The Current Trend of Radiation Therapy for Patients with Localized Prostate Cancer
Abstract
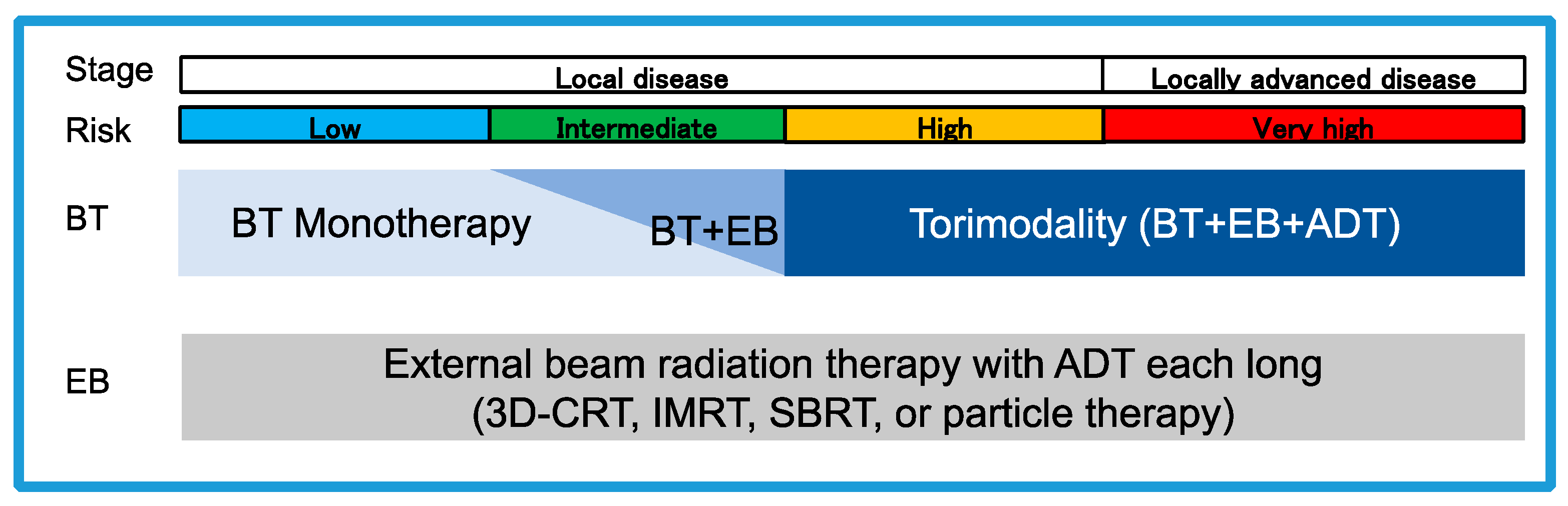
:1. Introduction
2. Radiotherapy for Each Risk of Localized Prostate Cancer
2.1. Low-Risk Prostate Cancer
2.2. Intermediate-Risk Prostate Cancer
2.3. High-Risk Prostate Cancer
2.4. Very High-Risk Prostate Cancer
3. Technical Advancement of Radiotherapy
3.1. IMRT
3.2. IGRT
3.3. VMAT
3.4. SBRT
3.5. Brachytherapy
3.5.1. Permanent Brachytherapy
3.5.2. HDR Brachytherapy
3.5.3. Trimodalilty Brachytherapy (Trimodality)
3.6. Particle Radiotherapy
4. Management for AEs Caused by Radiotherapy
4.1. Urinary Problems
4.2. Bowel Problems
4.3. Erectile Dysfunction
4.4. Fatigue
4.5. Secondary Cancers
4.6. Spacer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, M.; Gouveia, A.G.; Cury, F.L.; Moideen, N.; Bratti, V.F.; Patrocinio, H.; Berlin, A.; Mendez, L.C.; Moraes, F.Y. Practical considerations for prostate hypofractionation in the developing world. Nat. Rev. Urol. 2021, 18, 669–685. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Saskin, R.; Choo, R.; Herschorn, S.; Kodama, R.T.; Satkunasivam, R.; Shah, P.S.; Danjoux, C.; Nam, R.K. Surgery Versus Radiotherapy for Clinically-localized Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef]
- Pilepich, M.V.; Caplan, R.; Byhardt, R.W.; Lawton, C.A.; Gallagher, M.J.; Mesic, J.B.; Hanks, G.E.; Coughlin, C.T.; Porter, A.; Shipley, W.U.; et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. J. Clin. Oncol. 1997, 15, 1013–1021. [Google Scholar] [CrossRef]
- Warde, P.; Mason, M.; Ding, K.; Kirkbride, P.; Brundage, M.; Cowan, R.; Gospodarowicz, M.; Sanders, K.; Kostashuk, E.; Swanson, G.; et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet 2011, 378, 2104–2111. [Google Scholar] [CrossRef]
- Giberti, C.; Gallo, F.; Schenone, M.; Gastaldi, E.; Cortese, P.; Ninotta, G.; Becco, D. Robotic prostatectomy versus brachytherapy for the treatment of low risk prostate cancer. Can. J. Urol. 2017, 24, 8728–8733. [Google Scholar]
- Lawton, C.A.; Hunt, D.; Lee, W.R.; Gomella, L.; Grignon, D.; Gillin, M.; Morton, G.; Pisansky, T.M.; Sandler, H. Long-term results of a phase II trial of ultrasound-guided radioactive implantation of the prostate for definitive management of localized adenocarcinoma of the prostate (RTOG 98-05). Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1–7. [Google Scholar] [CrossRef]
- Mason, M.D.; Parulekar, W.R.; Sydes, M.R.; Brundage, M.; Kirkbride, P.; Gospodarowicz, M.; Cowan, R.; Kostashuk, E.C.; Anderson, J.; Swanson, G.; et al. Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J. Clin. Oncol. 2015, 33, 2143–2150. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Kuban, D.A.; Levy, L.B.; Potters, L.; Beyer, D.C.; Blasko, J.C.; Moran, B.J.; Ciezki, J.P.; Zietman, A.L.; Pisansky, T.M.; et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 327–333. [Google Scholar] [CrossRef]
- King, C. Stereotactic body radiotherapy for prostate cancer: Current results of a phase II trial. Front. Radiat. Ther. Oncol. 2011, 43, 428–437. [Google Scholar]
- Chen, J.; Yuan, Y.; Fang, M.; Zhu, Y.; Sun, X.; Lou, Y.; Xin, Y.; Zhou, F. Androgen deprivation therapy and radiotherapy in intermediate-risk prostate cancer: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1074540. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Jones, B.; Jackson, M.W.; Yeh, N.; Waxweiler, T.V.; Maroni, P.; Kavanagh, B.D.; Raben, D. Survival Outcomes of Dose-Escalated External Beam Radiotherapy versus Combined Brachytherapy for Intermediate and High Risk Prostate Cancer Using the National Cancer Data Base. J. Urol. 2016, 195, 1453–1458. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Davis, B.J.; Nguyen, P.L.; Showalter, T.N.; Hoskin, P.J.; Yoshioka, Y.; Morton, G.C.; Horwitz, E.M. The evolution of brachytherapy for prostate cancer. Nat. Rev. Urol. 2017, 14, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.U.; Hunt, D.; McGowan, D.G.; Amin, M.B.; Chetner, M.P.; Bruner, D.W.; Leibenhaut, M.H.; Husain, S.M.; Rotman, M.; Souhami, L.; et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 2011, 365, 107–118. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Bjornlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gregucci, F.; Carbonara, R.; Surgo, A.; Ciliberti, M.P.; Curci, D.; Ciocia, A.; Brana, L.; Ludovico, G.M.; Scarcia, M.; Portoghese, F.; et al. Extreme hypofractionated stereotactic radiotherapy for elderly prostate cancer patients: Side effects preliminary analysis of a phase II trial. Radiol. Med. 2023, 128, 501–508. [Google Scholar] [CrossRef]
