Maintenance Therapy Post-Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia
Abstract
1. Introduction
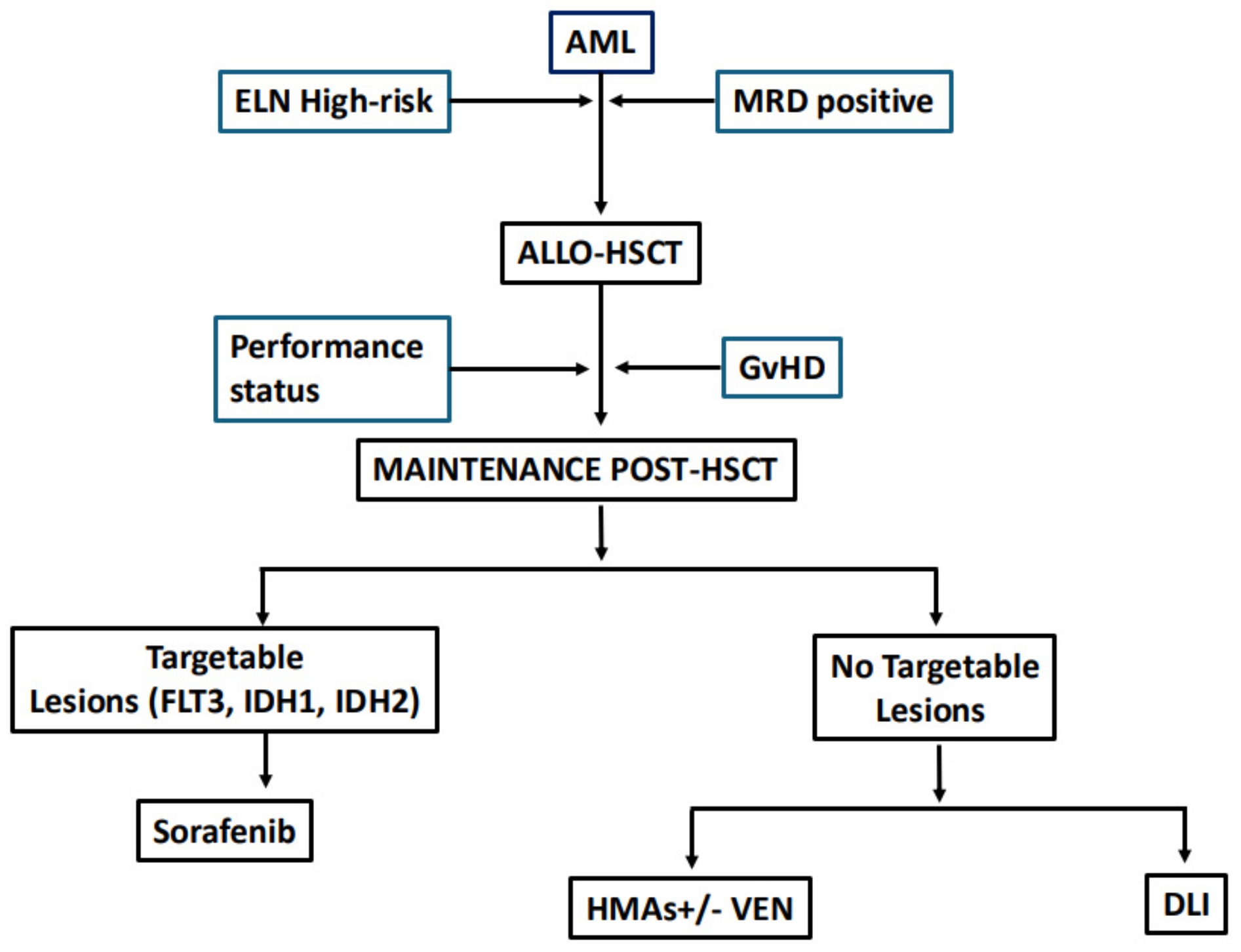
2. Maintenance Therapy in AML: Is It Time to Refine the Definition?
3. Who Are the Candidates for Maintenance Treatment?
4. Targeted Drugs
4.1. FLT3 Inhibitors (FLT3i)
4.2. IDH Inhibitors
4.3. Eprenetapopt
5. Hypomethylating Agents (Hma) Alone or in Combination
HMAs in Combination with Venetoclax
6. Donor Lymphocyte Infusion (DLI)
- The indication for DLI is for patients with high-risk relapse, according to one of the following three factors:
- high-risk biological features;
- transplantation in refractory or advanced stage;
- ex vivo lymphocyte depletion as GvHD prophylaxis.
