NTCP Calculations of Five Different Irradiation Techniques for the Treatment of Thymoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Planning
2.3. Planning Dose Parameters
2.4. Radiobiological Parameters
2.5. Statistical Analysis
3. Results
3.1. Planning Dose Parameters
3.2. Radiobiological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirvani, S.M.; Gomez, D.R.; Fuller, C.D.; Thomas, C.R., Jr. Mediastinum and trachea. In Principles and Practice of Radiation Oncology, 7th ed.; Halperin, E.C., Wazer, D.E., Perez, C.A., Brady, L.A., Eds.; Wolter Kluwer: Philadelphia, PA, USA, 2019; pp. 1173–1197. [Google Scholar]
- Engels, E.A. Epidemiology of thymoma and associated malignancies. J. Thorac. Oncol. 2010, 5, S260–S265. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Thymomas and Thymic Carcinomas, Version 2. 2022. Available online: http://www.nccn.org (accessed on 10 November 2022).
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S. Thymic epithelial tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, 40–45. [Google Scholar] [CrossRef]
- Gomez, D.; Komaki, R. Technical advances of radiation therapy for thymic malignancies. J. Thorac. Oncol. 2010, 5, S336–S343. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Boice, J.D., Jr.; Travis, W.D. Second primary cancers after thymoma. Int. J. Cancer 2003, 107, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, D.; Cozzi, L.; Loi, M.; Franzese, C.; Reggiori, G.; Mancosu, P.; Clivio, A.; Fogliata, A.; Scorsetti, M. Volumetric