Timing of Surgery and Social Determinants of Health Related to Pathologic Complete Response after Total Neoadjuvant Therapy for Rectal Adenocarcinoma: Retrospective Study of National Cancer Database
Abstract
1. Introduction
2. Materials and Methods
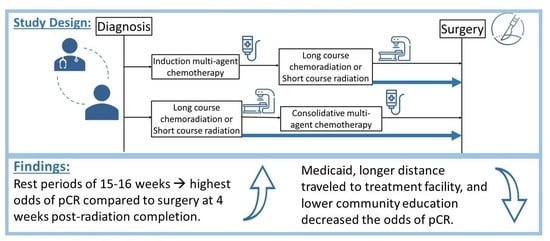
2.1. Study Design
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics
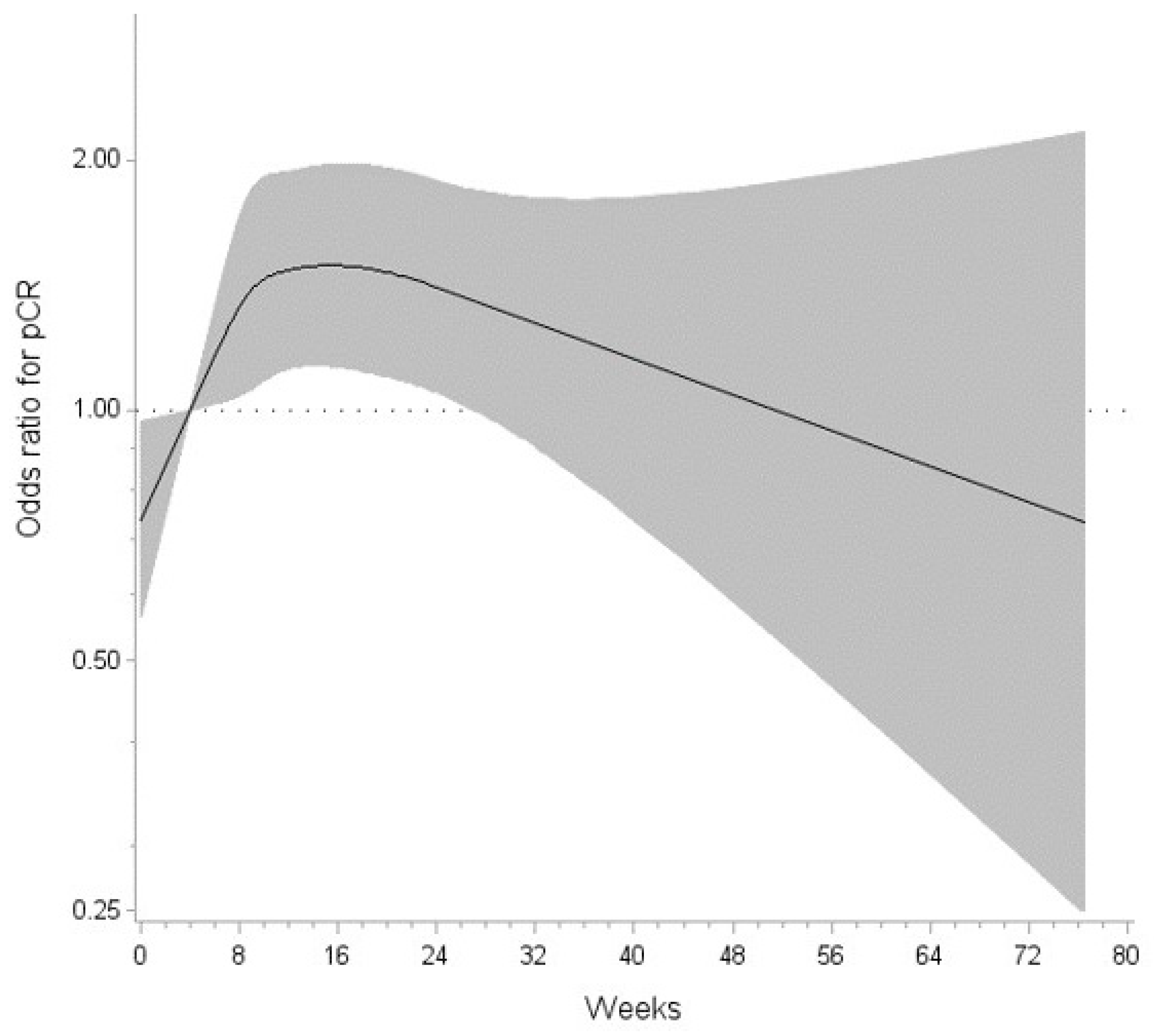
3.2. Factors Associated with pCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Rest Period (Weeks) | OR | (95% CI) |
|---|---|---|
| 0.1531428528 | 0.747 | (0.577, 0.968) |
| 4.0 (ref) | 1.000 | (1.000, 1.000) |
| 4.1348570251 | 1.010 | (1.001, 1.019) |
| 14.701713867 | 1.494 | (1.132, 1.972) |
| 14.85485672 | 1.494 | (1.131, 1.973) |
| 15.007999573 | 1.494 | (1.131, 1.974) |
| 15.161142426 | 1.494 | (1.131, 1.975) |
| 15.314285278 | 1.494 | (1.130, 1.976) |
| 15.467428131 | 1.494 | (1.129, 1.977) |
| 15.620570984 | 1.494 | (1.129, 1.978) |
| 15.773713837 | 1.494 | (1.128, 1.978) |
| 15.926856689 | 1.494 | (1.127, 1.979) |
| 26.95314209 | 1.357 | (1.001, 1.840) |
| 76.571426392 | 0.733 | (0.249, 2.159) |
References
- Johnson, G.G.R.J.; Park, J.; Helewa, R.M.; Goldenberg, B.A.; Nashed, M.; Hyun, E. Total neoadjuvant therapy for rectal cancer: A guide for surgeons. Can. J. Surg. 2023, 66, E196–E201. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Rectal Cancer Version 2.2016. Available online: https://www.nccn.org (accessed on 2 June 2022).
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Lefevre, J.H.; Mineur, L.; Kotti, S.; Rullier, E.; Rouanet, P.; de Chaisemartin, C.; Meunier, B.; Mehrdad, J.; Cotte, E.; Desrame, J.; et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J. Clin. Oncol. 2016, 34, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. National Cancer Database. Available online: https://www.facs.org/quality-programs/cancer/ncdb (accessed on 2 June 2022).
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef]
- Arndt, K.; Ore, A.S.; Quinn, J.; Fabrizio, A.; Crowell, K.; Messaris, E.; Cataldo, T. Outcomes Following Recent and Distant Neoadjuvant Radiation in Rectal Cancer: An Institutional Retrospective Review and Analysis of NSQIP. Clin. Color. Cancer 2023, 22, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Shahjehan, F.; Kasi, P.M.; Habermann, E.; Day, C.N.; Colibaseanu, D.T.; Mathis, K.L.; Larson, D.W.; Merchea, A. Trends and outcomes of sphincter-preserving surgery for rectal cancer: A national cancer database study. Int. J. Color. Dis. 2019, 34, 239–245. [Google Scholar] [CrossRef]