- Prophylactic DLI is indicated in transplants using a non-myeloablative conditioning regimen or in the absence of molecular targets, independently of the conditioning regimen;
- The first infusion should be administered after immunosuppression for >30 days; it is mandatory that there be an absence of active GvHD and infections;
- The interval from HSCT to the first DLI was reported to be 4–6 months; however, an early application is recommended in high-risk cases;
- The dose of infusions would increase 0.5–1 log and the interval would be 4–6 weeks;
- MRD, chimerism monitoring, and the onset of GvHD would drive the DLI frequencies; however, 1–3 doses are recommended.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 22, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Sánchez-Ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; de la Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dholaria, B.; Savani, B.N.; Hamilton, B.K.; Oran, B.; Liu, H.D.; Tallman, M.S.; Ciurea, S.O.; Holtzman, N.G.; Ii, G.L.P.; Devine, S.M.; et al. Hematopoietic Cell Transplantation in the Treatment of Newly Diagnosed Adult Acute Myeloid Leukemia: An Evidence-Based Review from the American Society of Transplantation and Cellular Therapy. Transplant. Cell. Ther. 2021, 27, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Breccia, M.; Foa, R.; Jabbour, E.; Kadia, T.M. Maintenance therapy in AML: The past, the present and the future. Am. J. Hematol. 2019, 94, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 24, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Chen, Y.B. How I treat with maintenance therapy after allogeneic HCT. Blood 2023, 141, 39–48. [Google Scholar] [CrossRef]
- Al-Shaibani, E.; Novitzky-Basso, I.; Mattsson, J.; Kim, D.D.H. Post-transplant maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation harmonizing multiple therapeutic modalities including targeted therapy, immunotherapy and cellular therapy. Int. J. Hematol. 2023, 118, 1–17. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Meshinchi, S.; Stirewalt, D.L.; Alonzo, T.A.; Boggon, T.J.; Gerbing, R.B.; Rocnik, J.L.; Lange, B.J.; Gilliland, D.G.; Radich, J.P. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood 2008, 111, 4930–4933. [Google Scholar] [CrossRef]
- Santos, F.P.; Jones, D.; Qiao, W.; Cortes, J.E.; Ravandi, F.; Estey, E.E.; Verma, D.; Kantarjian, H.; Borthakur, G. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer 2011, 117, 2145–2155. [Google Scholar] [CrossRef]
- Takahashi, S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: Biology and therapeutic implications. J. Hematol. Oncol. 2011, 4, 13. [Google Scholar] [CrossRef]
- Choudhary, C.; Schwable, J.; Brandts, C.; Tickenbrock, L.; Sargin, B.; Kindler, T.; Fischer, T.; Berdel, W.E.; Muller-Tidow, C.; Serve, H. AML-Associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood 2005, 106, 265–273. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Bug, G.; Baron, F.; Brissot, E.; Ciceri, F.; Dalle, I.A.; Döhner, H.; Esteve, J.; Floisand, Y.; Giebel, S.; et al. Clinical practice recommendationon hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: A position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2020, 11507–11516. [Google Scholar]
- Antar, A.I.; Otrock, Z.K.; Jabbour, E.; Mohty, M.; Bazarbachi, A. FLT3 inhibitors in acute myeloid leukemia: Ten frequently asked questions. Leukemia 2020, 34, 682–696. [Google Scholar] [CrossRef]
- Mathew, N.R.; Baumgartner, F.; Braun, L.; O’Sullivan, D.; Thomas, S.; Waterhouse, M.; AMüller, T.; Hanke, K.; Taromi, S.; Apostolova, P.; et al. Sorafenib Promotes Graft-Versus-Leukemia Activity in Mice and Humans Through IL-15 Production in FLT3-ITD-Mutant Leukemia Cells. Nat. Med. 2018, 24, 282–291. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 26, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Wang, Y.; Yang, K.; Shao, R.; Huang, F.; Fan, Z.; Chi, P.; Xu, Y.; Xu, N.; Deng, L.; et al. Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: Long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2023, 10, e600–e611. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Atallah, E.