Are Canadian Women Prepared for the Transition to Primary HPV Testing in Cervical Screening? A National Survey of Knowledge, Attitudes, and Beliefs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedure
2.3. Measures
2.3.1. Screening History
2.3.2. Knowledge Scales
2.3.3. Attitudes and Beliefs Scales
2.4. Analyses
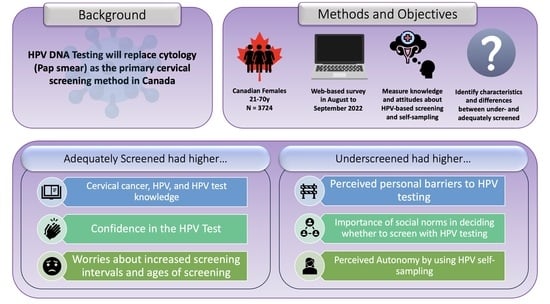
3. Results
3.1. Participants
3.2. Cervical Cancer Knowledge
3.3. HPV Testing Knowledge
3.4. HPV Knowledge
3.5. HPV Testing Attitudes and Beliefs
3.5.1. Personal Barriers
3.5.2. Social Norms
3.5.3. Confidence
3.5.4. Worries
3.6. HPV Self-Sampling Attitudes and Beliefs
3.6.1. Concerns
3.6.2. Autonomy
4. Discussion
4.1. Knowledge
4.2. HPV Testing Attitudes and Beliefs
4.3. HPV Self-Sampling Attitudes and Beliefs
4.4. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. Can. Med. Assoc. J. 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- Cervical Cancer Statistics. Available online: https://cancer.ca/en/cancer-information/cancer-types/cervical/statistics (accessed on 27 June 2022).
- Cervical Cancer Screening in Canada: Monitoring & Evaluation of Quality Indicators. Available online: https://s22457.pcdn.co/wp-content/uploads/2019/01/Cervical-Cancer-Screen-Quality-Indicators-Report-2016-EN.pdf (accessed on 10 July 2023).
- Action Plan for the Elimination of Cervical Cancer, 2020–2030. Available online: https://www.partnershipagainstcancer.ca/topics/elimination-cervical-cancer-action-plan/ (accessed on 30 August 2022).
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.-H.; et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: Acting on evidence to change the current paradigm. Prev. Med. 2017, 98, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Isidean, S.D.; Mayrand, M.-H.; Ramanakumar, A.V.; Gilbert, L.; Reid, S.L.; Rodrigues, I.; Ferenczy, A.; Ratnam, S.; Coutlée, F.; Franco, E.L.; et al. Human papillomavirus testing versus cytology in primary cervical cancer screening: End-of-study and extended follow-up results from the Canadian cervical cancer screening trial. Int. J. Cancer 2016, 139, 2456–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouvard, V.; Wentzensen, N.; Mackie, A.; Berkhof, J.; Brotherton, J.; Giorgi-Rossi, P.; Kupets, R.; Smith, R.; Arrossi, S.; Bendahhou, K.; et al. The IARC Perspective on Cervical Cancer Screening. N. Engl. J. Med. 2021, 385, 1908–1918. [Google Scholar] [CrossRef]
- WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. Available online: https://www.who.int/publications/i/item/9789240030824 (accessed on 7 February 2022).
- Final Recommendation Statement, Cervical Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening (accessed on 9 March 2022).
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; E Kennedy, C.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef]
- King, E.; Busolo, D. The Role of Primary Care Nurse Practitioners in Reducing Barriers to Cervical Cancer Screening: A Literature Review. Can. Oncol. Nurs. J. 2022, 32, 233–244. [Google Scholar] [CrossRef]
- Cervical Cancer: PHW Apology over Screening Changes. Available online: https://www.bbc.com/news/uk-wales-59878409 (accessed on 18 March 2022).
- Obermair, H.M.; Dodd, R.H.; Bonner, C.; Jansen, J.; McCaffery, K. ‘It has saved thousands of lives, so why change it?’ Content analysis of objections to cervical screening programme changes in Australia. BMJ Open 2018, 8, e019171. [Google Scholar] [CrossRef]
- Griffin-Mathieu, G.; Haward, B.; Tatar, O.; Zhu, P.; Perez, S.; Shapiro, G.K.; McBride, E.; Thompson, E.L.; Smith, L.W.; Lofters, A.K.; et al. Ensuring a Successful Transition from Cytology to Human Papillomavirus–Based Primary Cervical Cancer Screening in Canada by Investigating the Psychosocial Correlates of Women’s Intentions: Protocol for an Observational Study. JMIR Res. Protoc. 2022, 11, e38917. [Google Scholar] [CrossRef]
- Cervical Screening in Canada: 2021/2022 Environmental Scan. Available online: https://www.partnershipagainstcancer.ca/topics/cervical-cancer-screening-in-canada-2021-2022/summary/ (accessed on 30 May 2023).
