Deciphering the Dilemma: Choosing the Optimal Total Neoadjuvant Treatment Strategy for Locally Advanced Rectal Cancer
Abstract
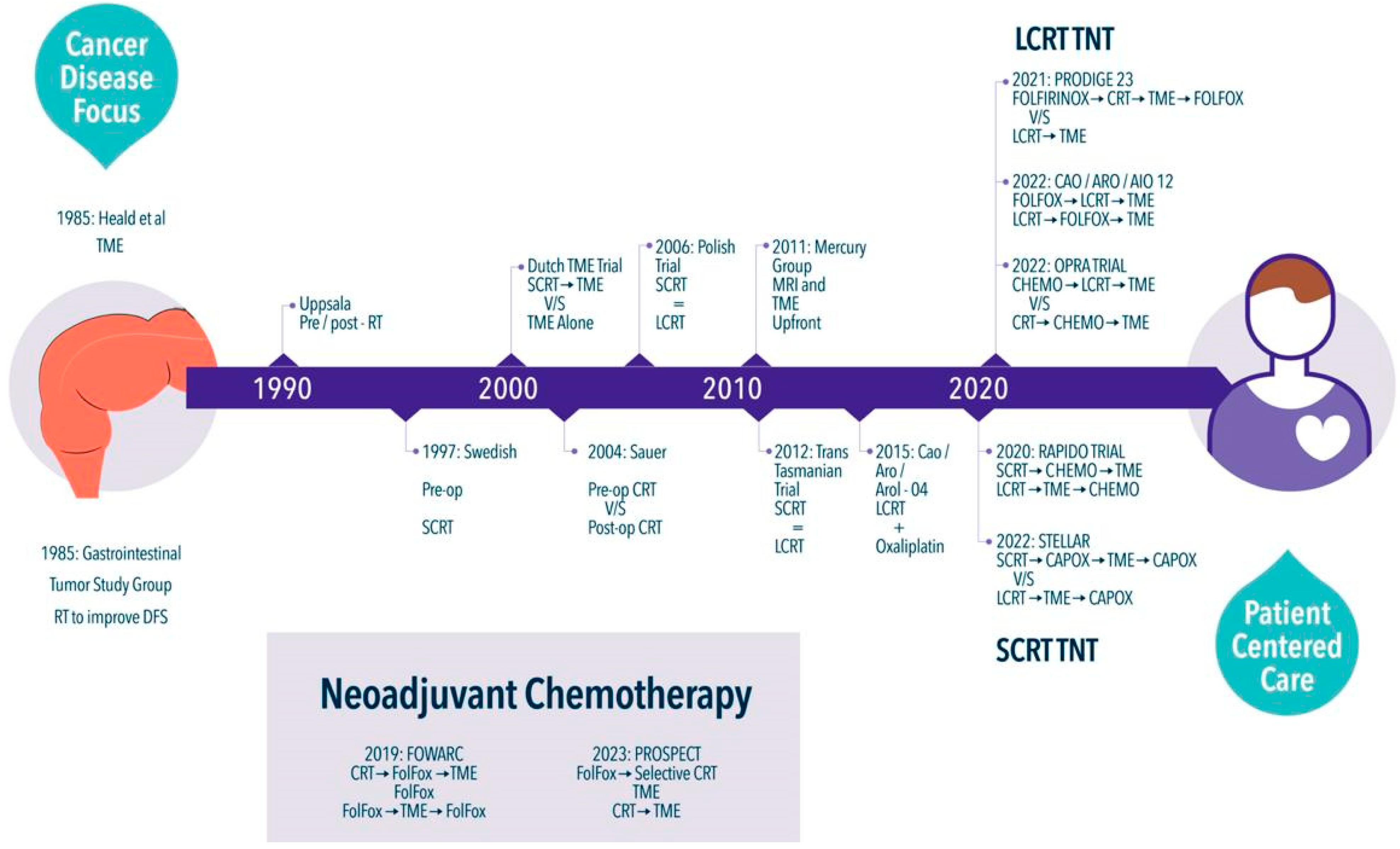
:1. Introduction
2. A Long Ride
3. Time to Make a Change
4. Total Neoadjuvant Therapy
5. LCRT-Based TNT
6. SCRT-Based TNT
7. Neoadjuvant Chemotherapy
8. Non-Operative Management (NOM)
9. Microsatellite Instability-High (MSI-H) / Deficient Mismatch Repair (dMMR) in LARC Patients
10. Which Neoadjuvant Treatment Is the Best?
Author Contributions
Funding
Conflicts of Interest
Abbreviation/Glossary List
| cCR | Complete Clinical Response |
| CRC | Colorectal Cancer |
| CRM | Circumferential Resection Margin |
| CRT | Chemoradiotherapy |
| CT | Chemotherapy |
| DFS | Disease-free Survival |
| EMVI | Extramural Vascular Invasion |
| LARC | Locally Advanced Rectal Cancer |
| LCRT | Long Course Chemoradiotherapy |
| LR | Local Recurrence |
| MFS | Metastasis-free Survival |
| MRF | Mesorectal Fascia |
| NAT | Neoadjuvant Therapy |
| OS | Overall Survival |
| pCR | Pathological Complete Response |
| RCT | Randomized Controlled Trial |
| RT | Radiotherapy |
| SCRT | Short Course Radiotherapy |
| TME | Total Mesorectal Excision |
| TNT | Total Neoadjuvant Therapy |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bhimani, N.; Wong, G.Y.; Molloy, C.; Dieng, M.; Hugh, T.J. Cost of colorectal cancer by treatment type from different health economic perspectives: A systematic review. Eur. J. Surg. Oncol. 2022, 48, 2082–2093. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Galandiuk, S. Rectal Cancer: New Challenges. Dis. Colon Rectum 2023, 66, 863–864. [Google Scholar] [CrossRef] [PubMed]
- McMullen, C.K.; Bulkley, J.E.; Altschuler, A.; Wendel, C.S.M.; Grant, M.R.; Hornbrook, M.C.; Sun, V.R.; Krouse, R.S. Greatest Challenges of Rectal Cancer Survivors: Results of a Population-Based Survey. Dis. Colon Rectum 2016, 59, 1019–1027. [Google Scholar] [CrossRef]
- Smith, H.G.; Nilsson, P.J.; Shogan, B.D.; Harji, D.; Gambacorta, M.A.; Romano, A.; Brandl, A.; Qvortrup, C. Neoadjuvant treatment of colorectal cancer: Comprehensive review. BJS Open. 2024, 8, zrae038. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Z.; Lengyel, C.G. Challenges in the management of colorectal cancer in low- and middle-income countries. Cancer Treat. Res. Commun. 2023, 35, 100705. [Google Scholar] [CrossRef] [PubMed]
- Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N. Engl. J. Med. 1985, 312, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- Heald, R.J.; Moran, B.J.; Ryall, R.D.; Sexton, R.; MacFarlane, J.K. Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998, 133, 894–899. [Google Scholar] [CrossRef]
