Immunotherapeutic Strategies Targeting Breast Cancer Stem Cells
Abstract
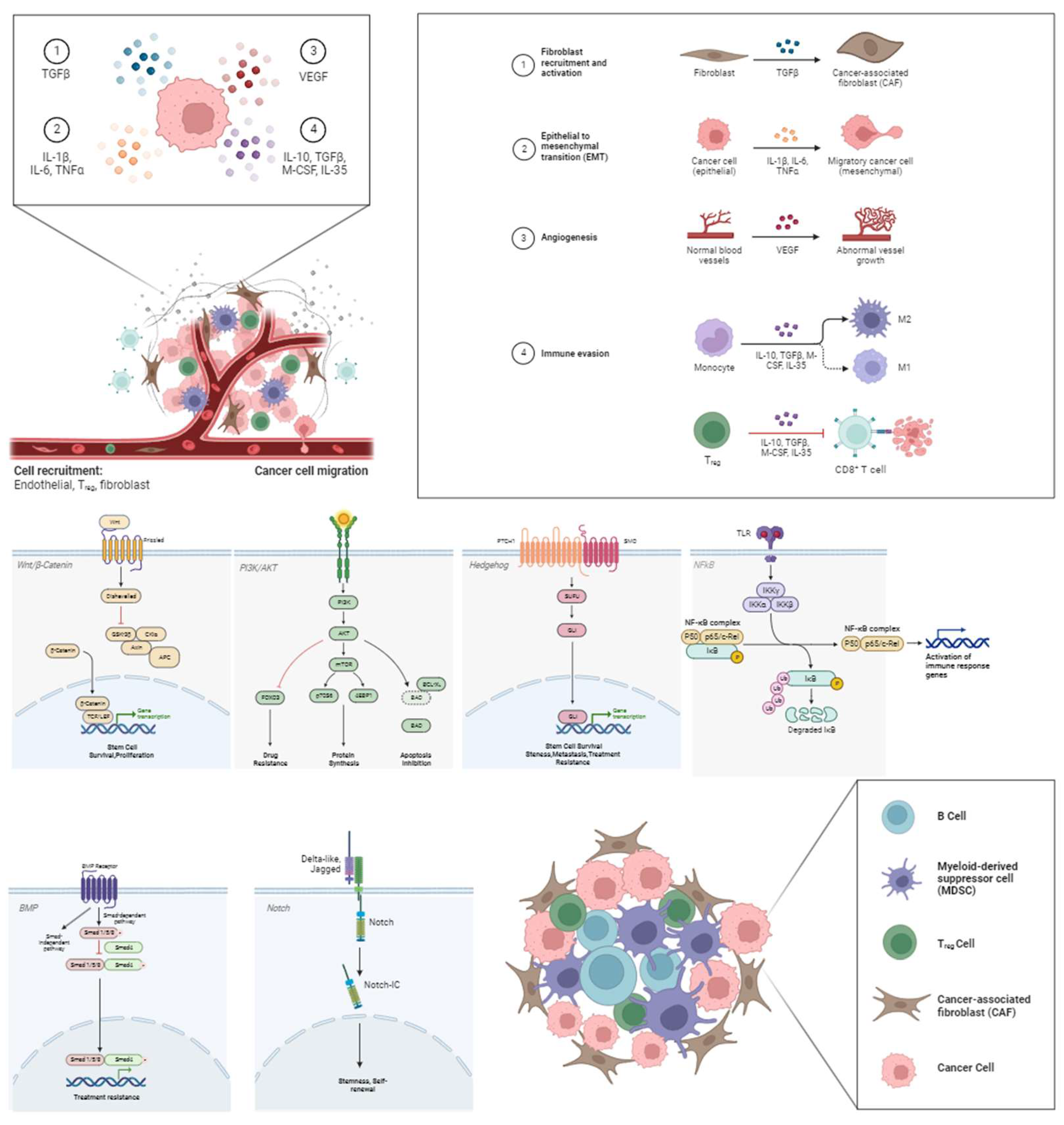
1. Introduction
2. CSC Immune Checkpoint
2.1. Immune Checkpoint Molecules
2.2. Dendritic Cells (DCs)
2.3. Tumor-Associated Macrophages (TAMs)
2.4. NK Ligands
2.5. Cytokines
3. CSC Immune Evasion
4. Immunotherapeutic Strategies
4.1. Adoptive Cell Therapy (ACT)
4.2. Oncolytic Virotherapy (OVT)
4.3. DC-Based Vaccines
4.4. Other Immunotherapeutic Approaches
4.4.1. NK Cells
4.4.2. Mesenchymal Stem Cells (MSCs)
4.4.3. Signaling Regulation
4.4.4. Metabolism Regulation
5. Limitations and Challenges
5.1. Adoptive Cell Therapy (ACT)
5.2. Oncolytic Virotherapy (OVT)
5.3. DC-Based Vaccines
5.4. NK Cells
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 28 October 2023).