- Greenberger, B.A.; Chen, V.E.; Den, R.B. Combined Modality Therapies for High-Risk Prostate Cancer: Narrative Review of Current Understanding and New Directions. Front. Oncol. 2019, 9, 1273. [Google Scholar] [CrossRef]
- Hanks, G.E.; Pajak, T.F.; Porter, A.; Grignon, D.; Brereton, H.; Venkatesan, V.; Horwitz, E.M.; Lawton, C.; Rosenthal, S.A.; Sandler, H.M.; et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92-02. J. Clin. Oncol. 2003, 21, 3972–3978. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, Y.; Chen, J.; Sun, G.; Zhang, X.; Zhao, J.; Zhu, X.; Zhang, H.; Zhu, S.; Dai, J.; et al. The efficacy and safety of radical prostatectomy and radiotherapy in high-risk prostate cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2020, 18, 42. [Google Scholar] [CrossRef]
- Burgess, L.; Roy, S.; Morgan, S.; Malone, S. A Review on the Current Treatment Paradigm in High-Risk Prostate Cancer. Cancers 2021, 13, 4257. [Google Scholar] [CrossRef]
- Jackson, W.C.; Hartman, H.E.; Dess, R.T.; Birer, S.R.; Soni, P.D.; Hearn, J.W.D.; Reichert, Z.R.; Kishan, A.U.; Mahal, B.A.; Zumsteg, Z.S.; et al. Addition of Androgen-Deprivation Therapy or Brachytherapy Boost to External Beam Radiotherapy for Localized Prostate Cancer: A Network Meta-Analysis of Randomized Trials. J. Clin. Oncol. 2020, 38, 3024–3031. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Harrison, A.S.; Trabulsi, E.J.; Gomella, L.G.; Showalter, T.N.; Hurwitz, M.D.; Dicker, A.P.; Den, R.B. Evolution of advanced technologies in prostate cancer radiotherapy. Nat. Rev. Urol. 2013, 10, 565–579. [Google Scholar] [CrossRef]
- Mitchell, J.M. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N. Engl. J. Med. 2013, 369, 1629–1637. [Google Scholar] [CrossRef]
- Ezzell, G.A.; Galvin, J.M.; Low, D.; Palta, J.R.; Rosen, I.; Sharpe, M.B.; Xia, P.; Xiao, Y.; Xing, L.; Yu, C.X.; et al. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med. Phys. 2003, 30, 2089–2115. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Q.; Zheng, T.; Shi, H.; Liu, Y.; Feng, S.; Hao, M.; Ye, L.; Wu, X.; Yang, C. The Effectiveness of Intensity Modulated Radiation Therapy versus Three-Dimensional Radiation Therapy in Prostate Cancer: A Meta-Analysis of the Literatures. PLoS ONE 2016, 11, e0154499. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients with Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e180039. [Google Scholar] [CrossRef]
- Hall, W.A.; Deshmukh, S.; Bruner, D.W.; Michalski, J.M.; Purdy, J.A.; Bosch, W.; Bahary, J.P.; Patel, M.P.; Parliament, M.B.; Lock, M.I.; et al. Quality of Life Implications of Dose-Escalated External Beam Radiation for Localized Prostate Cancer: Results of a Prospective Randomized Phase 3 Clinical Trial, NRG/RTOG 0126. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 83–92. [Google Scholar] [CrossRef]
- Viani, G.A.; Viana, B.S.; Martin, J.E.; Rossi, B.T.; Zuliani, G.; Stefano, E.J. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 2016, 122, 2004–2011. [Google Scholar] [CrossRef]
- Incrocci, L.; Wortel, R.C.; Alemayehu, W.G.; Aluwini, S.; Schimmel, E.; Krol, S.; van der Toorn, P.P.; Jager, H.; Heemsbergen, W.; Heijmen, B.; et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1061–1069. [Google Scholar] [CrossRef]
- Aluwini, S.; Pos, F.; Schimmel, E.; Krol, S.; van der Toorn, P.P.; de Jager, H.; Alemayehu, W.G.; Heemsbergen, W.; Heijmen, B.; Incrocci, L. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Mossop, H.; Syndikus, I.; Khoo, V.; Bloomfield, D.; Parker, C.; Logue, J.; Scrase, C.; Patterson, H.; Birtle, A.; et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015, 16, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.; Simpson, E.L.; Hemingway, P.; Stevenson, M.D.; Rees, A. Intensity-modulated radiotherapy for the treatment of prostate cancer: A systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1–108. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Kataria, T. Image guidance in radiation therapy: Techniques and applications. Radiol. Res. Pract. 2014, 2014, 705604. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Yan, D.; McGrath, S.; Dilworth, J.T.; Liang, J.; Ye, H.; Krauss, D.J.; Martinez, A.A.; Kestin, L.L. Adaptive image-guided radiotherapy (IGRT) eliminates the risk of biochemical failure caused by the bias of rectal distension in prostate cancer treatment planning: Clinical evidence. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 947–952. [Google Scholar] [CrossRef]