- Påhlman, L.; Glimelius, B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann. Surg. 1990, 211, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Trial, S.R.C.; Cedermark, B.; Dahlberg, M.; Glimelius, B.; Påhlman, L.; E Rutqvist, L.; Wilking, N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; Van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.-A.; et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.G.M.; Quirke, P.; Heald, R.J.; Moran, B.; Blomqvist, L.; Swift, I.; Sebag-Montefiore, D.J.; Tekkis, P.; Brown, G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: A prospective, multicenter, European study. Ann. Surg. 2011, 253, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Etienne, P.L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2023, 41 (Suppl. S17), LBA3504. [Google Scholar] [CrossRef]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, A.; Cervantes, A.; Crolla, R.M.P.H.; et al. Locoregional Failure During and after Short-course Radiotherapy followed by Chemotherapy and Surgery Compared with Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 278, e766–e772. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.-C. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, R.; Junginger, T.; Ptok, H.; Strassburg, J.; Maurer, C.A.; Brosi, P.; Sauer, J.; Baral, J.; Kreis, M.; Wollschlaeger, D.; et al. Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM Rectal Cancer Trial. Br. J. Surg. 2018, 105, 1519–1529. [Google Scholar]
- Kennedy, E.D.; Simunovic, M.; Jhaveri, K.; Kirsch, R.; Brierley, J.; Drolet, S.; Brown, C.; Vos, P.M.; Xiong, W.; MacLean, T.; et al. Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients with “Good Prognosis” Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial. JAMA Oncol. 2019, 5, 961–966. [Google Scholar] [CrossRef]
- Breugom, A.J.; Swets, M.; Bosset, J.F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; Broek, C.B.M.v.D.; et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Haustermans, K.; Price, T.J.; Nordlinger, B.; Hofheinz, R.; Daisne, J.; Janssens, J.; Brenner, B.; Schmidt, P.; Reinel, H.; et al. Preoperative Chemoradiotherapy and Postoperative Chemotherapy with Capecitabine +/− Oxaliplatin in Locally Advanced Rectal Cancer: Interim Analysis for Disease-Free Survival of Petacc 6. Ann. Oncol. 2014, 25, iv170. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef]
- Aschele, C.; Lonardi, S.; Cionini, L.; Pinto, C.; Cordio, S.S.; Rosati, G.; Bianchi, A.S.; Tagliagambe, A.; Frisinghelli, M.; Zagonel, V.; et al. Final results of STAR-01: A randomized phase III trial comparing preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer. J. Clin. Oncol. 2016, 34 (Suppl. S15), 3521. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Lee, K.-W.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer after Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Verheij, F.S.; Omer, D.M.; Williams, H.; Lin, S.T.; Qin, L.X.; Buckley, J.T.; Thompson, H.M.; Yuval, J.B.; Kim, J.K.; Dunne, R.F.; et al. Long-Term Results of Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J. Clin. Oncol. 2024, 42, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Riou, O.; Gourgou, S.; Conroy, T. Comment on “Locoregional Failure During and After Short-Course Radiotherapy Followed by Chemotherapy and Surgery Compared to Long-Course Chemoradiotherapy and Surgery: A Five-Year Follow-Up of the RAPIDO Trial”: The RAPIDO Trial Does Not Achieve Its Primary Endpoint. Ann. Surg. Open 2023, 4, e288. [Google Scholar] [PubMed]
