L. Meta-analysis of alkaline phosphatase and prognosis for osteosarcoma. Eur. J. Cancer Care 2017, 26, e12536. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, K.-H.; Moon, S.-H.; Jang, J.; Kim, H.S.; Suh, J.S.; Yang, W.I. Reassessment of alkaline phosphatase as serum tumor marker with high specificity in osteosarcoma. Cancer Med. 2017, 6, 1311–1322. [Google Scholar] [CrossRef]
- Bacci, G.; Longhi, A.; Ferrari, S.; Lari, S.; Manfrini, M.; Donati, D.; Forni, C.; Versari, M. Prognostic significance of serum alkaline phosphatase in osteosarcoma of the extremity treated with neoadjuvant chemotherapy: Recent experience at Rizzoli Institute. Oncol. Rep. 2002, 9, 171–175. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, C.; Lou, J.; Huang, Y.; Ren, T.; Guo, W. Development of a Nomogram for Predicting the Efficacy of Preoperative Chemotherapy in Osteosarcoma. Int. J. Gen. Med. 2021, 14, 4819–4827. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Yin, F.; Guo, P.; Yang, X.; Liu, J.; Han, Y.; Ren, Z. Systemic Inflammatory Markers for Predicting Overall Survival in Patients with Osteosarcoma: A Systematic Review and Meta-Analysis. Mediat. Inflamm. 2021, 2021, 3456629. [Google Scholar] [CrossRef]