- Wang, S.; Tang, W.; Luo, H.; Jin, F.; Wang, Y. The role of image-guided radiotherapy in prostate cancer: A systematic review and meta-analysis. Clin. Transl. Radiat. Oncol. 2023, 38, 81–89. [Google Scholar] [CrossRef]
- Becker-Schiebe, M.; Abaci, A.; Ahmad, T.; Hoffmann, W. Reducing radiation-associated toxicity using online image guidance (IGRT) in prostate cancer patients undergoing dose-escalated radiation therapy. Rep. Pract. Oncol. Radiother. 2016, 21, 188–194. [Google Scholar] [CrossRef]
- Bedford, J.L.; Warrington, A.P. Commissioning of volumetric modulated arc therapy (VMAT). Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 537–545. [Google Scholar] [CrossRef]
- Thwaites, D.I.; Tuohy, J.B. Back to the future: The history and development of the clinical linear accelerator. Phys. Med. Biol. 2006, 51, R343–R362. [Google Scholar] [CrossRef]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Arcangeli, G.; Arcangeli, S.; Pinzi, V.; Benassi, M.; Benassi, M.; Strigari, L. Optimal scheduling of hypofractionated radiotherapy for localized prostate cancer: A systematic review and metanalysis of randomized clinical trials. Cancer Treat. Rev. 2018, 70, 22–29. [Google Scholar] [CrossRef]
- Zhou, K.; Renouf, M.; Perrocheau, G.; Magne, N.; Latorzeff, I.; Pommier, P.; Crehange, G.; Paumier, A.; Bera, G.; Martin, J.; et al. Cost-effectiveness of hypofractionated versus conventional radiotherapy in patients with intermediate-risk prostate cancer: An ancillary study of the PROstate fractionated irradiation trial—PROFIT. Radiother. Oncol. 2022, 173, 306–312. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Hunte, S.O.; Clark, C.H.; Zyuzikov, N.; Nisbet, A. Volumetric modulated arc therapy (VMAT): A review of clinical outcomes-what is the clinical evidence for the most effective implementation? Br. J. Radiol. 2022, 95, 20201289. [Google Scholar] [CrossRef]
- Gomez-Aparicio, M.A.; Valero, J.; Caballero, B.; Garcia, R.; Hernando-Requejo, O.; Montero, A.; Gomez-Iturriaga, A.; Zilli, T.; Ost, P.; Lopez-Campos, F.; et al. Extreme Hypofractionation with SBRT in Localized Prostate Cancer. Curr. Oncol. 2021, 28, 2933–2949. [Google Scholar] [CrossRef]
- Ricco, A.; Hanlon, A.; Lanciano, R. Propensity Score Matched Comparison of Intensity Modulated Radiation Therapy vs Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Survival Analysis from the National Cancer Database. Front. Oncol. 2017, 7, 185. [Google Scholar] [CrossRef]
- Tree, A.C.; Ostler, P.; van der Voet, H.; Chu, W.; Loblaw, A.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; Staffurth, J.; et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022, 23, 1308–1320. [Google Scholar] [CrossRef]
- Jackson, W.C.; Silva, J.; Hartman, H.E.; Dess, R.T.; Kishan, A.U.; Beeler, W.H.; Gharzai, L.A.; Jaworski, E.M.; Mehra, R.; Hearn, J.W.D.; et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 778–789. [Google Scholar] [CrossRef]
- Ito, M.; Yoshioka, Y.; Takase, Y.; Suzuki, J.; Takahashi, H.; Minami, Y.; Sakuragi, A.; Oshima, Y.; Okuda, T.; Suzuki, K. Stereotactic body radiation therapy for prostate cancer: A study comparing 3-year genitourinary toxicity between CyberKnife and volumetric-modulated arc therapy by propensity score analysis. Radiat. Oncol. 2023, 18, 39. [Google Scholar] [CrossRef]
- King, C.R. LDR vs. HDR brachytherapy for localized prostate cancer: The view from radiobiological models. Brachytherapy 2002, 1, 219–226. [Google Scholar] [CrossRef]
- King, M.T.; Keyes, M.; Frank, S.J.; Crook, J.M.; Butler, W.M.; Rossi, P.J.; Cox, B.W.; Showalter, T.N.; Mourtada, F.; Potters, L.; et al. Low dose rate brachytherapy for primary treatment of localized prostate cancer: A systemic review and executive summary of an evidence-based consensus statement. Brachytherapy 2021, 20, 1114–1129. [Google Scholar] [CrossRef]
- Saito, S.; Yagi, Y.; Nishiyama, T.; Nakamura, K.; Toya, K.; Yorozu, A. Brachytherapy with permanent seed implantation. Nihon Rinsho 2016, 74 (Suppl. 3), 531–536. [Google Scholar] [CrossRef]
- Prada, P.J.; Juan, G.; Gonzalez-Suarez, H.; Fernandez, J.; Jimenez, I.; Amon, J.; Cepeda, M. Prostate-specific antigen relapse-free survival and side-effects in 734 patients with up to 10 years of follow-up with localized prostate cancer treated by permanent iodine implants. BJU Int. 2010, 106, 32–36. [Google Scholar] [CrossRef]