- Vailati, B.B.; Cerdán-Santacruz, C.; São Julião, G.P.; Corbi, L.; Perez, R.O. Short-Course Radiation and Consolidation Chemotherapy for Rectal Cancer-the Rise and Fall of a Treatment Strategy-Rest in Peace. Dis. Colon Rectum 2023, 66, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.J.; van Etten, B.; Hospers, G.A.P.; Marijnen, C.A.M.; Meershoek-Klein Kranenberg, E.; Roodvoets, A.G.H.; van de Velde, C.J.M.; Glimelius, B. Comment on the RAPIDO Trial Point-Counterpoint Debate. Dis. Colon Rectum 2024, 67, e126. [Google Scholar] [CrossRef]
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Kryński, J.; Michalski, W.; Olędzki, J.; Kuśnierz, J.; Zając, L.; et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef]
- Raldow, A.C.; Chen, A.B.; Russell, M.; Lee, P.P.; Hong, T.S.; Ryan, D.P.; Cusack, J.C.; Wo, J.Y. Cost-effectiveness of Short-Course Radiation Therapy vs Long-Course Chemoradiation for Locally Advanced Rectal Cancer. JAMA Netw. Open 2019, 2, e192249. [Google Scholar] [CrossRef] [PubMed]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J. Clin. Oncol. 2019, 37, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.J.; Ahlberg, M.; Kordnejad, S.; Holm, T.; Martling, A. Organ preservation following short-course radiotherapy for rectal cancer. BJS Open 2021, 5, zrab093. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Pietrzak, L.; Partycki, M.; Szczepkowski, M.; Wyrwicz, L.; Rupiński, M.; Rutkowski, A.; Mróz, A. The feasibility of short-course radiotherapy in a watch-and-wait policy for rectal cancer. Acta Oncol. 2017, 56, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, Y.L.; Lambregts, D.M.J.; van den Berg, J.; Grotenhuis, B.A.; Aalbers, A.; Van Triest, B.; Beets-Tan, R.G.; van de Belt, M.; Dokter, S.; Balduzzi, S.; et al. Radiotherapy, atezolizumab, and bevacizumab in rectal cancers with the aim of organ preservation: The TARZAN study. J. Clin. Orthod. 2023, 41 (Suppl. S4), 158. [Google Scholar] [CrossRef]
- Quezada, F.F.; Diaz-Feldman, L.E.; Manriquez, E.; Caire, N.; Carvajal, G. No operation after short course radiotherapy followed by consolidation chemotherapy in locally advanced rectal cancer: The prospective, single arm NOAHS-ARC trial. J. Clin. Orthod. 2024, 42 (Suppl. S3), TPS237. [Google Scholar] [CrossRef]
- Kavun, A.; Veselovsky, E.; Lebedeva, A.; Belova, E.; Kuznetsova, O.; Yakushina, V.; Grigoreva, T.; Mileyko, V.; Fedyanin, M.; Ivanov, M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Fernandes, G.D.S.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.K.; Siqueira, S.; Coudry, R.; Santos, J.; Alves, M.; Hoff, P.M.; Riechelmann, R.P. Response to Chemotherapy and Prognosis in Metastatic Colorectal Cancer With DNA Deficient Mismatch Repair. Clin. Color. Cancer 2017, 16, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Rectal Cancer Version 3.2024.© National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed [july 09, 2024]. To view the most recent and complete version of the guideline, go online to NCCN.org.