- Haward, B.; Tatar, O.; Zhu, P.; Griffin-Mathieu, G.; Perez, S.; Shapiro, G.K.; McBride, E.; Zimet, G.D.; Rosberger, Z. Development and validation of the cervical cancer knowledge scale and HPV testing knowledge scale in a sample of Canadian women. Prev. Med. Rep. 2022, 30, 102017. [Google Scholar] [CrossRef]
- Perez, S.; Tatar, O.; Ostini, R.; Shapiro, G.K.; Waller, J.; Zimet, G.; Rosberger, Z. Extending and validating a human papillomavirus (HPV) knowledge measure in a national sample of Canadian parents of boys. Prev. Med. 2016, 91, 43–49. [Google Scholar] [CrossRef]
- Waller, J.; Ostini, R.; Marlow, L.A.; McCaffery, K.; Zimet, G. Validation of a measure of knowledge about human papillomavirus (HPV) using item response theory and classical test theory. Prev. Med. 2012, 56, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Tatar, O.; Haward, B.; Zhu, P.; Griffin-Mathieu, G.; Perez, S.; McBride, E.; Lofters, A.K.; Smith, L.W.; Mayrand, M.H.; Daley, E.M.; et al. Understanding the Challenges of HPV-Based Cervical Screening: Development and Validation of HPV Testing and Self-Sampling Attitudes and Beliefs Scales. Curr Oncol. 2023, 30, 1206–1219. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows (Version 24.0); IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- List of Ethnic Origins. Available online: https://www23.statcan.gc.ca/imdb/p3VD.pl?Function=getVD&TVD=402936 (accessed on 13 January 2022).
- Kasting, M.L.; Wilson, S.; Zollinger, T.W.; Dixon, B.E.; Stupiansky, N.W.; Zimet, G.D. Differences in cervical cancer screening knowledge, practices, and beliefs: An examination of survey responses. Prev. Med. Rep. 2017, 5, 169–174. [Google Scholar] [CrossRef]
- Harries, J.; Scott, S.E.; Walter, F.M.; Mwaka, A.D.; Moodley, J. Women’s appraisal, interpretation and help-seeking for possible symptoms of breast and cervical cancer in South Africa: A qualitative study. BMC Women’s Health 2020, 20, 251. [Google Scholar] [CrossRef]
- Thompson, E.L.; Wheldon, C.W.; Rosen, B.L.; Maness, S.B.; Kasting, M.L.; Massey, P.M. Awareness and knowledge of HPV and HPV vaccination among adults ages 27–45 years. Vaccine 2020, 38, 3143–3148. [Google Scholar] [CrossRef]
- Preti, M.; Rosso, S.; Micheletti, L.; Libero, C.; Sobrato, I.; Giordano, L.; Busso, P.; Gallio, N.; Cosma, S.; Bevilacqua, F.; et al. Risk of HPV-related extra-cervical cancers in women treated for cervical intraepithelial neoplasia. BMC Cancer 2020, 20, 972. [Google Scholar] [CrossRef]
- How to Prevent 9 Different Cancers & Genital Warts. Available online: https://hpvglobalaction.org/en/educational-resources/ (accessed on 12 July 2023).