- Yapar, A.; Tokgöz, M.A.; Yapar, D.; Atalay, İ.B.; Ulucaköy, C.; Güngör, B.Ş. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt. Dis. Relat. Surg. 2021, 32, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N. Osteosarcoma: Diagnostic dilemmas in histopathology and prognostic factors. Indian. J. Orthop. 2014, 48, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Wang, X.; Feng, J.; Zhao, X. The Prognostic Role of Glasgow Prognostic Score and C-reactive Protein to Albumin Ratio for Sarcoma: A System Review and Meta-Analysis. Dis. Markers 2020, 2020, 8736509. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Rutkowski, P.; Kamińska, J.; Kowalska, M.; Ruka, W.; Steffen, J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: Correlations with local tumor extent and prognosis. J. Surg. Oncol. 2003, 84, 151–159. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Matsubara, T.; Asanuma, K.; Uchida, A.; Sudo, A. Clinical significance of pretreatment serum C-reactive protein level in soft tissue sarcoma. Cancer 2012, 118, 1055–1061. [Google Scholar] [CrossRef]
- Vasquez, L.; Tarrillo, F.; Oscanoa, M.; Maza, I.; Geronimo, J.; Paredes, G.; Silva, J.M.; Sialer, L. Analysis of Prognostic Factors in High-Grade Osteosarcoma of the Extremities in Children: A 15-Year Single-Institution Experience. Front. Oncol. 2016, 6, 22. [Google Scholar] [CrossRef]
- Maretty-Kongstad, K.; Aggerholm-Pedersen, N.; Keller, J.; Safwat, A. A Validated Prognostic Biomarker Score for Adult Patients with Nonmetastatic Soft Tissue Sarcomas of the Trunk and Extremities. Transl. Oncol. 2017, 10, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, F.; Wang, F.; Li, Y. Serum C-reactive protein and overall survival of patients with osteosarcoma. Tumour Biol. 2015, 36, 5663–5666. [Google Scholar] [CrossRef]
- Ferrari, S.; Mercuri, M.; Picci, P.; Bertoni, F.; Brach del Prever, A.; Tienghi, A.; Mancini, A.; Longhi, A.; Rimondini, S.; Donati, D.; et al. Nonmetastatic osteosarcoma of the extremity: Results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with high-dose methotrexate, intraarterial or intravenous cisplatin, doxorubicin, and salvage chemotherapy based on histologic tumor response. Tumori 1999, 85, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Smrke, A.; Anderson, P.M.; Gulia, A.; Gennatas, S.; Huang, P.H.; Jones, R.L. Future Directions in the Treatment of Osteosarcoma. Cells 2021, 10, 172. [Google Scholar] [CrossRef] [PubMed]