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood 2022, 139, 3366–3375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Gilteritinib as Post-Transplant Maintenance for AML With Internal Tandem Duplication Mutation of FLT3. J. Clin. Oncol. 2024, 42, 1766–1775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Erba, H.P.; Montesinos, P.; Kim, H.-J.; Patkowska, E.; Vrhovac, R.; Žák, P.; Wang, P.-N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1571–1583. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Ravandi, F.; Agresta, S.; Konopleva, M.; Takahashi, K.; Kadia, T.; Routbort, M.; Patel, K.P.; Mark, B.; Pierce, S.; et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am. J. Hematol. 2015, 90, 732–736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, E.C.; Li, S.; Eisfeld, A.-K.; Luskin, M.R.; Mims, A.; Jones, D.; Antin, J.H.; Cutler, C.S.; Koreth, J.; Ho, V.T.; et al. Outcomes for patients with IDH-mutated acute myeloid leukemia undergoing allogeneic hematopoietic cell transplantation. Transplant. Cell. Ther. 2021, 479, 479.e1–479.e7. [Google Scholar]
- Salhotra, A.; Afkhami, M.; Yang, D.; Mokhtari, S.; Telatar, M.; Gu, D.; Pillai, R.K.; Weisenburger, D.D.; Murata-Collins, J.; Weigel, D.; et al. Allogeneic hematopoietic cell transplantation outcomes in patients carrying isocitrate dehydrogenase mutations. Clin. Lymphoma Myeloma Leuk. 2019, 19, e400–e405. [Google Scholar] [CrossRef]
- Mishra, A.; Tamari, R.; DeZern, A.E.; Byrne, M.T.; Gooptu, M.; Chen, Y.-B.; Deeg, H.J.; Sallman, D.; Gallacher, P.; Wennborg, A.; et al. Eprenetapopt Plus Azacitidine After Allogeneic Hematopoietic Stem-Cell Transplantation for TP53-Mutant Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2022, 34, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.T.; Kurian, T.J.; Patel, A.D.; Tamari, R.; Hong, S.; Abdelhakim, H.; Klein, V.; Rojas, P.; Madhavan, R.; Kent, A.; et al. Non-relapse mortality in TP53-mutated MDS/AML—A multi-center collaborative study. Blood 2021, 138 (Suppl. S1), 2922. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef]
- Manobianco, S.A.; Rakiewicz, T.; Wilde, L.; Palmisiano, N.D. Novel Mechanisms for Post-Transplant Maintenance Therapy in Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 892289. [Google Scholar] [CrossRef]
- de Lima, M.; Giralt, S.; Thall, P.F.; de Padua Silva, L.; Jones, R.B.; Komanduri, K.; Braun, T.M.; Nguyen, H.Q.; Champlin, R.; Garcia-Manero, G. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: A dose and schedule finding study. Cancer 2010, 116, 5420–5431. [Google Scholar] [CrossRef]
- Goodyear, O.C.; Dennis, M.; Jilani, N.Y.; Loke, J.; Siddique, S.; Ryan, G.; Nunnick, J.; Khanum, R.; Raghavan, M.; Cook, M.; et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012, 119, 3361–3369. [Google Scholar] [CrossRef]
- Schroeder, T.; Fröbel, J.; Cadeddu, R.-P.; Czibere, A.; Dienst, A.; Platzbecker, U.; Bug, G.; Uharek, L.; Fenk, R.; Germing, U.; et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia 2013, 27, 1910–1913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oran, B.; de Lima, M.; Garcia-Manero, G.; Thall, P.F.; Lin, R.; Popat, U.; Alousi, A.M.; Hosing, C.; Giralt, S.; Rondon, G.; et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020, 21, 5580–5588, Erratum in Blood Adv. 2021, 5, 1755–1756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Zhou, Z.; Chen, L.; Wang, X. Comparison of Azacitidine and Decitabine in Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Network Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2021, 6, e530–e544. [Google Scholar] [CrossRef] [PubMed]
- Kungwankiattichai, S.; Ponvilawan, B.; Roy, C.; Tunsing, P.; Kuchenbauer, F.; Owattanapanich, W. Maintenance With Hypomethylating Agents After Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 801632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Lima, M.; Oran, B.; Champlin, R.E.; Papadopoulos, E.B.; Giralt, S.A.; Scott, B.L.; William, B.M.; Hetzer, J.; Laille, E.; Hubbell, B.; et al. CC-486 (oral azacitidine) maintenance therapy is well tolerated after allogeneic hematopoietic stem cell transplantation (alloHSCT) in patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML). Biol. Blood Marrow Transplant. 2016, 22, S312–S313. [Google Scholar] [CrossRef]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.M.; Knoth, H.; Röllig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef]