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Tatar, O.; Thompson, E.; Naz, A.; Perez, S.; Shapiro, G.K.; Wade, K.; Zimet, G.; Gilca, V.; Janda, M.; Kahn, J.; et al. Factors associated with human papillomavirus (HPV) test acceptability in primary screening for cervical cancer: A mixed methods research synthesis. Prev. Med. 2018, 116, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Nothacker, J.; Nury, E.; Mathieu, M.R.; Raatz, H.; Meerpohl, J.J.; Schmucker, C. Women’s attitudes towards a human papillomavirus-based cervical cancer screening strategy: A systematic review. BMJ Sex. Reprod. Health 2022, 48, 295–306. [Google Scholar] [CrossRef]
- Islam, R.M.; Billah, B.; Hossain, N.; Oldroyd, J. Barriers to Cervical Cancer and Breast Cancer Screening Uptake in Low-Income and Middle-Income Countries: A Systematic Review. Asian Pac. J. Cancer Prev. 2017, 18, 1751–1763. [Google Scholar] [CrossRef]
- Zhu, P.; Tatar, O.; Haward, B.; Griffin-Mathieu, G.; Perez, S.; Smith, L.; Brotherton, J.; Ogilvie, G.; Rosberger, Z. Assessing Canadian women’s preferences for cervical cancer screening: A brief report. Front. Public Health 2022, 10, 962039. [Google Scholar] [CrossRef] [PubMed]
- Cervical Cancer Screening Programme. Available online: https://www.rivm.nl/en/cervical-cancer-screening-programme (accessed on 20 June 2023).
- Chidyaonga-Maseko, F.; Chirwa, M.L.; Muula, A.S. Underutilization of cervical cancer prevention services in low and middle income countries: A review of contributing factors. Pan Afr. Med J. 2015, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Oliffe, J.L.; Kelly, M.T.; Krist, J. Bridging Barriers to Cervical Cancer Screening in Transgender Men: A Scoping Review. Am. J. Men’s Health 2020, 14, 1557988320925691. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, M.; Lee, S.; Goopy, S.; Yang, H.; Rumana, N.; Abedin, T.; Turin, T.C. Barriers to cervical cancer screening faced by immigrant women in Canada: A systematic scoping review. BMC Women’s Health 2018, 18, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirubarajan, A.; Leung, S.; Li, X.; Yau, M.; Sobel, M. Barriers and facilitators for cervical cancer screening among adolescents and young people: A systematic review. BMC Women’s Health 2021, 21, 122. [Google Scholar] [CrossRef]
- Bryant, J.; Patterson, K.; Vaska, M.; Chiang, B.; Letendre, A.; Bill, L.; Yang, H.; Kopciuk, K. Cancer Screening Interventions in Indigenous Populations: A Rapid Review. Curr. Oncol. 2021, 28, 1728–1743. [Google Scholar] [CrossRef]
- Östensson, E.; Alder, S.; Elfström, K.M.; Sundström, K.; Zethraeus, N.; Arbyn, M.; Andersson, S. Barriers to and Facilitators of Compliance with Clinic-Based Cervical Cancer Screening: Population-Based Cohort Study of Women Aged 23-60 Years. PLoS ONE 2015, 10, e0128270. [Google Scholar] [CrossRef] [Green Version]
- Bennett, K.F.; Waller, J.; Chorley, A.J.; Ferrer, R.A.; Haddrell, J.B.; Marlow, L.A. Barriers to cervical screening and interest in self-sampling among women who actively decline screening. J. Med. Screen. 2018, 25, 211–217. [Google Scholar] [CrossRef]
- McBride, E.; Tatar, O.; Rosberger, Z.; Rockliffe, L.; Marlow, L.A.V.; Moss-Morris, R.; Kaur, N.; Wade, K.; Waller, J. Emotional response to testing positive for human papillomavirus at cervical cancer screening: A mixed method systematic review with meta-analysis. Heal. Psychol. Rev. 2020, 15, 395–429. [Google Scholar] [CrossRef]
- Kahn, J.A.; Slap, G.B.; Bernstein, D.I.; Kollar, L.M.; Tissot, A.M.; Hillard, P.A.; Rosenthal, S.L. Psychological, Behavioral, and Interpersonal Impact of Human Papillomavirus and Pap Test Results. J. Women’s Health 2005, 14, 650–659. [Google Scholar] [CrossRef]