- Prestidge, B.R.; Winter, K.; Sanda, M.G.; Amin, M.; Bice, W.S.; Michalski, J.; Ibbott, G.S.; Crook, J.M.; Catton, C.N.; Gay, H.A.; et al. Initial Report of NRG Oncology/RTOG 0232: A Phase 3 Study Comparing Combined External Beam Radiation and Transperineal Interstitial Permanent Brachytherapy with Brachytherapy Alone for Selected Patients with Intermediate-Risk Prostatic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, S4. [Google Scholar] [CrossRef]
- McLaughlin, P.W.; Narayana, V. Progress in Low Dose Rate Brachytherapy for Prostate Cancer. Semin. Radiat. Oncol. 2020, 30, 39–48. [Google Scholar] [CrossRef]
- Kissel, M.; Crehange, G.; Graff, P. Stereotactic Radiation Therapy versus Brachytherapy: Relative Strengths of Two Highly Efficient Options for the Treatment of Localized Prostate Cancer. Cancers 2022, 14, 2226. [Google Scholar] [CrossRef]
- Mendez, L.C.; Morton, G.C. High dose-rate brachytherapy in the treatment of prostate cancer. Transl. Androl. Urol. 2018, 7, 357–370. [Google Scholar] [CrossRef]
- Anderson, E.M.; Kim, S.; Sandler, H.M.; Kamrava, M. High-dose-rate fractionated brachytherapy monotherapy for localized prostate cancer: A systematic review and meta-analysis. J. Contemp. Brachyther. 2021, 13, 365–372. [Google Scholar] [CrossRef]
- Morton, G.; McGuffin, M.; Chung, H.T.; Tseng, C.L.; Helou, J.; Ravi, A.; Cheung, P.; Szumacher, E.; Liu, S.; Chu, W.; et al. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: Efficacy results from a randomized phase II clinical trial of one fraction of 19 Gy or two fractions of 13.5 Gy. Radiother. Oncol. 2020, 146, 90–96. [Google Scholar] [CrossRef]
- Carpenter, T.J.; Forsythe, K.; Kao, J.; Stone, N.N.; Stock, R.G. Outcomes for patients with extraprostatic prostate cancer treated with trimodality therapy, including brachytherapy, external beam radiotherapy, and hormone therapy. Brachytherapy 2011, 10, 261–268. [Google Scholar] [CrossRef]
- Hattangadi, J.A.; Chen, M.H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Predictors of the use of supplemental androgen suppression therapy and external beam radiation in men with high-risk prostate cancer undergoing brachytherapy in community practice. Brachytherapy 2011, 10, 369–375. [Google Scholar] [CrossRef]
- Oh, J.; Tyldesley, S.; Pai, H.; McKenzie, M.; Halperin, R.; Duncan, G.; Morton, G.; Keyes, M.; Hamm, J.; Morris, W.J. An Updated Analysis of the Survival Endpoints of ASCENDE-RT. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1061–1070. [Google Scholar] [CrossRef]
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 286–295. [Google Scholar] [CrossRef]
- Konaka, H.; Egawa, S.; Saito, S.; Yorozu, A.; Takahashi, H.; Miyakoda, K.; Fukushima, M.; Dokiya, T.; Yamanaka, H.; Stone, N.N.; et al. Tri-Modality therapy with I-125 brachytherapy, external beam radiation therapy, and short- or long-term hormone therapy for high-risk localized prostate cancer (TRIP): Study protocol for a phase III, multicenter, randomized, controlled trial. BMC Cancer 2012, 12, 110. [Google Scholar] [CrossRef]
- Du, T.Q.; Liu, R.; Zhang, Q.; Luo, H.; Chen, Y.; Tan, M.; Wang, Q.; Wu, X.; Liu, Z.; Sun, S.; et al. Does particle radiation have superior radiobiological advantages for prostate cancer cells? A systematic review of in vitro studies. Eur. J. Med. Res. 2022, 27, 306. [Google Scholar] [CrossRef]
- Bryant, C.M.; Henderson, R.H.; Nichols, R.C.; Mendenhall, W.M.; Hoppe, B.S.; Vargas, C.E.; Daniels, T.B.; Choo, C.R.; Parikh, R.R.; Giap, H.; et al. Consensus Statement on Proton Therapy for Prostate Cancer. Int. J. Part. Ther. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Poon, D.M.C.; Wu, S.; Ho, L.; Cheung, K.Y.; Yu, B. Proton Therapy for Prostate Cancer: Challenges and Opportunities. Cancers 2022, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, Q.; Li, P.; Fu, S. Landscape of Carbon Ion Radiotherapy in Prostate Cancer: Clinical Application and Translational Research. Front. Oncol. 2021, 11, 760752. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Kubo, N.; Sato, H.; Miyasaka, Y.; Matsui, H.; Ito, K.; Suzuki, K.; Ohno, T. Quality of life in prostate cancer patients receiving particle radiotherapy: A review of the literature. Int. J. Urol. 2020, 27, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, T.; Tsuji, H.; Kawamura, H.; Ohno, T.; Toyama, S.; Shioyama, Y.; Nakayama, Y.; Nemoto, K.; Tsujii, H.; Kamada, T. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: A report from the Japan Carbon ion Radiation Oncology Study Group (J-CROS). Radiother. Oncol. 2016, 121, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Yao, L.; Han, X.; Yan, W.; Liu, Y.; Fu, Y.; Wang, Y.; Huang, M.; Zhang, Q.; et al. Clinical Efficacy and Safety of Proton and Carbon Ion Radiotherapy for Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 709530. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Cotter, S.E.; Gargollo, P.C.; Zietman, A.L.; Dahl, D.M.; Smith, M.R. Management of complications of prostate cancer treatment. CA Cancer J. Clin. 2008, 58, 196–213. [Google Scholar] [CrossRef]