modulated arc therapy versus intensity-modulated proton therapy in the postoperative irradiation of thymoma. J. Cancer Res. Clin. Oncol. 2020, 146, 2267–2276. [Google Scholar] [CrossRef]
- Jallbout, W.; Jbara, R.; Rizk, C.; Youssef, B. On the risk of secondary cancer from thymoma radiotherapy. Phys. Med. Biol. 2022, 67, 155015. [Google Scholar] [CrossRef]
- Konig, L.; Horner-Rieber, J.; Forsthoefel, M.; Haering, P.; Meixner, E.; Eichkorn, T.; Kramer, A.; Mielke, T.; Tonndorf-Martini, E.; Haefner, M.F.; et al. Secondary malignancy risk following proton vs. X-ray radiotherapy of thymic epithelial tumors: A comparative modeling study of thoracic organ-specific cancer risk. Cancers 2022, 14, 2409. [Google Scholar] [CrossRef] [PubMed]
- Lidestahl, A.; Johansson, G.; Siegbahn, A.; Lind, P.A. Estimated risk of radiation-induced cancer after thymoma treatments with proton- or X-ray beams. Cancers 2021, 13, 5153. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, V.; Craig, T.; Bezjak, A.; Van Dyk, J. Dose-volume analysis of lung complications in the radiation treatment of malignant thymoma: A retrospective review. Radiother. Oncol. 2003, 67, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Lin, L.; Litzky, L.A.; Berman, A.T.; Simone, C.B. Predicted rate of secondary malignancies following adjuvant proton versus photon radiation therapy for thymoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 427–433. [Google Scholar] [CrossRef]