- Knops-Dullens, T.; de Vries, N.; de Vries, H. Reasons for non-attendance in cervical cancer screening programmes: An application of the Integrated Model for Behavioural Change. Eur. J. Cancer Prev. 2007, 16, 436–445. [Google Scholar] [CrossRef]
- Williams, R.C.; Simonds, H.; Roomaney, R. Knowledge, misinformation, stigma, and disclosure hesitancy among women receiving curative treatment for cervical cancer at a tertiary hospital in South Africa. S. Afr. J. Psychol. 2023. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Peng, T.-Q. Nature and Diffusion of Gynecologic Cancer–Related Misinformation on Social Media: Analysis of Tweets. J. Med. Internet Res. 2018, 20, e11515. [Google Scholar] [CrossRef]
- Virtanen, A.; Nieminen, P.; Niironen, M.; Luostarinen, T.; Anttila, A. Self-sampling experiences among non-attendees to cervical screening. Gynecol. Oncol. 2014, 135, 487–494. [Google Scholar] [CrossRef]
- Racey, C.S.; Gesink, D.C. Barriers and Facilitators to Cervical Cancer Screening Among Women in Rural Ontario, Canada: The Role of Self-Collected HPV Testing. J. Rural. Health 2015, 32, 136–145. [Google Scholar] [CrossRef]
- Zehbe, I.; Wakewich, P.; King, A.-D.; Morrisseau, K.; Tuck, C. Self-administered versus provider-directed sampling in the Anishinaabek Cervical Cancer Screening Study (ACCSS): A qualitative investigation with Canadian First Nations women. BMJ Open 2017, 7, e017384. [Google Scholar] [CrossRef] [Green Version]
- McDowell, M.; Pardee, D.J.; Peitzmeier, S.; Reisner, S.L.; Agénor, M.; Alizaga, N.; Bernstein, I.; Potter, J. Cervical Cancer Screening Preferences Among Trans-Masculine Individuals: Patient-Collected Human Papillomavirus Vaginal Swabs Versus Provider-Administered Pap Tests. LGBT Health 2017, 4, 252–259. [Google Scholar] [CrossRef]
- Griner, S.B.; Vamos, C.A.; Puccio, J.A.; Perrin, K.M.; Beckstead, J.W.; Daley, E.M. “I’ll Just Pick It up…”: Women’s Acceptability of Self-Sampling Methods for Sexually Transmitted Infection Screening. Sex. Transm. Dis. 2019, 46, 762–767. [Google Scholar] [CrossRef]
- Hanley, S.J.; Fujita, H.; Yokoyama, S.; Kunisawa, S.; Tamakoshi, A.; Dong, P.; Kobayashi, N.; Watari, H.; Kudo, M.; Sakuragi, N. HPV self-sampling in Japanese women: A feasibility study in a population with limited experience of tampon use. J. Med. Screen. 2016, 23, 164–170. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [Green Version]
- Megersa, B.S.; Bussmann, H.; Bärnighausen, T.; Muche, A.A.; Alemu, K.; Deckert, A. Community cervical cancer screening: Barriers to successful home-based HPV self-sampling in Dabat district, North Gondar, Ethiopia. A qualitative study. PLoS ONE 2020, 15, e0243036. [Google Scholar] [CrossRef] [PubMed]





| Full Sample (N = 3724) | Adequately Screened (n = 1871) | Underscreened (n = 1853) | p-Value (Effect Size) 1 | |
|---|---|---|---|---|
| Age (years), M (SD) | 44.97 (14.73) | 46.44 (13.9) | 43.50 (15.33) | <0.001 (d = 0.20) |
| Region, n (%) 2 | <0.001 | |||
| Western and Territories | 1182 (31.7) | 615 (32.9) | 567 (30.6) | ns |
| Ontario | 1453 (39.0) | 759 (40.6) | 694 (37.5) | ns |
| Quebec | 826 (22.2) | 352 (18.8) | 474 (25.6) | h = 0.16 |
| Atlantic | 263 (7.1) | 145 (7.7) | 118 (6.4) | ns |
| Area, n (%) | 0.954 | |||
| Rural | 743 (20.0) | 374 (20.0) | 369 (19.9) | ̶ |
| Urban | 2981 (80.0) | 1497 (80.0) | 1484 (80.1) | |
| Ethnicity 3, n (%) | <0.001 | |||
| North American Indigenous | 123 (3.3) | 67 (3.6) | 56 (3.0) | ns |