- Matzinger, O.; Duclos, F.; van den Bergh, A.; Carrie, C.; Villa, S.; Kitsios, P.; Poortmans, P.; Sundar, S.; van der Steen-Banasik, E.M.; Gulyban, A.; et al. Acute toxicity of curative radiotherapy for intermediate- and high-risk localised prostate cancer in the EORTC trial 22991. Eur. J. Cancer 2009, 45, 2825–2834. [Google Scholar] [CrossRef]
- Chorbinska, J.; Krajewski, W.; Zdrojowy, R. Urological complications after radiation therapy-nothing ventured, nothing gained: A Narrative Review. Transl. Cancer Res. 2021, 10, 1096–1118. [Google Scholar] [CrossRef]
- Chang, P.; Regan, M.M.; Ferrer, M.; Guedea, F.; Patil, D.; Wei, J.T.; Hembroff, L.A.; Michalski, J.M.; Saigal, C.S.; Litwin, M.S.; et al. Relief of Urinary Symptom Burden after Primary Prostate Cancer Treatment. J. Urol. 2017, 197, 376–384. [Google Scholar] [CrossRef]
- Faithfull, S.; Lemanska, A.; Aslet, P.; Bhatt, N.; Coe, J.; Drudge-Coates, L.; Feneley, M.; Glynn-Jones, R.; Kirby, M.; Langley, S.; et al. Integrative review on the non-invasive management of lower urinary tract symptoms in men following treatments for pelvic malignancies. Int. J. Clin. Pract. 2015, 69, 1184–1208. [Google Scholar] [CrossRef]
- Syndikus, I.; Morgan, R.C.; Sydes, M.R.; Graham, J.D.; Dearnaley, D.P.; Collaborators, M.R. Late gastrointestinal toxicity after dose-escalated conformal radiotherapy for early prostate cancer: Results from the UK Medical Research Council RT01 trial (ISRCTN47772397). Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Dalsania, R.M.; Shah, K.P.; Stotsky-Himelfarb, E.; Hoffe, S.; Willingham, F.F. Management of Long-Term Toxicity From Pelvic Radiation Therapy. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 147–157. [Google Scholar] [CrossRef]
- Lehto, U.S.; Tenhola, H.; Taari, K.; Aromaa, A. Patients’ perceptions of the negative effects following different prostate cancer treatments and the impact on psychological well-being: A nationwide survey. Br. J. Cancer 2017, 116, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Magrino, T.J.; Johnstone, P.A. Rectal bleeding after radiation therapy for prostate cancer: Endoscopic evaluation. Radiology 2000, 217, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Do, N.L.; Nagle, D.; Poylin, V.Y. Radiation proctitis: Current strategies in management. Gastroenterol. Res. Pract. 2011, 2011, 917941. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Fort, M.K.; Rogers, M.J.; Santiago, R.; Mahase, S.S.; Mendez, M.; Zheng, Y.; Kong, X.; Kashanian, J.A.; Niaz, M.J.; McClelland, S., 3rd; et al. Prostatic irradiation-induced sexual dysfunction: A review and multidisciplinary guide to management in the radical radiotherapy era (Part I defining the organ at risk for sexual toxicities). Rep. Pract. Oncol. Radiother. 2020, 25, 367–375. [Google Scholar] [CrossRef]
- Helgason, A.R.; Fredrikson, M.; Adolfsson, J.; Steineck, G. Decreased sexual capacity after external radiation therapy for prostate cancer impairs quality of life. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 33–39. [Google Scholar] [CrossRef]
- Bokhour, B.G.; Clark, J.A.; Inui, T.S.; Silliman, R.A.; Talcott, J.A. Sexuality after treatment for early prostate cancer: Exploring the meanings of “erectile dysfunction”. J. Gen. Intern. Med. 2001, 16, 649–655. [Google Scholar] [CrossRef]
- Incrocci, L. Radiotherapy for prostate cancer and sexual health. Transl. Androl. Urol. 2015, 4, 124–130. [Google Scholar]
- Pisansky, T.M.; Pugh, S.L.; Greenberg, R.E.; Pervez, N.; Reed, D.R.; Rosenthal, S.A.; Mowat, R.B.; Raben, A.; Buyyounouski, M.K.; Kachnic, L.A.; et al. Tadalafil for prevention of erectile dysfunction after radiotherapy for prostate cancer: The Radiation Therapy Oncology Group [0831] randomized clinical trial. JAMA 2014, 311, 1300–1307. [Google Scholar] [CrossRef]
- McMahon, C.G. Erectile dysfunction. Intern. Med. J. 2014, 44, 18–26. [Google Scholar] [CrossRef]