- Yan, D.; Ning, L.; Chen, Y.; Ke, S.; Huang, H.; Wang, L.; Yan, S. Analysis of deep inspiration breath-hold technique to improve dosimetric and clinical advantages in postoperative intensity-modulated radiation therapy for thymomas. Quant. Imaging Med. Surg. 2022, 12, 4239–4247. [Google Scholar] [CrossRef]
- Bosse, C.; Narayanasamy, G.; Saenz, D.; Myers, P.; Kirby, N.; Rasmussen, K.; Mavroidis, P.; Papanikolaou, N.; Stathakis, S. Dose calculation comparisons between three modern treatment planning systems. J. Med. Phys. 2020, 45, 143–147. [Google Scholar] [PubMed]
- Clements, M.; Schupp, N.; Tattersall, M.; Brown, A.; Larson, R. Monaco treatment planning system tools and optimization processes. Med. Dosim. 2018, 43, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Matsali, E.; Pappas, E.P.; Lyraraki, E.; Lymperopoulou, G.; Mazonakis, M.; Karaiskos, P. Assessment of radiation-induced bladder and bowel cancer risks after conventionally and hypo-fractionated radiotherapy for the preoperative management of rectal carcinoma. J. Pers. Med. 2022, 12, 1442. [Google Scholar] [CrossRef]
- Mazonakis, M.; Lyraraki, E.; Tolia, M.; Damilakis, J. 3D-CRT, IMRT and VMAT for flank irradiation due to pediatric Wilms tumor: A comparative planning study with XCAT phantoms. Phys. Med. 2022, 103, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mazonakis, M.; Lyraraki, E.; Damilakis, J. Lifetime radiation-induced sarcoma risk in patients subjected to IMRT or VMAT for uterine cervix carcinoma. Phys. Eng. Sci. Med. 2021, 44, 573–579. [Google Scholar] [CrossRef]
- Haefner, M.F.; Verma, V.; Bougatf, N.; Mielke, T.; Tonndorf-Martini, E.; Konig, L.; Rwigema, J.C.M.; Simone, C.B.; Ulhmann, L.; Eichorn, F.; et al. Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol. 2018, 57, 1713–1720. [Google Scholar] [CrossRef]
- Gay, H.A.; Niemierko, A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys. Med. 2007, 23, 115–125. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mazonakis, M.; Tzanis, E.; Lyraraki, E.; Damilakis, J. Automatic radiobiological comparison of radiation therapy plans: An application to gastric cancer. Cancers 2022, 14, 6098. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; Zhu, Z.J.; Zhang, X.Y.; Zhang, Y.; Hong, J.; Shi, T.T.; Han, J.H. Dosimetric analysis of postoperative radiotherapy for thymoma. Cancer Radiother. 2022, 26, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, B.; Lin, Y.; Xu, Z.; Huang, M.; Li, X.; Wu, X.; Chen, Y. Stereotactic body radiotherapy boost with the cyberknife for locally advanced cervical cancer: Dosimetric analysis and potential clinical benefits. Cancers 2022, 14, 5166. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.A.; Wulff, J.; Jazmati, D.; Geismar, D.; Baumer, C.; Kramer, P.H.; Steinmeier, T.; Schleithoff, S.S.; Tschirdewahn, S.; Hadaschik, B.; et al. Is an endorectal balloon beneficial for rectal sparing after spacer implantation in prostate cancer patients treated with hypofractionated intensity-modulated proton beam therapy? A dosimetric and radiobiological comparison study. Curr. Oncol. 2023, 30, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Loap, P.; Vitolo, V.; Barcellini, A.; De Marzi, L.; Mirandola, A.; Fiore, M.R.; Vischioni, B.; Jereczek-Fossa, B.A.; Girard, N.; Kirova, Y.; et al. Hadrontherapy for thymic epithelial tumors: Implementation in clinical practice. Front. Oncol. 2021, 11, 738320. [Google Scholar] [CrossRef] [PubMed]
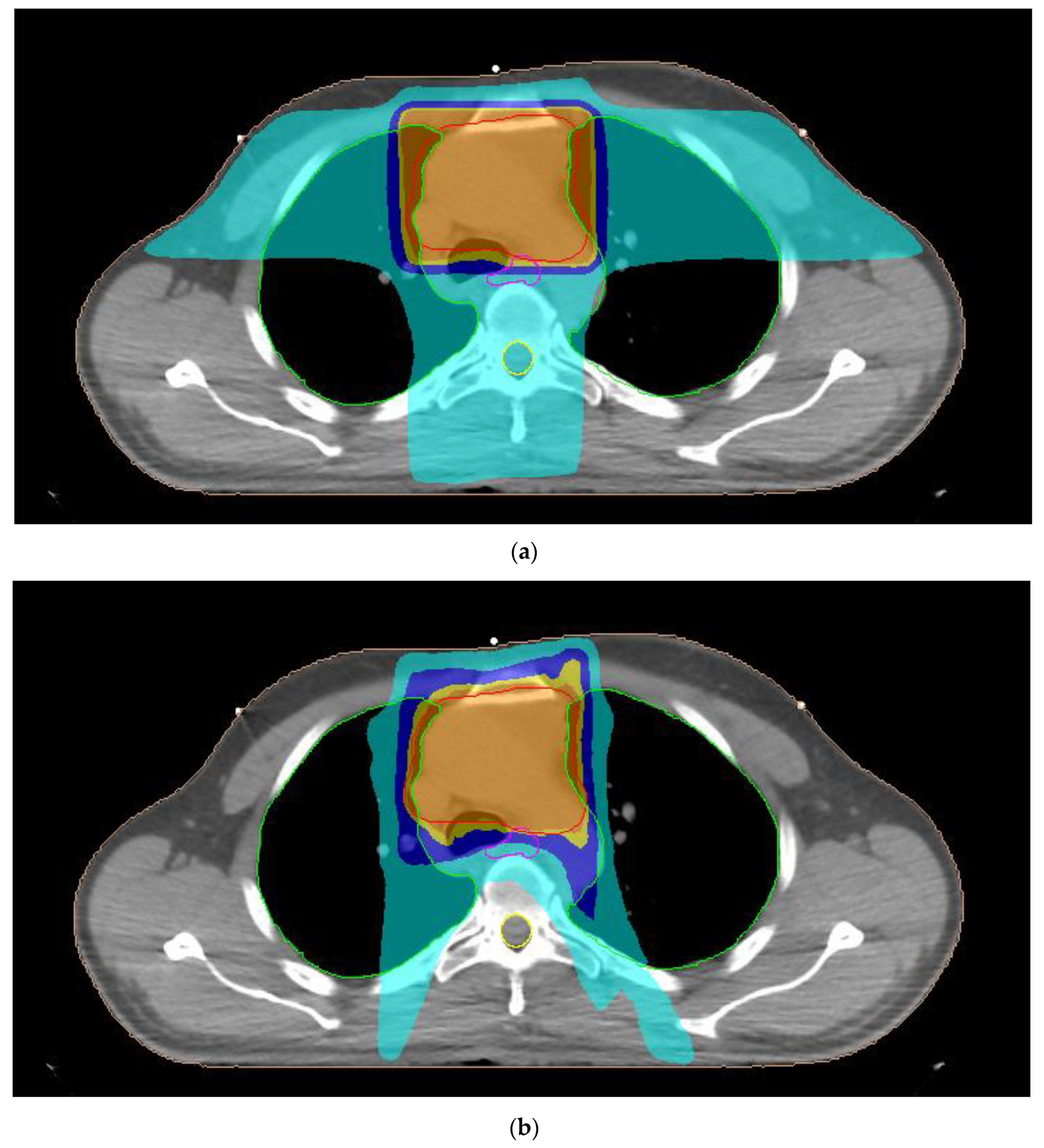
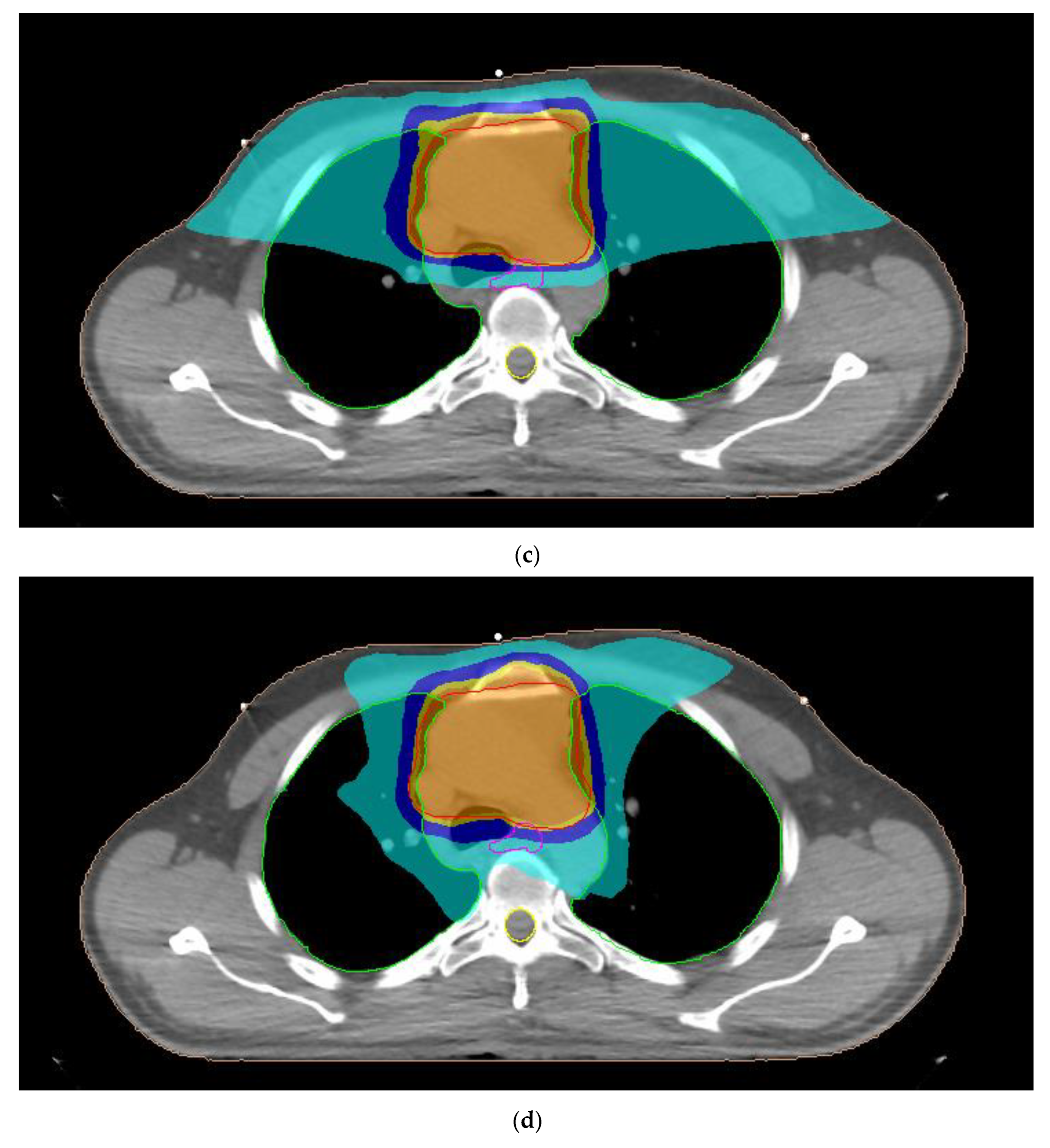
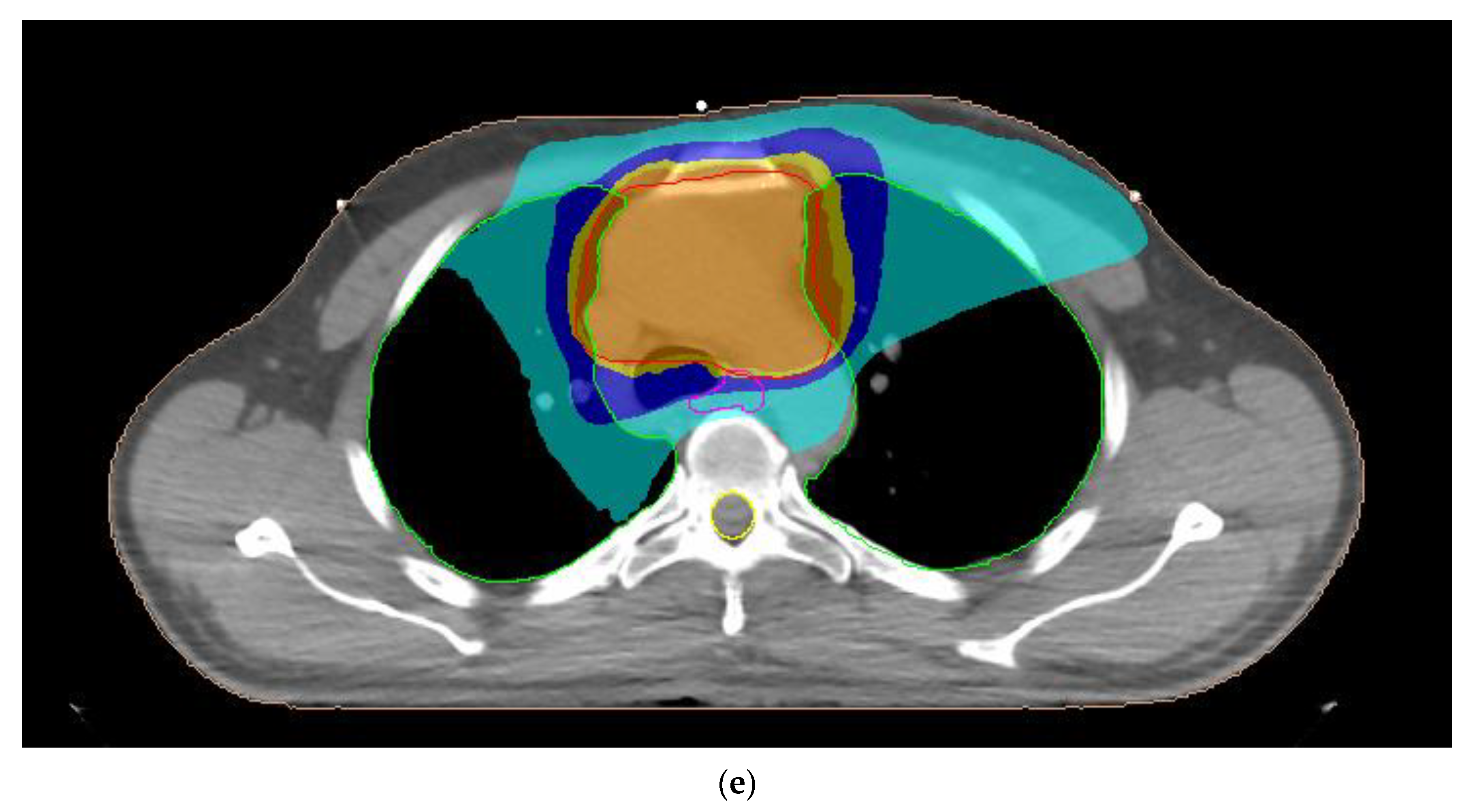

| Organ-at-Risk | Parameter |
|---|---|
| Lungs | Dav < 20 Gy |
| V20Gy < 35% | |
| V5Gy < 65% | |
| Heart | Dav < 26 Gy |
| V30Gy < 45% | |
| Esophagus | Dav < 34 Gy |
| V50Gy < 50% | |
| Spinal cord | Dmax < 45 Gy |
| Organ-at-Risk | α | γ50 | TD50 (Gy) | α/β (Gy) | Endpoint |
|---|---|---|---|---|---|
| Lungs | 1 | 2 | 24.5 | 3 | Pneumonitis |
| Heart | 3 | 3 | 50 | 2.5 | Pericarditis |
| Esophagus | 19 | 4 | 68 | 3 | Perforation/Stricture |
| Technique | TC (%) | HI | CN |
|---|---|---|---|
| 3D-CRT | 58.4 ± 10.7 | 1.11 ± 0.02 | 0.48 ± 0.10 |
| 7F-IMRT | 95.6 ± 0.6 | 1.06 ± 0.01 | 0.83 ± 0.03 |