- Pulte, D.; Jansen, L.; Brenner, H. Population-Level Differences in Rectal Cancer Survival in Uninsured Patients Are Partially Explained by Differences in Treatment. Oncologist 2017, 22, 351–358. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 5997) | No pCR (n = 4888) | pCR (n = 1109) | pCR Rate | p-Value | |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD 1) | 56.5 (11.4) | 56.6 (11.5) | 56.3 (11.2) | 0.483 | |
| 18–39 years old | 464 (7.7%) | 380 (7.8%) | 84 (7.6%) | 18.1% | 0.221 |
| 40–49 years old | 1089 (18.2%) | 879 (18.0%) | 210 (18.9%) | 19.3% | |
| 50–59 years old (Ref) | 1979 (33.0%) | 1622 (33.2%) | 357 (32.2%) | 18.0% | |
| 60–69 years old | 1705 (28.4%) | 1378 (28.2%) | 327 (29.5%) | 19.2% | |
| 70–79 years old | 669 (11.2%) | 546 (11.2%) | 123 (11.1%) | 18.4% | |
| 80+ years old | 91 (1.5%) | 83 (1.7%) | 8 (0.7%) | 0.7% | |
| Sex | |||||
| Male (Ref) | 3736 (62.3%) | 3220 (62.2%) | 516 (62.9%) | 13.8% | 0.689 |
| Female | 2261 (37.7%) | 1957 (37.8%) | 304 (37.1%) | 13.5% | |
| Race/Ethnicity | |||||
| Non-Hispanic White (Ref) | 4529 (75.5%) | 3682 (75.3%) | 847 (76.4%) | 18.7% | 0.871 |
| Hispanic/Latinx | 625 (10.4%) | 516 (10.6%) | 79 (9.8%) | 17.4% | |
| Non-Hispanic Black | 454 (7.6%) | 375 (7.7%) | 109 (7.1%) | 17.4% | |
| Non-Hispanic Asian/Pacific Islander | 286 (4.8%) | 230 (4.7%) | 56 (5.1%) | 19.6% | |
| Other/unknown | 103 (1.7%) | 85 (1.7%) | 18 (1.6%) | 17.5% | |
| Community education measure 2 | |||||
| >15.3% | 993 (16.6%) | 836 (17.1%) | 157 (14.2%) | 15.8% | 0.011 |
| 9.1–15.2% | 1342 (22.4%) | 1109 (22.7%) | 233 (21.0%) | 17.4% | |
| 5.0–9.0% | 1534 (25.6%) | 1239 (25.4%) | 295 (26.6%) | 19.2% | |
| <5.0% (ref) | 1210 (20.2%) | 952 (19.5%) | 258 (23.3%) | 21.3% | |
| Unknown | 918 (15.3%) | 752 (15.4%) | 952 (15.0%) | 18.1% | |
| Income | |||||
| <USD 46,277 | 749 (12.5%) | 629 (12.9%) | 120 (10.8%) | 16.0% | 0.107 |
| USD 46,277–USD 57,856 | 1015 (16.9%) | 824 (16.9%) | 191 (17.2%) | 18.8% | |
| USD 57,856–USD 74,062 | 1282 (21.4%) | 1057 (21.6%) | 225 (20.3%) | 17.6% | |
| USD 74,062 or more (Ref) | 2018 (33.7%) | 1612 (33.0%) | 406 (36.6%) | 20.1% | |
| Unknown | 933 (15.6%) | 766 (15.7%) | 167 (15.1%) | 17.9% | |
| Year of Diagnosis | |||||
| 2016 (Ref) | 396 (6.6%) | 338 (6.1%) | 58 (9.8%) | 14.7% | 0.018 |
| 2017 | 518 (8.6%) | 439 (8.5%) | 79 (9.6%) | 15.3% | |
| 2018 | 1339 (22.3%) | 1088 (21.9%) | 251 (25.1%) | 18.8% | |
| 2019 | 1897 (31.6%) | 1552 (32.8%) | 345 (24.3%) | 18.2% | |