- Platzbecker, U.; Middeke, J.M.; Sockel, K.; Herbst, R.; Fransecky, L.R.; Wolf, D.; Martin, S.; Krämer, A.; Noppeney, R.; Novotny, J.; et al. Azacitidine for Pre-Emptive Treatment of Measurable-Residual Disease in MDS/AML Patients at High Risk of Hematological Relapse: Results of the Second Cohort of the RELAZA2 Trial. Blood 2019, 134, 644. [Google Scholar] [CrossRef]
- Woo, J.; Deeg, H.J.; Storer, B.; Yeung, C.; Fang, M.; Mielcarek, M.; Scott, B.L. Factors Determining Responses to Azacitidine in Patients with Myelodysplastic Syndromes and Acute Myeloid Leukemia with Early Post-Transplantation Relapse: A Prospective Trial. Biol. Blood Marrow Transplant. 2017, 23, 176179. [Google Scholar] [CrossRef][Green Version]
- Gao, L.; Zhang, Y.; Wang, S.; Kong, P.; Su, Y.; Hu, J.; Jiang, M.; Bai, H.; Lang, T.; Wang, J.; et al. Effect of rhG-CSF Combined with Decitabine Prophylaxis on Relapse of Patients with High-Risk MRD-Negative AML after HSCT: An Open-Label, Multicenter, Randomized Controlled Trial. J. Clin. Oncol. 2020, 38, 4249–4259. [Google Scholar] [CrossRef]
- Wei, Y.; Xiong, X.; Li, X.; Lu, W.; He, X.; Jin, X.; Sun, R.; Lyu, H.; Yuan, T.; Sun, T.; et al. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome. Cancer Sci. 2021, 9, 3636–3644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 1, 7–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, A.H.; Strickland, S.A., Jr.; Hou, J.-Z.; Fiedler, W.; Lin, T.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. 2019, 15, 1277–1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kent, A.; Schwartz, M.; McMahon, C.; Amaya, M.; Smith, C.A.; Tobin, J.; Marciano, K.; Rezac, R.; Bosma, G.; Pollyea, D.A.; et al. Venetoclax is safe and tolerable as post-transplant maintenance therapy for AML patients at high risk for relapse. Bone Marrow Transpl. 2023, 58, 849–854. [Google Scholar] [CrossRef]
- Parks, K.; Diebold, K.; Salzman, D.; Di Stasi, A.; Al-Kadhimi, Z.; Espinoza-Gutarra, M.; Bhatia, R.; Jamy, O. Low-dose decitabine plus venetoclax as post-transplant maintenance for high-risk myeloid malignancies. eJHaem 2024, 3, 560–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pagliuca, S.; Schmid, C.; Santoro, N.; Simonetta, F.; Battipaglia, G.; Guillaume, T.; Greco, R.; Onida, F.; Sánchez-Ortega, I.; Yakoub-Agha, I.; et al. Donor lymphocyte infusion after allogeneic haematopoietic cell transplantation for haematological malignancies: Basic considerations and best practice recommendations from the EBMT. Lancet Haematol. 2024, 6, e448–e458. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Kuball, J.; Bug, G. Defining the Role of Donor Lymphocyte Infusion in High-Risk Hematologic Malignancies. J. Clin. Oncol. 2021, 5, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Frederik, F.J.H.; Schmid, C.; Kolb, H.J.; Locatelli, F.; Kuball, J. Delayed transfer of immune cells or the art of donor lymphocyte infusion. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; The EBMT: Maastricht, The Netherlands, 2019; pp. 443–448. [Google Scholar]
- Levine, J.E.; Braun, T.; Penza, S.L.; Beatty, P.; Cornetta, K.; Martino, R.; Drobyski, W.R.; Barrett, A.J.; Porter, D.L.; Giralt, S.; et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J. Clin. Oncol. 2002, 2, 405–412. [Google Scholar] [CrossRef]