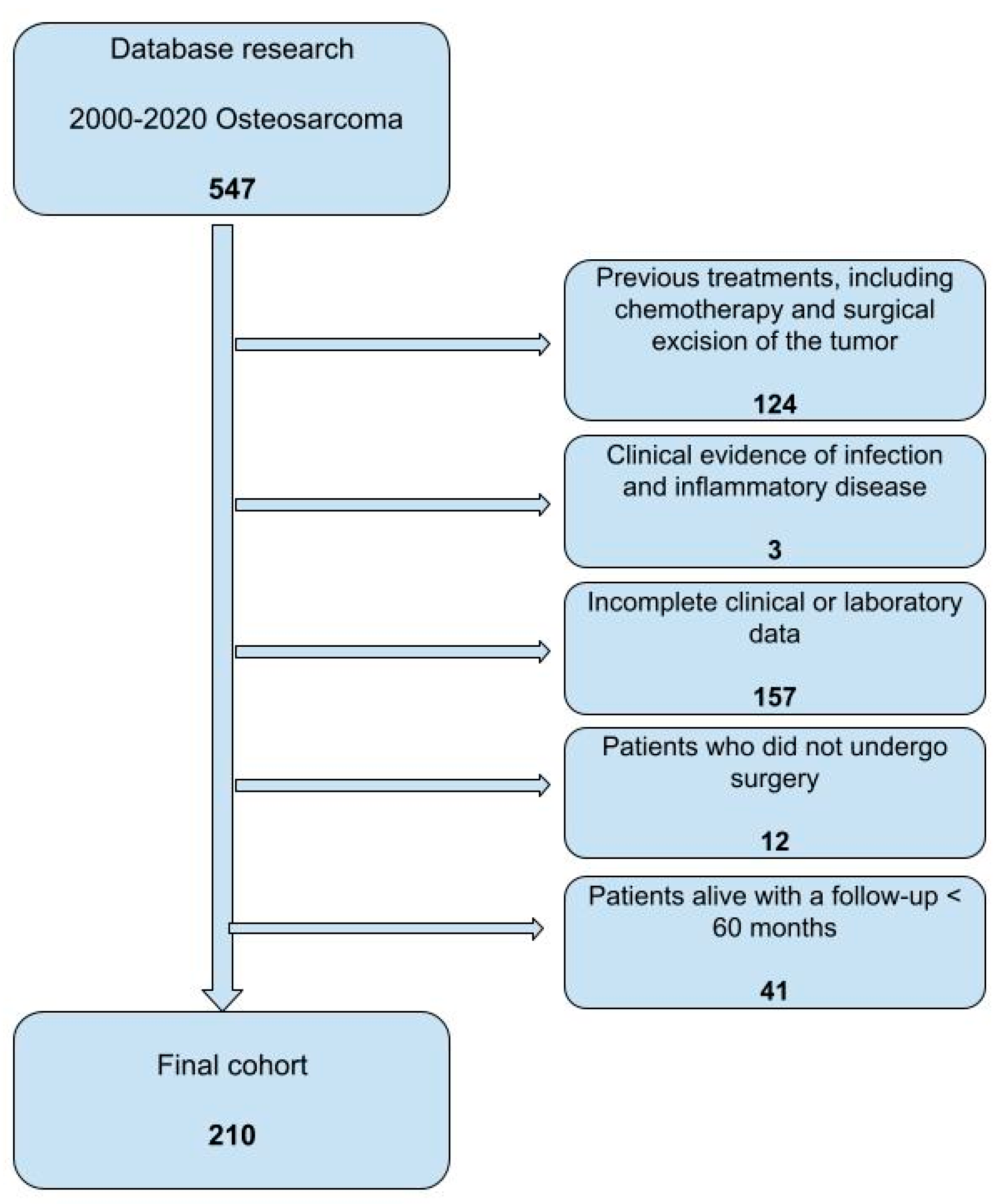
| Variables | ||
|---|---|---|
| Age (years) | ||
| Median (IQ range) | 13 (11–16) years | |
| Absolute frequencies | Relative frequencies | |
| Gender | ||
| male | 114 | 54.3 |
| female | 96 | 45.7 |
| Neoadjuvant CHT | ||
| yes | 206 | 98.1 |
| no | 4 | 1.9 |
| Adjuvant CHT | ||
| yes | 208 | 99.0 |
| no | 2 | 1.0 |
| Tumor location | ||
| femur | 110 | 52.4 |
| tibia | 69 | 32.8 |
| humerus | 16 | 7.6 |
| fibula | 8 | 3.8 |
| ulna | 4 | 1.9 |
| radius | 2 | 1.0 |
| pelvis | 1 | 0.5 |
| Tumor size (n) | ||
| >150 mL | 168 | 80.0 |
| missing | 41 | 19.5 |
| ≤150 mL | 1 | 0.5 |
| Metastatic disease | ||
| no | 159 | 75.7 |
| yes | 51 | 24.3 |
| N Tot | Death in 5 Years (N) | Overall Survival at 5 Years [95% CI] | p-Value 1 | |
|---|---|---|---|---|
| Disease at presentation | <0.001 | |||
| Localized | 159 | 31 | 80.5 [73.6, 85.9] | |
| Metastatic | 51 | 33 | 35.3 [23.5, 49.2] | |
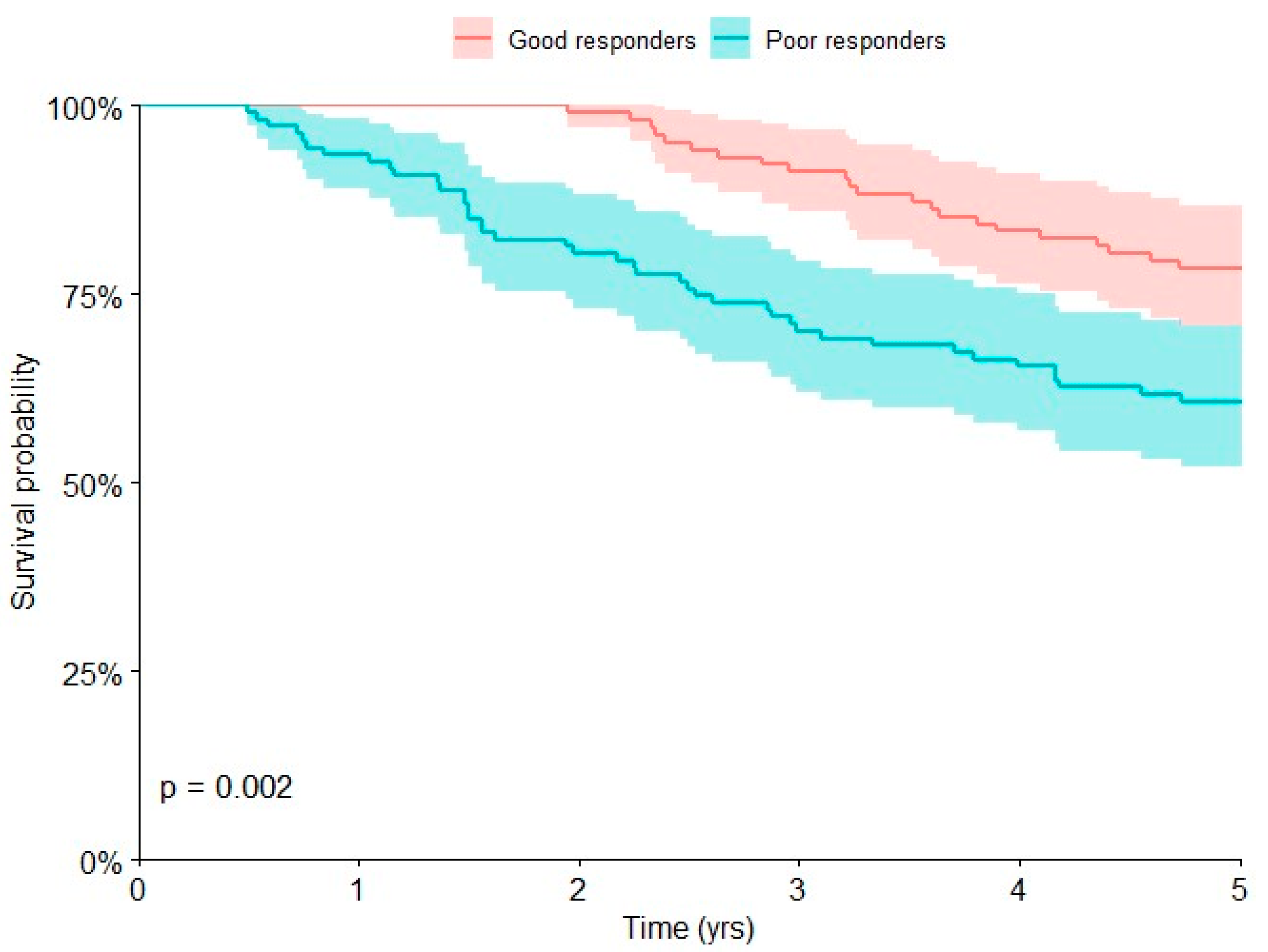
| Response to chemotherapy | 0.002 | |||
| Good | 102 | 22 | 78.4 [69.4, 85.4] | |
| Poor | 107 | 42 | 60.7 [51.2, 69.5] | |