- Mahmood, J.; Shamah, A.A.; Creed, T.M.; Pavlovic, R.; Matsui, H.; Kimura, M.; Molitoris, J.; Shukla, H.; Jackson, I.; Vujaskovic, Z. Radiation-induced erectile dysfunction: Recent advances and future directions. Adv. Radiat. Oncol. 2016, 1, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Richards, M.; A’Hern, R.; Hardy, J. Fatigue in patients with cancers of the breast or prostate undergoing radical radiotherapy. J. Pain. Symptom Manag. 2001, 22, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Golfam, M.; Samant, R.; Eapen, L.; Malone, S. Effects of radiation and total androgen blockade on serum hemoglobin, testosterone, and erythropoietin in patients with localized prostate cancer. Curr. Oncol. 2012, 19, e258–e263. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Nice, E.; Huang, C.; Zhang, W.; Tang, Y. Circadian rhythms and cancers: The intrinsic links and therapeutic potentials. J. Hematol. Oncol. 2022, 15, 21. [Google Scholar] [CrossRef]
- Hsiao, C.P.; Daly, B.; Saligan, L.N. The Etiology and management of radiotherapy-induced fatigue. Expert. Rev. Qual. Life Cancer Care 2016, 1, 323–328. [Google Scholar] [CrossRef]
- Kaushik, D.; Shah, P.K.; Mukherjee, N.; Ji, N.; Dursun, F.; Kumar, A.P.; Thompson, I.M.; Mansour, A.M., Jr.; Jha, R.; Yang, X.; et al. Effects of yoga in men with prostate cancer on quality of life and immune response: A pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2022, 25, 531–538. [Google Scholar] [CrossRef]
- Mustian, K.M.; Sprod, L.K.; Janelsins, M.; Peppone, L.J.; Mohile, S. Exercise Recommendations for Cancer-Related Fatigue, Cognitive Impairment, Sleep problems, Depression, Pain, Anxiety, and Physical Dysfunction: A Review. Oncol. Hematol. Rev. 2012, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef]
- Neefjes, E.C.; van der Vorst, M.J.; Blauwhoff-Buskermolen, S.; Verheul, H.M. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013, 18, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Barsevick, A.M.; Newhall, T.; Brown, S. Management of cancer-related fatigue. Clin. J. Oncol. Nurs. 2008, 12, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.J.; Soloway, M.S. Secondary cancer after radiotherapy for prostate cancer: Should we be more aware of the risk? Eur. Urol. 2007, 52, 973–982. [Google Scholar] [CrossRef]
- Ozawa, Y.; Yagi, Y.; Nakamura, K.; Hattori, S.; Nishiyama, T.; Momma, T.; Yorozu, A.; Saito, S. Secondary bladder cancer during long-term follow-up after iodine-125 permanent seed implantation for localized prostate cancer. Brachytherapy 2022, 21, 451–459. [Google Scholar] [CrossRef]
- Sountoulides, P.; Koletsas, N.; Kikidakis, D.; Paschalidis, K.; Sofikitis, N. Secondary malignancies following radiotherapy for prostate cancer. Ther. Adv. Urol. 2010, 2, 119–125. [Google Scholar] [CrossRef]
- Wallis, C.J.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: Systematic review and meta-analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef]
- Hegemann, N.S.; Schlesinger-Raab, A.; Ganswindt, U.; Horl, C.; Combs, S.E.; Holzel, D.; Gschwend, J.E.; Stief, C.; Belka, C.; Engel, J. Risk of second cancer following radiotherapy for prostate cancer: A population-based analysis. Radiat. Oncol. 2017, 12, 2. [Google Scholar] [CrossRef]
- Pithadia, K.J.; Advani, P.G.; Citrin, D.E.; Bekelman, J.E.; Withrow, D.R.; Berrington de Gonzalez, A.; Morton, L.M.; Schonfeld, S.J. Comparing Risk for Second Primary Cancers After Intensity-Modulated vs 3-Dimensional Conformal Radiation Therapy for Prostate Cancer, 2002–2015. JAMA Oncol. 2023, 9, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Whalley, D.; Hruby, G.; Alfieri, F.; Kneebone, A.; Eade, T. SpaceOAR Hydrogel in Dose-escalated Prostate Cancer Radiotherapy: Rectal Dosimetry and Late Toxicity. Clin. Oncol. (R. Coll. Radiol.) 2016, 28, e148–e154. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Efstathiou, J.A.; Bhattacharyya, S.K.; Payne, H.A.; Woodward, E.; Pinkawa, M. Association of the Placement of a Perirectal Hydrogel Spacer with the Clinical Outcomes of Men Receiving Radiotherapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e208221. [Google Scholar] [CrossRef]