- Dominietto, A.; Pozzi, S.; Miglino, M.; Albarracin, F.; Piaggio, G.; Bertolotti, F.; Grasso, R.; Zupo, S.; Raiola, A.M.; Gobbi, M.; et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood 2007, 109, 5063–5064. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Brecht, A.; Stadler, M.; Finke, J.; Baron, F.; Collin, M.; Bug, G.; et al. Long-term results and GvHD after prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia. Bone Marrow Transplant. 2022, 2, 215–223. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M.; Stadler, M.; Finke, J.; Hurst, E.; Baron, F.; Ringden, O.; et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—A matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2019, 5, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Le Floch, A.-C.; Granata, A.; Fürst, S.; Faucher, C.; Lemarie, C.; Harbi, S.; Bramanti, S.; Calmels, B.; El-Cheikh, J.; et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation for high-risk AML. Bone Marrow Transplant. 2017, 52, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Jedlickova, Z.; Schmid, C.; Koenecke, C.; Hertenstein, B.; Baurmann, H.; Schwerdtfeger, R.; Tischer, J.; Kolb, H.J.; Schleuning, M. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 663–667. [Google Scholar] [CrossRef]
- Cauchois, R.; Castagna, L.; Pagliardini, T.; Harbi, S.; Calmels, B.; Bramanti, S.; Granata, A.; Lemarie, C.; Maisano, V.; Legrand, F.; et al. Prophylactic donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation for high risk haematological malignancies: A retrospective bicentric analysis of serial infusions of increasing doses of CD3+ cells. Br. J. Haematol. 2019, 3, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Mooyaart, J.E.; Devillier, R.; Koc, Y.; Vydra, J.; Castagna, L.; Gülbas, Z.; Martin, J.D.; Araujo, M.C.; Kulagin, A.; et al. Donor lymphocyte infusions after haploidentical stem cell transplantation with PTCY: A study on behalf of the EBMT cellular therapy & immunobiology working party. Bone Marrow Transplant. 2023, 58, 54–60. [Google Scholar]
| Study | Phase | Agents | Schedule | Results | Side Effects |
|---|---|---|---|---|---|
| NCT02474290 | III | Sorafenib vs. placebo | Sorafenib 400 mg bid for 6 months | Improved 2-year RFS (78.9% vs. 56.6%) and OS (82.1% vs. 68.0%) with sorafenib | myelosuppression |
| NCT02997202 | III | Gilteritinib vs. placebo | Gilteritinib 120 mg for 24 months | Improved RFS in MRD+ (HR = 0.515, 95% CI: 0.316, 0.838, p = 0.0065) with gilteritinib | Important myelosuppression |
| NCT03564821 | I | Ivosidenib | Ivosidenib 500 mg and 250 mg | 2-year relapse = 19% 2-year PFS = 81% 2-year OS = 88% | No increased GvHD |
| NCT03515512 | I | Enasidenib | Enasidenib 100 mg and 50 mg | Relapse = 16% 2-year PFS = 69% 2-year OS = 74% | No increased GvHD |
| NCT03931291 | II | APR246 + AZA in | 1-year RFS = 59.9% 1-year OS = 78.8% | No increased GvHD | |
| NCT00887068 | III | AZA vs. placebo | AZA 32 mg/m2 for 5 days | RFS: 2.07 years (AZA) vs. 1.28 years (placebo), p = 0.43 | myelosuppression |
| ChiCTR1900025374 | II | DEC/VEN | DEC 15 mg/m2 for 3 days Ven 200 mg days 1–21 | 1-year relapse = 15.3% 1-year OS = 85.2% | Reversible myelosuppression |
| NCT03613532 | I | AZA/VEN | AZA 36 mg/m2 for 5 days Ven 400 mg days 1–14 | 2-year relapse = 41% 2-year OS = 67% | Reversible myelosuppression |
| DLI Dose for Matched Related Donor | DLI Dose for Matched Unrelated Donor | DLI Dose for Mismatched Unrelated Donor or Haploidentical Donor | |
|---|---|---|---|
| 3 months | 1 × 105 cells/kg × 1–3 doses | 1 × 105 cells/kg 1–3 doses | 1 × 105 cells/kg 1–3 doses |
| 6 months | 1 × 106 cells/kg | 1 × 106 cells/kg | 5 × 105 cells/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canichella, M.; Molica, M.; Mazzone, C.; de Fabritiis, P. Maintenance Therapy Post-Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia. Curr. Oncol. 2024, 31, 6050-6060. https://doi.org/10.3390/curroncol31100451
Canichella M, Molica M, Mazzone C, de Fabritiis P. Maintenance Therapy Post-Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia. Current Oncology. 2024; 31(10):6050-6060. https://doi.org/10.3390/curroncol31100451
Chicago/Turabian StyleCanichella, Martina, Matteo Molica, Carla Mazzone, and Paolo de Fabritiis. 2024. "Maintenance Therapy Post-Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia" Current Oncology 31, no. 10: 6050-6060. https://doi.org/10.3390/curroncol31100451
APA StyleCanichella, M., Molica, M., Mazzone, C., & de Fabritiis, P. (2024). Maintenance Therapy Post-Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia. Current Oncology, 31(10), 6050-6060. https://doi.org/10.3390/curroncol31100451