| 5F-IMRT | 95.3 ± 0.4 | 1.06 ± 0.01 | 0.82 ± 0.03 |
| FA-VMAT | 95.4 ± 0.6 | 1.07 ± 0.01 | 0.82 ± 0.03 |
| PA-VMAT | 95.7 ± 0.4 | 1.06 ± 0.01 | 0.82 ± 0.02 |
| Organ-At-Risk | Parameter | 3D-CRT | 7F-IMRT | 5F-IMRT | FA-VMAT | PA-VMAT |
|---|---|---|---|---|---|---|
| Lungs | Dav (Gy) | 11.4 ± 2.7 | 10.9 ± 2.9 | 11.0 ± 2.9 | 11.3 ± 2.8 | 11.3 ± 3.0 |
| V20Gy (%) | 26.3 ± 6.4 | 20.9 ± 7.4 | 21.5 ± 7.5 | 21.9 ± 7.1 | 21.2 ± 6.2 | |
| V5Gy (%) | 45.8 ± 11.3 | 46.7 ± 11.2 | 47.8 ± 12.5 | 49.3 ± 12.3 | 48.8 ± 13.2 | |
| Heart | Dav (Gy) | 10.2 ± 6.0 | 8.6 ± 5.3 | 8.5 ± 5.2 | 8.6 ± 5.1 | 8.7 ± 5.7 |
| V30Gy (%) | 14.8 ± 11.1 | 11.4 ± 8.4 | 10.9 ± 8.2 | 10.9 ± 7.9 | 11.5 ± 8.8 | |
| Esophagus | Dav (Gy) | 18.9 ± 5.1 | 16.8 ± 4.2 | 16.6 ± 4.2 | 17.0 ± 4.2 | 16.5 ± 4.3 |
| V50Gy (%) | 6.1 ± 6.9 | 6.4 ± 6.0 | 6.8 ± 5.9 | 6.4 ± 5.7 | 6.1 ± 5.3 | |
| Spinal cord | Dmax (Gy) | 30.6 ± 3.7 | 17.9 ± 1.0 | 16.6 ± 1.2 | 17.5 ± 0.6 | 17.5 ± 0.9 |
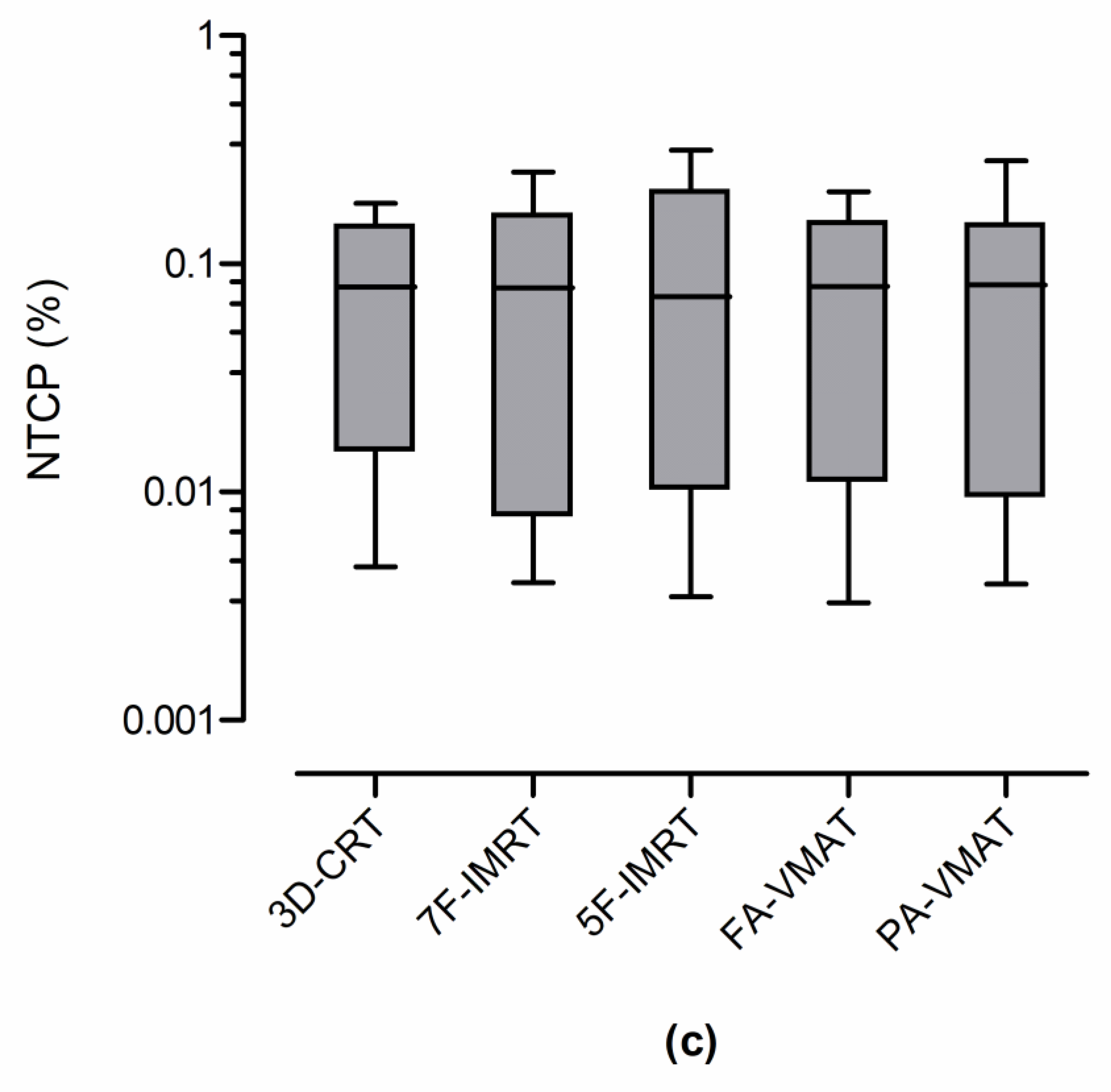
| Technique | NTCP (%) | ||
|---|---|---|---|
| Lungs | Heart | Esophagus | |
| 3D-CRT | 0.38 ± 0.44 | 0.06 ± 0.11 | 0.08 ± 0.07 |
| 7F-IMRT | 0.34 ± 0.46 | 0.04 ± 0.07 | 0.09 ± 0.09 |
| 5F-IMRT | 0.39 ± 0.62 | 0.03 ± 0.06 | 0.10 ± 0.11 |
| FA-VMAT | 0.43 ± 0.57 | 0.03 ± 0.06 | 0.09 ± 0.07 |
| PA-VMAT | 0.49 ± 0.72 | 0.04 ± 0.07 | 0.09 ± 0.09 |
| Techniques | p-Value | ||
|---|---|---|---|
| Lungs | Heart | Esophagus | |
| 3D-CRT vs. 7F-IMRT | 0.083 | 0.007 * | 0.320 |