| 2020 | 1847 (30.8%) | 1471 (30.7%) | 376 (31.2%) | 20.4% | |
| Facility type | |||||
| Academic/Research Program (Ref) | 2290 (38.2%) | 1874 (38.3%) | 416 (37.5%) | 18.2% | 0.842 |
| Comprehensive Community Cancer Program | 1757 (29.3%) | 1438 (29.4%) | 319 (28.8%) | 18.2% | |
| Integrated Network Cancer Program | 1256 (20.9%) | 1012 (20.7%) | 244 (22.0%) | 19.4% | |
| Community Cancer Program | 230 (3.8%) | 184 (3.8%) | 46 (4.2%) | 20.0% | |
| Unknown | 464 (7.7%) | 380 (7.8%) | 84 (7.6%) | 18.1% | |
| Metro/urban/rural area | |||||
| Metro/urban (Ref) | 5549 (92.5%) | 4512 (92.3%) | 1037 (93.5%) | 18.7% | 0.279 |
| Rural | 253 (4.2%) | 209 (4.3%) | 44 (4.0%) | 17.4% | |
| Unknown | 28 (3.3%) | 167 (3.4%) | 28 (2.5%) | 14.4% | |
| Distance traveled | |||||
| 0–30 miles (Ref) | 3925 (65.5%) | 3173 (64.9%) | 752 (67.8%) | 19.2% | 0.094 |
| More than 30 miles | 1197 (20.0%) | 1001 (20.5%) | 196 (17.7%) | 16.4% | |
| Unknown | 875 (14.6%) | 714 (14.6% | 161 (14.5%) | 18.4% | |
| Primary payer | |||||
| Private insurance/managed care (Ref) | 3452 (57.6%) | 2783 (56.9%) | 669 (60.3%) | 19.4% | 0.019 |
| Medicare | 1495 (24.9%) | 1218 (24.9%) | 277 (25.0%) | 18.5% | |
| Medicaid | 653 (10.9%) | 563 (11.5%) | 90 (8.1%) | 13.8% | |
| Uninsured | 241 (4.0%) | 199 (4.1%) | 42 (3.8%) | 17.4% | |
| Other/Unknown | 156 (2.6%) | 125 (2.6%) | 31 (2.8%) | 19.9% |
| Overall (N = 5997) | No pCR (n = 4888) | pCR (n = 1109) | pCR Rate | p-Value | |
|---|---|---|---|---|---|
| Clinical Stage | |||||
| Stage I (T2N0M0) | 63 (1.1%) | 48 (1.0%) | 15 (1.4%) | 23.8% | 0.547 |
| Stage II | 1027 (17.1%) | 839 (17.2%) | 188 (17.0%) | 18.3% | |
| Stage III (Ref) | 4907 (81.8%) | 4001 (81.9%) | 906 (81.7%) | 18.5% | |
| Initial tumor size (mm) | |||||
| Median (IQR) | 50 (35–66) | 50 (35–66) | 50 (38–66) | 0.48 | |
| Charlson–Deyo score | |||||
| 0 (Ref) | 4815 (80.3%) | 3916 (80.1%) | 899 (81.1%) | 18.7% | 0.473 |
| 1 or more | 1182 (19.7%) | 972 (19.9%) | 210 (19.0%) | 17.8% | |
| Order of chemotherapy | |||||
| Induction chemotherapy (Ref) | 5177 (86.3%) | 4221 (86.2%) | 966 (87.1%) | 18.7% | 0.403 |
| Consolidative chemotherapy | 820 (13.7%) | 677 (13.9%) | 143 (12.9%) | 17.4% | |
| Radiation dosing | |||||
| 25 Gy in 5 fx | 538 (9.0%) | 427 (8.7%) | 111 (10.0%) | 20.65 | 0.216 |
| 45–50 Gy in 25–28 fx | 4597 (76.7%) | 3740 (76.5%) | 857 (77.3%) | 18.6% | |
| Nonstandard dose (Ref) | 605 (10.1%) | 504 (10.3%) | 101 (9.1%) | 16.7% | |