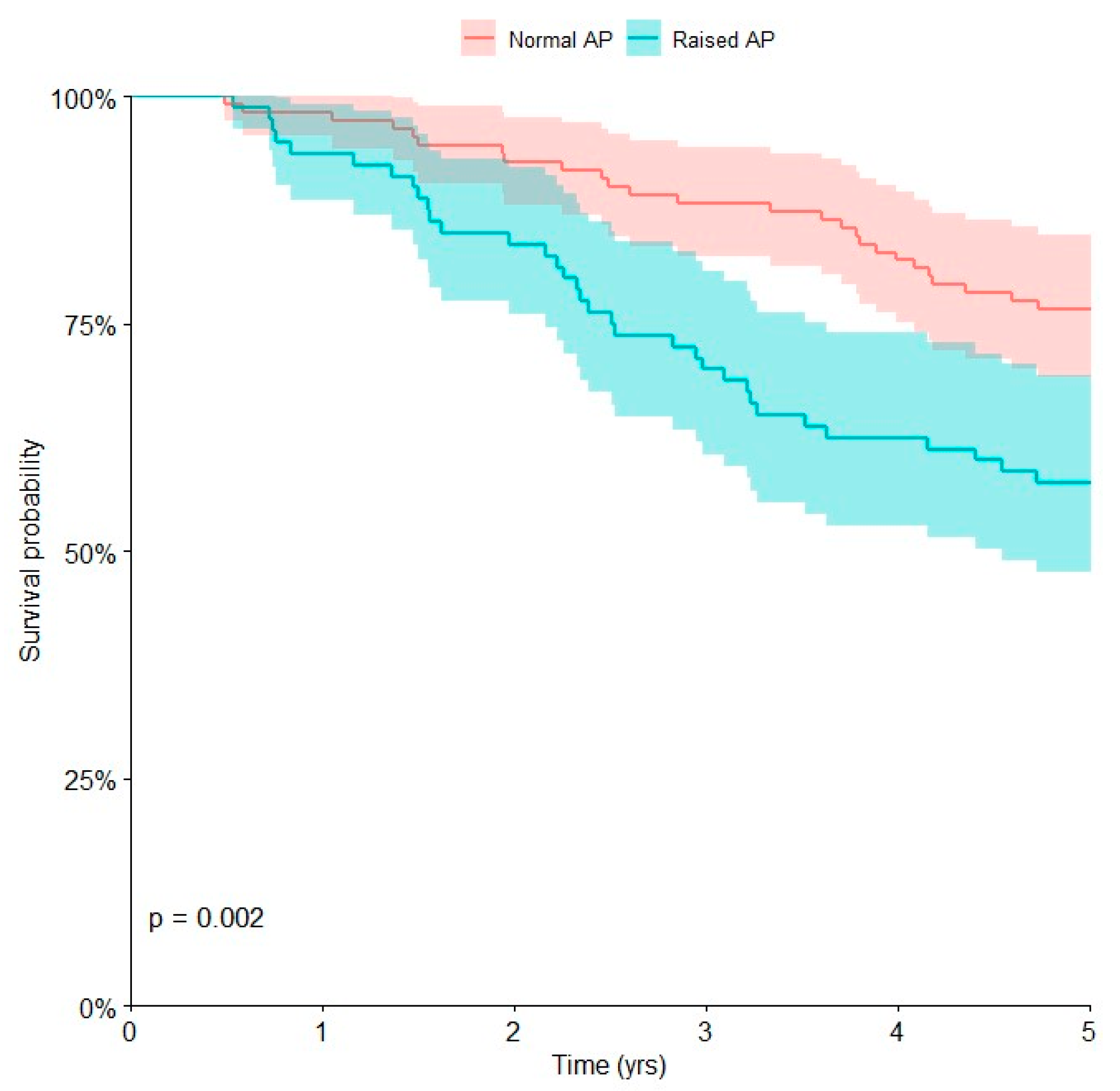
| Alkaline phosphatase | 0.002 | |||
| Normal | 111 | 26 | 76.6 [67.8, 83.5] | |
| Raised | 80 | 34 | 57.5 [46.5, 67.8] |
| HR 1 | 95% CI 1 | p-Value | |
|---|---|---|---|
| Disease at presentation | |||
| Localized | — | — | |
| Metastatic | 3.71 | 2.19, 6.29 | <0.001 |
| Alkaline phosphatase (AP) | |||
| Normal | — | — | |
| Raised | 1.73 | 1.02, 2.94 | 0.042 |
| Chemotherapy-induced tumor necrosis | |||
| Good responders | — | — | |
| Poor responders | 2.40 | 1.41, 4.08 | 0.001 |
| N Tot | Local Recurrence-Free in 5 Years (N) | Local Recurrence-Free Survival at 5 Years [95% CI] | p-Value 1 | |
|---|---|---|---|---|
| Margins | 0.007 | |||
| Adequate | 197 | 19 | 89.2 [8.37, 93.1] | |
| Inadequate | 6 | 2 | 53.3 [13.9, 89.0] | |
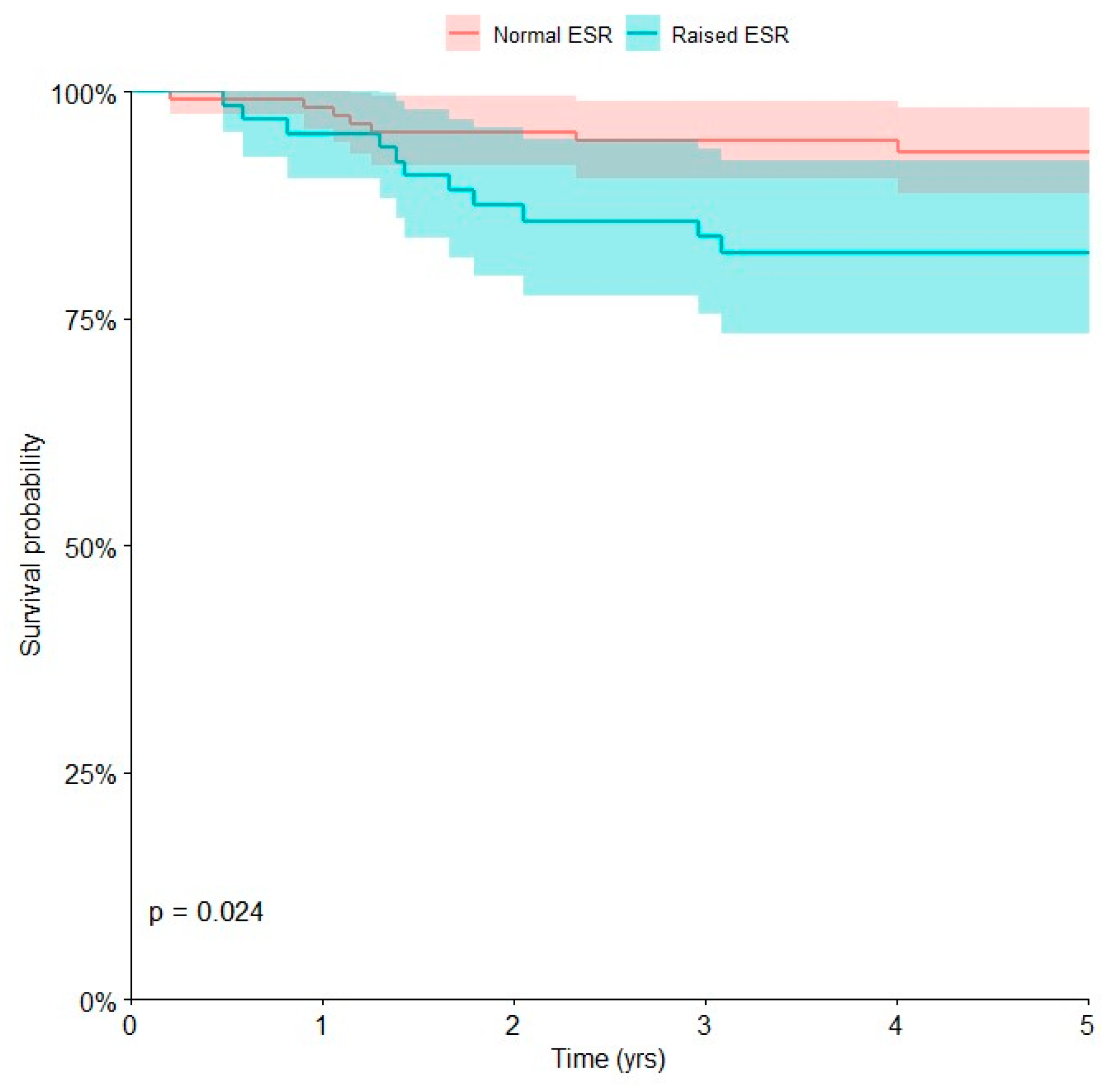
| Erythrocyte sedimentation rate (ESR) | 0.024 | |||
| Normal | 117 | 7 | 93.4 [86.8, 96.9] | |
| Raised | 66 | 11 | 82.3 [70.8, 90.0] |
| HR 1 | 95% CI 1 | p-Value | |
|---|---|---|---|
| Erythrocyte sedimentation rate (ESR) | |||
| Normal | — | — | |
| Raised | 3.58 | 1.29, 9.98 | 0.015 |
| Margins | |||
| Adequate | — | — | |