| North American-Other | 1653 (44.4) | 840 (44.9) | 813 (43.9) | ns |
| European | 1120 (30.1) | 618 (33.0) | 502 (27.1) | h = 0.13 |
| Asian | 529 (14.2) | 203 (10.8) | 326 (17.6) | h = 0.19 |
| Other 4 | 299 (8.0) | 143 (7.6) | 156 (8.4) | ns |
| Visible minority, n (%) | <0.001 | |||
| Yes | 741 (19.9) | 306 (16.4) | 435 (23.5) | h = 0.18 |
| No | 2983 (80.1) | 1565 (83.6) | 1418 (76.5) | |
| Primary Language, n (%) | <0.001 | |||
| English | 2870 (77.1) | 1510 (80.7) | 1360 (73.4) | h = 0.17 |
| French | 666 (17.9) | 293 (15.7) | 373 (20.1) | h = 0.12 |
| Other | 188 (5.0) | 68 (3.6) | 120 (6.5) | h = 0.13 |
| Living in Canada more than 10 years, n (%) | <0.001 | |||
| Yes | 3457 (92.8) | 1768 (94.5) | 1689 (91.1) | h = 0.13 |
| No | 267 (7.2) | 103 (5.5) | 164 (8.9) | |
| Completed post-secondary education, n (%) | 0.092 | |||
| Yes | 2697 (72.4) | 1378 (73.7) | 1319 (71.2) | ̶ |
| No | 1027 (27.6) | 493 (26.3) | 534 (28.8) | |
| Gender identity, n (%) | 0.010 | |||
| Female/woman | 3676 (98.7) | 1855 (99.1) | 1821 (98.3) | h = 0.08 |
| Gender diverse 5 | 48 (1.3) | 16 (0.9) | 32 (1.7) | |
| Sexual Orientation, n (%) | <0.001 | |||
| Heterosexual | 3302 (88.7) | 1698 (90.8) | 1604 (86.6) | h = 0.13 |
| Bisexual | 218 (5.9) | 99 (5.3) | 119 (6.4) | ns |
| Other 6 | 204 (5.5) | 74 (4.0) | 130 (7.0) | h = 0.14 |
| Relationship/marital status, n (%) | <0.001 | |||
| In a relationship | 2414 (64.8) | 1329 (71.0) | 1085 (58.6) | h = 0.26 |
| Single | 1310 (35.2) | 542 (29.0) | 768 (41.4) | |
| Household income (CAD), n (%) | <0.001 | |||
| 39,999 or less | 852 (22.9) | 343 (18.3) | 509 (27.5) | h = 0.22 |
| Between 40,000 and 79,999 | 1243 (33.4) | 643 (34.4) | 600 (32.4) | ns |
| 80,000 or more | 1495 (40.1) | 830 (44.4) | 665 (35.9) | h = 0.17 |
| Prefer not to answer | 134 (3.6) | 55 (2.9) | 79 (4.3) | h = 0.07 |
| Employment status, n (%) | < 0.01 | |||
| Employed | 2333 (62.6) | 1220 (65.2) | 1113 (60.1) | h = 0.11 |
| Not employed | 1390 (37.4) | 651(34.7) | 740 (39.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haward, B.; Tatar, O.; Zhu, P.; Griffin-Mathieu, G.; McBride, E.; Waller, J.; Brotherton, J.; Lofters, A.; Mayrand, M.-H.; Perez, S.; et al. Are Canadian Women Prepared for the Transition to Primary HPV Testing in Cervical Screening? A National Survey of Knowledge, Attitudes, and Beliefs. Curr. Oncol. 2023, 30, 7055-7072. https://doi.org/10.3390/curroncol30070512
Haward B, Tatar O, Zhu P, Griffin-Mathieu G, McBride E, Waller J, Brotherton J, Lofters A, Mayrand M-H, Perez S, et al. Are Canadian Women Prepared for the Transition to Primary HPV Testing in Cervical Screening? A National Survey of Knowledge, Attitudes, and Beliefs. Current Oncology. 2023; 30(7):7055-7072. https://doi.org/10.3390/curroncol30070512
Chicago/Turabian StyleHaward, Ben, Ovidiu Tatar, Patricia Zhu, Gabrielle Griffin-Mathieu, Emily McBride, Jo Waller, Julia Brotherton, Aisha Lofters, Marie-Hélène Mayrand, Samara Perez, and et al. 2023. "Are Canadian Women Prepared for the Transition to Primary HPV Testing in Cervical Screening? A National Survey of Knowledge, Attitudes, and Beliefs" Current Oncology 30, no. 7: 7055-7072. https://doi.org/10.3390/curroncol30070512
APA StyleHaward, B., Tatar, O., Zhu, P., Griffin-Mathieu, G., McBride, E., Waller, J., Brotherton, J., Lofters, A., Mayrand, M.-H., Perez, S., & Rosberger, Z. (2023). Are Canadian Women Prepared for the Transition to Primary HPV Testing in Cervical Screening? A National Survey of Knowledge, Attitudes, and Beliefs. Current Oncology, 30(7), 7055-7072. https://doi.org/10.3390/curroncol30070512