- Bjoreland, U.; Notstam, K.; Fransson, P.; Soderkvist, K.; Beckman, L.; Jonsson, J.; Nyholm, T.; Widmark, A.; Thellenberg Karlsson, C. Hyaluronic acid spacer in prostate cancer radiotherapy: Dosimetric effects, spacer stability and long-term toxicity and PRO in a phase II study. Radiat. Oncol. 2023, 18, 1. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Patient’s Number | PCa Characteristics | Dose (Gy) | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PROG/ACR 95-09 | 2010 | 196 vs. 197 | low (58%), intermediate (37%), and high (4%) risk | 79.2 vs. 70.2 | - | 10-year | 82.6% vs. 68.0% | 6-month grade ≥ 2 GU toxicity | 29% vs. 25% | 6-month grade ≥ 2 GI toxicity | 24% vs. 13% |
| GETUG 06 | 2011 | 153 vs. 153 | intermediate (28.9%), and high (71.1%) risk | 80 vs. 70 | - | 5-year | 72% vs. 61% | grade ≥ 2 GU toxicity | 17.5% vs. 10% | grade ≥ 2 GI toxicity | 19.5% vs. 14% |
| MRC RT01 | 2014 | 422 vs. 421 | low (19%), intermediate (37%), and high (43%) risk | 74 vs. 64 | physician decision | 10-year | 55% vs. 43% | ||||
| Dutch CKVO96-10 | 2014 | 333 vs. 331 | low (17.9%), intermediate (27.0%), and high (55.1%) risk | 78 vs. 68 | - | 10-year | 49% vs. 43% | ||||
| RTOG 0126 | 2018 | 748 vs. 751 | low or intermediate risk | 79.2 vs. 70.2 | - | 8-year | 80% vs. 75% | 5-year grade ≥ 2 GU toxicity | 12% vs. 7% | 5-year grade ≥ 2 GI toxicity | 21% vs. 15% |
| MD Anderson study | 2019 | 151 vs. 150 | low (20.6%), intermediate (45.8%), and high (33.6%) risk | 78 vs. 70 | - | 15-year | 92.9% vs. 87.7% | ||||
| FLAME Trial | 2021 | 284 vs. 287 | low (1.1%), intermediate (15.1%), and high (83.9%) risk | 77 + focal boost vs. 77 | physician decision | 5-year | 92% vs. 85% | late grade ≥ 2 GU toxicity | 27.8% vs. 23.0% | late grade ≥ 2 GI toxicity | 12.7% vs. 12.2% |
| Study | Year | Patient’s Number | PCa Characteristics | Dose (Gy) | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02257827 | 2016 | 109 vs. 106 | low (43.7%), intermediate (21.9%), and high (34.4%) risk | 70 (IMRT) vs. 70 (3DCRT) | 2 years in intermediate- and high-risk patients | 5-year | 95.4% vs. 94.3% | 6-month grade ≥ 2 GU toxicity | 3% vs. 4% | 6-month grade ≥ 2 GI toxicity | 1% vs. 9% |
| The PROFIT trial | 2017 | 608 vs. 598 | intermediate risk | 60 (IMRT) vs. 78 (3DCRT) | only 6% of all patients | 5-year | 85% vs. 85% | 6-month grade ≥ 3 GU toxicity | 2.1% vs. 3.0% | grade ≥ 3 GI toxicity | 1.5% vs. 2.7% |
| RTOG 0126 | 2018 | 748 vs. 751 | low or intermediate risk | 79.2 vs. 70.2 | - | 8-year | 80% vs. 75% | 5-year grade ≥ 2 GU toxicity | 12% vs. 7% | 5-year grade ≥ 2 GI toxicity | 21% vs. 15% |
| POP-RT | 2021 | 110 vs. 114 | high risk | 68 + 50 vs. 68 | 2 years | 5 year | 95.0% vs. 81.2% | late grade ≥ 2 GU toxicity | 20.0% vs. 9.0% | late grade ≥ 2 GI toxicity | 8.2% vs. 4.5% |
| Study | Year | Patient’s Number | PCa Characteristics | Dose (Gy) | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HYPRO trial | 2016 | 407 vs. 397 | intermediate (26.2%) and high (73.8%) risk | 64.6 in 19 f vs. 78.0 in 39 f | each institutional protocol | 5-year | 80.5% vs. 77.1% | ||||
| CHHiP trial | 2016 | 1074 and 1077 vs. 1065 | low (15.0%), intermediate (73.0%), and high (12.0%) risk | 60 in 20 f or 57 in 19 f vs. 74 in 37 f | 3–6 months | 5-year | 90.6%, 85.9% vs. 88.3% | 2-year grade ≥ 2 GU toxicity | 2%, 1% vs. 1% | 2-year grade ≥ 2 GI toxicity | 3%, 2% vs. 4% |
| Study | Year | Patient’s Number | PCa Characteristics | Treatment Methods | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hannover study | 2016 | 102 vs. 96 | low (15.2%), intermediate (34.3%), and high (50.5%) risk | IGRT vs. non-IGRT | physician decision | late grade ≥ 2 GU toxicity | 34% vs. 34% | late grade ≥ 2 GI toxicity | 19% vs. 31% | ||
| RIC-trial | 2018 | 125 vs. 125 | intermediate (39.2%), and high (60.8%) risk | IGRT daily vs. IGRT weekly | 6 months in intermediate- and 3 years in high-risk | 89.3% vs. 84.6% | |||||
| STIC-IGRT trial | 2018 | 234 vs. 236 | low (0.6%), intermediate (69.1%), and high (32.0%) risk | IGRT daily vs. IGRT weekly | physician decision | 5-year | 91% vs. 79% | 5-year grade ≥ 2 GU toxicity | 14% vs. 18% | 5-year grade ≥ 2 GI toxicity | 10% vs. 13% |
| CHHiP | 2020 | 137 and 108 vs. 48 | low (11.9%), intermediate (77.5%), and high (10.6%) risk | IGRT-S and -R vs. non-IGRT | 3–6 months | 2-year grade ≥ 2 GU toxicity | 4.6%, 3.9% vs. 8.4% | 2-year grade ≥ 2 GI toxicity | 8.3%, 5.8% vs. 8.3% | ||
| Study | Year | Patient’s Number | PCa Characteristics | Dose (Gy) | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marilia Medical School | 2016 | 109 vs. 106 | low (43.7%), intermediate (21.9%) and high (34.4%) | 70 (IMRT) vs. 70 (3DCRT) | 6 months in intermediate- and 2 years in high-risk | 5-year | 95.4% vs. 94.3% | 6-month grade ≥ 2 GI toxicity | 1% vs. 9% | 6-month grade ≥ 2 GU toxicity | 3% vs. 4% |