| Trial | Regimen | Main outcome | Secondary | Outcomes | ||
|---|---|---|---|---|---|---|
| 3y DFS * | 3y MFS * | 3y OS | pCR * | 3y LRR | ||
| PRODIGE 23 | CT → LCRT → TME → CT | 76% | 79% | 91% | 28% | 4% |
| N = 461 | LCRT → TME → CT | 69% | 72% | 88% | 12% | 6% |
| pCR | 3y DFS | 3y OS | 3y DM | 3y LRR | ||
| CAO/ARO/AIO 12 | CT → LCRT → TME | 17% | 73% | 92% | 18% | 6% |
| N = 306 | LCRT → CT → TME | 25% * | 73% | 92% | 16% | 5% |
| 5y DFS | 5y MFS | 5y OS | 5y OP * | 5y LRFS | ||
| OPRA | CT → LCRT → TME or WW | 71% | 80% | 88% | 39% | 94% |
| N = 324 | LCRT → CT → TME or WW | 69% | 78% | 85% | 54% | 90% |
| 5y DrTF * | 5y DM * | 5y OS | pCR * | 5y LRR * | ||
| RAPIDO | SCRT → CT → TME | 27.8% | 23% | 81.7% | 28% | 10.2% |
| N = 920 | LCRT → TME → CT | 34% | 30.4% | 80.2% | 14% | 6.1% |
| 3y DFS | 3y DM | 3y OS * | pCR+cCR * | 3y LRR | ||
| STELLAR | SCRT → CT → TME → CT | 64.5% (non inferior) | 22% | 86% | 21.8% | 8.4% |
| N = 591 | LCRT → TME → CT | 62.3% | 24% | 75% | 12.3% | 11% |
| 5y DFS | 5y OS | pCR | 5y LRR | |||
| PROSPECT | CT → Selective LCRT → TME ± CT | 80.8% (non inferior) | - | 89.5% | 21.9% | 1.8% |
| N = 1128 | LCRT → TME ± CT | 78.6% | - | 90.2% | 24.3% | 1.6% |
| 3y DFS | 3y OS | pCR | 3y LRR | |||
| FOWARC | LCRT → TME → 5FU | 72.9% | - | 91.3% | 14% | 8% |
| N = 495 | LCRT + CT → TME → CT | 77.2% | - | 89.1% | 27.5% | 7% |
| CT → TME → CT | 73.5% | - | 90.7% | 6.5% | 8.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manriquez, E.; Solé, S.; Silva, J.; Hermosilla, J.P.; Romero, R.; Quezada-Diaz, F. Deciphering the Dilemma: Choosing the Optimal Total Neoadjuvant Treatment Strategy for Locally Advanced Rectal Cancer. Curr. Oncol. 2024, 31, 4292-4304. https://doi.org/10.3390/curroncol31080320
Manriquez E, Solé S, Silva J, Hermosilla JP, Romero R, Quezada-Diaz F. Deciphering the Dilemma: Choosing the Optimal Total Neoadjuvant Treatment Strategy for Locally Advanced Rectal Cancer. Current Oncology. 2024; 31(8):4292-4304. https://doi.org/10.3390/curroncol31080320
Chicago/Turabian StyleManriquez, Erik, Sebastián Solé, Javiera Silva, Juan Pablo Hermosilla, Rubén Romero, and Felipe Quezada-Diaz. 2024. "Deciphering the Dilemma: Choosing the Optimal Total Neoadjuvant Treatment Strategy for Locally Advanced Rectal Cancer" Current Oncology 31, no. 8: 4292-4304. https://doi.org/10.3390/curroncol31080320
APA StyleManriquez, E., Solé, S., Silva, J., Hermosilla, J. P., Romero, R., & Quezada-Diaz, F. (2024). Deciphering the Dilemma: Choosing the Optimal Total Neoadjuvant Treatment Strategy for Locally Advanced Rectal Cancer. Current Oncology, 31(8), 4292-4304. https://doi.org/10.3390/curroncol31080320