| 3D-CRT vs. 5F-IMRT | 0.240 | 0.019 * | 0.320 |
| 3D-CRT vs. FA-VMAT | 0.517 | 0.042 * | 0.413 |
| 3D-CRT vs. PA-VMAT | 0.700 | 0.005 * | 0.320 |
| 7F-IMRT vs. 5F-IMRT | 0.765 | 0.966 | 0.083 |
| 7F-IMRT vs. FA-VMAT | 0.001 * | 0.966 | 0.517 |
| 7F-IMRT vs. PA-VMAT | 0.206 | 0.206 | 0.577 |
| 5F-IMRT vs. FA-VMAT | 0.465 | 0.700 | 0.413 |
| 5F-IMRT vs. PA-VMAT | 0.009 * | 0.831 | 0.123 |
| FA-VMAT vs. PA-VMAT | 0.966 | 0.577 | 0.517 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazonakis, M.; Kachris, S.; Tolia, M.; Damilakis, J. NTCP Calculations of Five Different Irradiation Techniques for the Treatment of Thymoma. Curr. Oncol. 2023, 30, 7740-7752. https://doi.org/10.3390/curroncol30080561
Mazonakis M, Kachris S, Tolia M, Damilakis J. NTCP Calculations of Five Different Irradiation Techniques for the Treatment of Thymoma. Current Oncology. 2023; 30(8):7740-7752. https://doi.org/10.3390/curroncol30080561
Chicago/Turabian StyleMazonakis, Michalis, Stefanos Kachris, Maria Tolia, and John Damilakis. 2023. "NTCP Calculations of Five Different Irradiation Techniques for the Treatment of Thymoma" Current Oncology 30, no. 8: 7740-7752. https://doi.org/10.3390/curroncol30080561
APA StyleMazonakis, M., Kachris, S., Tolia, M., & Damilakis, J. (2023). NTCP Calculations of Five Different Irradiation Techniques for the Treatment of Thymoma. Current Oncology, 30(8), 7740-7752. https://doi.org/10.3390/curroncol30080561