| HYPRO trial | 2016 | 407 vs. 397 | intermediate (26.2%) and high (73.8%) | 64.6 in 19 f (VMAT) vs. 78.0 in 39 f | each institutional protocol | 5-year | 80.5% vs. 77.1% | ||||
| CHHiP trial | 2016 | 1074 and 1077 vs. 1065 | low (15.0%), intermediate (73.0%), and high (12.0%) risk | 60 in 20 f or 57 in 19 f vs. 74 in 37 f | 3–6 months | 5-year | 90.6%, 85.9% vs. 88.3% | 2-year grade ≥ 2 GI toxicity | 3%, 2% vs. 4% | 2-year grade ≥ 2 GU toxicity | 2%, 1% vs. 1% |
| MD Anderson study | 2018 | 103 vs. 103 | low (25.7%), intermediate (66.2%), and high (0.9%) risk | 72 in 30 f vs. 75.6 in 42 f | for patients with PSA levels > 10 ng/mL or cT3 disease | 8-year | 89.3% vs. 84.6% | 8-year grade ≥ 2 GI toxicity | 12.6% vs. 5.0% | 8-year grade ≥ 2 GU toxicity | 15.1% vs. 16.4% |
| NCT00062309 | 2020 | 151 vs. 152 | low (9.2%), intermediate (62.4%) and high (28.4%) | 70.2 in 26 f vs. 76 in 38 f | 4 months in intermediate- and 2 years in high-risk | 10-year | 74.6% vs. 78.9% | ||||
| Study | Year | Patient’s Number | PCa Characteristics | Dose (Gy) | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HYPO-RT-PC | 2019 | 589 vs. 591 | intermediate (89%) and high (11%) risk | 42.7 in 7 f vs. 78.0 in 39 f | - | 5 year | 84% vs. 84% | 2-year grade ≥ 2 GU toxicity | 13% vs. 9% | 2-year grade ≥ 2 GI toxicity | 6% vs. 5% |
| PACE-B | 2022 | 416 vs. 433 | low (8.0%) and intermediate (92.0%) risk | 36.25 in 5 f vs. 78 in 39 f or 62 in 20 f | - | 2-year grade ≥ 2 GU toxicity | 3% vs. 2% | 2-year grade ≥ 2 GI toxicity | 2% vs. 3% | ||
| Study | Year | Treatment | Patient’s Number | PCa Characteristics | Treatment Methods | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| San Paolo Hospital | 2009 | LDR | 85 vs. 89 | BT vs. RP | - | 5-year | 91.0% vs. 91.7% | |||||
| RTOG 0232 | 2016 | LDR | 287 vs. 292 | Intermediate risk | EBRT + BT vs. BT | ? | 5-year | 85% vs. 86% (PFS) | ||||
| ISRCTN98241100 | 2012 | HDR | 110 vs. 106 | low (4.2%), intermediate (42.1%) and high (53.7%) | EBRT + HDR-BT vs. EBRT | 6 months in low/intermediate risk and up to 3 years in high-risk | 12-year | 69% vs. 49% | 6-year grade ≥ 3 GU toxicity | 11% vs. 4% | 6-year grade ≥ 3 GI toxicity | 0.9% vs. 0.8% |
| NCT01890096 | 2020 | HDR | 87 vs. 83 | low (19.4%) and intermediate (80.6%) risk | 19 Gy in 1 f vs. 27 Gy in 2 f | physician decision | 5-year | 73.5% vs. 95% | late grade ≥ 2 GU toxicity | 45% vs. 45% | ||
| ASCENDE-RT | 2017 | Torimodality | 198 vs. 200 | intermediate (30.7%) and high (69.3%) risk | EBRT + LDR-BT vs. DE-EBRT | 12 months | 7-year | 85% vs. 76% | late grade ≥ 2 GU toxicity | 32.8% vs. 20.6% | late grade ≥ 2 GI toxicity | 31.3% vs. 20.2% |
| Study | Year | Treatment | Patient’s Number | PCa Characteristics | Treatment Methods | ADT | bRFS (Phoenix) | Toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPI | 2016 | Proton and Carbon ion | 46 vs. 46 | low (23.1%), intermediate (59.3%) and high (17.6%) | Proton vs. Carbon ion | physician decision | 8-year | 50% vs. 26% | late grade ≥ 2 GU toxicity | 21.7% vs. 13.3% | late grade ≥ 2 GI toxicity | 8.7% vs. 2.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numakura, K.; Kobayashi, M.; Muto, Y.; Sato, H.; Sekine, Y.; Sobu, R.; Aoyama, Y.; Takahashi, Y.; Okada, S.; Sasagawa, H.; et al. The Current Trend of Radiation Therapy for Patients with Localized Prostate Cancer. Curr. Oncol. 2023, 30, 8092-8110. https://doi.org/10.3390/curroncol30090587
Numakura K, Kobayashi M, Muto Y, Sato H, Sekine Y, Sobu R, Aoyama Y, Takahashi Y, Okada S, Sasagawa H, et al. The Current Trend of Radiation Therapy for Patients with Localized Prostate Cancer. Current Oncology. 2023; 30(9):8092-8110. https://doi.org/10.3390/curroncol30090587
Chicago/Turabian StyleNumakura, Kazuyuki, Mizuki Kobayashi, Yumina Muto, Hiromi Sato, Yuya Sekine, Ryuta Sobu, Yu Aoyama, Yoshiko Takahashi, Syuhei Okada, Hajime Sasagawa, and et al. 2023. "The Current Trend of Radiation Therapy for Patients with Localized Prostate Cancer" Current Oncology 30, no. 9: 8092-8110. https://doi.org/10.3390/curroncol30090587
APA StyleNumakura, K., Kobayashi, M., Muto, Y., Sato, H., Sekine, Y., Sobu, R., Aoyama, Y., Takahashi, Y., Okada, S., Sasagawa, H., Narita, S., Kumagai, S., Wada, Y., Mori, N., & Habuchi, T. (2023). The Current Trend of Radiation Therapy for Patients with Localized Prostate Cancer. Current Oncology, 30(9), 8092-8110. https://doi.org/10.3390/curroncol30090587