| Unknown | 257 (4.3%) | 217 (4.4%) | 40 (3.6%) | 15.6% | |
| TNT regimen | |||||
| I-chemotherapy + L-XRT 1 (Ref) | 4184 (81.5%) | 3389 (81.0%) | 795 (19.0%) | 19.0% | 0.107 |
| L-XRT + C-chemotherapy 2 | 413 (8.0%) | 351 (85.0%) | 62 (15.0%) | 15.0% | |
| S-XRT + C-chemotherapy 3 | 289 (5.6%) | 225 (77.9%) | 64 (22.2%) | 22.2% | |
| I-chemotherapy + S-XRT 4 | 249 (4.9%) | 202 (81.1%) | 47 (18.9%) | 18.9% | |
| Tumor boost | |||||
| None or incomplete boost (Ref) | 3261 (54.4%) | 2670 (54.6%) | 591 (53.3%) | 18.1% | 0.074 |
| 5.4 Gy in 3 fx | 2485 (41.4%) | 2002 (41.0%) | 483 (43.6%) | 19.4% | |
| Unknown | 251 (4.2%) | 216 (4.4%) | 35 (3.2%) | 13.9% | |
| Type of surgery | |||||
| Total proctectomy (Ref) | 1432 (23.9%) | 1211 (24.8%) | 221 (19.9%) | 15.4% | <0.001 |
| Partial proctectomy | 3893 (64.9%) | 3108 (63.6%) | 785 (70.8%) | 20.2% | |
| Pull through with sphincter preservation | 381 (6.4%) | 304 (6.2%) | 77 (6.9%) | 20.2% | |
| Other proctectomy, unspecified | 291 (4.9%) | 265 (5.4%) | 26 (2.3%) | 8.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, M.; Goldman, J.; Appiah, D.; Abdulrahman, R.; Kidwell, J.; Shi, Z. Timing of Surgery and Social Determinants of Health Related to Pathologic Complete Response after Total Neoadjuvant Therapy for Rectal Adenocarcinoma: Retrospective Study of National Cancer Database. Curr. Oncol. 2024, 31, 1291-1301. https://doi.org/10.3390/curroncol31030097
Mai M, Goldman J, Appiah D, Abdulrahman R, Kidwell J, Shi Z. Timing of Surgery and Social Determinants of Health Related to Pathologic Complete Response after Total Neoadjuvant Therapy for Rectal Adenocarcinoma: Retrospective Study of National Cancer Database. Current Oncology. 2024; 31(3):1291-1301. https://doi.org/10.3390/curroncol31030097
Chicago/Turabian StyleMai, Megan, Jodi Goldman, Duke Appiah, Ramzi Abdulrahman, John Kidwell, and Zheng Shi. 2024. "Timing of Surgery and Social Determinants of Health Related to Pathologic Complete Response after Total Neoadjuvant Therapy for Rectal Adenocarcinoma: Retrospective Study of National Cancer Database" Current Oncology 31, no. 3: 1291-1301. https://doi.org/10.3390/curroncol31030097
APA StyleMai, M., Goldman, J., Appiah, D., Abdulrahman, R., Kidwell, J., & Shi, Z. (2024). Timing of Surgery and Social Determinants of Health Related to Pathologic Complete Response after Total Neoadjuvant Therapy for Rectal Adenocarcinoma: Retrospective Study of National Cancer Database. Current Oncology, 31(3), 1291-1301. https://doi.org/10.3390/curroncol31030097