| Inadequate | 11.3 | 1.37, 92.8 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basoli, S.; Cosentino, M.; Traversari, M.; Manfrini, M.; Tsukamoto, S.; Mavrogenis, A.F.; Bordini, B.; Donati, D.M.; Errani, C. The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities. Curr. Oncol. 2023, 30, 7043-7054. https://doi.org/10.3390/curroncol30070511
Basoli S, Cosentino M, Traversari M, Manfrini M, Tsukamoto S, Mavrogenis AF, Bordini B, Donati DM, Errani C. The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities. Current Oncology. 2023; 30(7):7043-7054. https://doi.org/10.3390/curroncol30070511
Chicago/Turabian StyleBasoli, Stefano, Monica Cosentino, Matteo Traversari, Marco Manfrini, Shinji Tsukamoto, Andreas F. Mavrogenis, Barbara Bordini, Davide Maria Donati, and Costantino Errani. 2023. "The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities" Current Oncology 30, no. 7: 7043-7054. https://doi.org/10.3390/curroncol30070511
APA StyleBasoli, S., Cosentino, M., Traversari, M., Manfrini, M., Tsukamoto, S., Mavrogenis, A. F., Bordini, B., Donati, D. M., & Errani, C. (2023). The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities. Current Oncology, 30(7), 7043-7054. https://doi.org/10.3390/curroncol30070511