- Knutson, T.P.; Lange, C.A. Tracking Progesterone Receptor-Mediated Actions in Breast Cancer. Pharmacol. Ther. 2014, 142, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Penault-Llorca, F.; Viale, G. Pathological and Molecular Diagnosis of Triple-Negative Breast Cancer: A Clinical Perspective. Ann. Oncol. 2012, 23, vi19–vi22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-Negative Breast Cancer Has Worse Overall Survival and Cause-Specific Survival than Non-Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2016, 161, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, M.; Jiang, Z.; Wang, X. A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer. Transl. Oncol. 2018, 11, 311–329. [Google Scholar] [CrossRef]
- Anders, C.; Carey, L.A. Understanding and Treating Triple-Negative Breast Cancer. Oncology 2008, 22, 1233–1243. [Google Scholar] [PubMed]
- Uchimiak, K.; Badowska-Kozakiewicz, A.M.; Sobiborowicz-Sadowska, A.; Deptała, A. Current State of Knowledge on the Immune Checkpoint Inhibitors in Triple-Negative Breast Cancer Treatment: Approaches, Efficacy, and Challenges. Clin. Med. Insights Oncol. 2022, 16, 117955492210998. [Google Scholar] [CrossRef]
- Tarekegn, K.; Keskinkilic, M.; Kristoff, T.J.; Evans, S.T.; Kalinsky, K. The Role of Immune Checkpoint Inhibition in Triple Negative Breast Cancer. Expert Rev. Anticancer Ther. 2023, 23, 1095–1106. [Google Scholar] [CrossRef]
- Sacituzumab Govitecan +/− Pembrolizumab in Metastatic TNBC. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04468061 (accessed on 24 April 2024).
- Domchek, S.M.; Postel-Vinay, S.; Im, S.-A.; Park, Y.H.; Delord, J.-P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and Durvalumab in Patients with Germline BRCA-Mutated Metastatic Breast Cancer (MEDIOLA): An Open-Label, Multicentre, Phase 1/2, Basket Study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Ernst, B.; Anderson, K.S. Immunotherapy for the Treatment of Breast Cancer. Curr. Oncol. Rep. 2015, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Gandini, S.; Trapani, D.; Criscitiello, C.; Curigliano, G. Immunotherapy Addition to Neoadjuvant Chem-otherapy for Early Triple Negative Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Crit. Rev. Oncol./Hematol. 2021, 159, 103223. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in Cancer Immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and Drug Resistance: The Mechanistic Link and Clinical Implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Viale, G.; Curigliano, G. Recent Advances in Triple Negative Breast Cancer: The Immunotherapy Era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Flaum, L.; Helenowski, I.; Santa-Maria, C.A.; Jain, S.; Rademaker, A.; Nelson, V.; Tsarwhas, D.; Cristofanilli, M.; Gradishar, W. Phase II Study of Pembrolizumab and Capecitabine for Triple Negative and Hormone Receptor-Positive, HER2−Negative Endocrine-Refractory Metastatic Breast Cancer. J. Immunother. Cancer 2020, 8, e000173. [Google Scholar] [CrossRef] [PubMed]
- Ablett, M.P.; Singh, J.K.; Clarke, R.B. Stem Cells in Breast Tumours: Are They Ready for the Clinic? Eur. J. Cancer 2012, 48, 2104–2116. [Google Scholar] [CrossRef] [PubMed]
- Dhanota, N.; Bal, A.; Singh, G.; Arora, S.K. Evaluation of Breast Cancer Stem Cells in Human Primary Breast Carcinoma and Their Role in Aggressive Behavior of the Disease. J. Clin. Transl. Res. 2021, 7, 687–700. [Google Scholar] [PubMed]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect When Combined with Anti-CTLA-4 Anti-body. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Prat, A.; Baselga, J. The Role of Hormonal Therapy in the Management of Hormonal-Receptor-Positive Breast Cancer with Co-Expression of HER2. Nat. Clin. Pract. Oncol. 2008, 5, 531–542. [Google Scholar] [CrossRef]
- Jia, H.; Truica, C.I.; Wang, B.; Wang, Y.; Ren, X.; Harvey, H.A.; Song, J.; Yang, J.M. Immunotherapy for Triple-Negative Breast Cancer: Existing Challenges and Exciting Prospects. Drug Resist. Updates 2017, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Geurts, V.; Kok, M. Immunotherapy for Metastatic Triple Negative Breast Cancer: Current Paradigm and Future Ap-proaches. Curr. Treat. Options Oncol. 2023, 24, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Z.; Wang, L. Progress and Challenges of Immunotherapy Predictive Biomarkers for Triple Negative Breast Cancer in the Era of Single-Cell Multi-Omics. Life 2023, 13, 1189. [Google Scholar] [CrossRef] [PubMed]
- Eyler, C.E.; Rich, J.N. Survival of the Fittest: Cancer Stem Cells in Therapeutic Resistance and Angiogenesis. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the Cancer Stem Cells—What Challenges Do They Pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Dudás, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.-I. Therapy Resistance Mediated by Cancer Stem Cells. Semin. Cancer Biol. 2018, 53, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Maccalli, C.; Rasul, K.I.; Elawad, M.; Ferrone, S. The Role of Cancer Stem Cells in the Modulation of An-ti-Tumor Immune Responses. Semin. Cancer Biol. 2018, 53, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Laurenti, E.; Oser, G.; van der Wath, R.C.; Blanco-Bose, W.; Jaworski, M.; Offner, S.; Dunant, C.F.; Eshkind, L.; Bockamp, E.; et al. Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repair. Cell 2008, 135, 1118–1129. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Rosell, R. Cancer Stem Cells and Immunoresistance: Clinical Implications and Solutions. Transl. Lung Cancer Res. 2015, 4, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Rutella, S.; Ferrone, S.; Maccalli, C. Cancer Stem Cells: The Players of Immune Evasion from Immunotherapy. Cancer Stem Cell Resist. Target. Ther. 2019, 19, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2017, 24, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Farzaneh, M. Signaling Pathways Governing Breast Cancer Stem Cells Behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Lu, N. A New Role for the PI3K/Akt Signaling Pathway in the Epithelial-Mesenchymal Transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ben Ammar, R.; Al-Saeedi, F.J.; Elsayed Mohamed, M.; Islam, M.; Al-Ramadan, S.Y. Thidiazuron Decreases Epithelial-Mesenchymal Transition Activity through the NF-KB and PI3K/AKT Signalling Pathways in Breast Cancer. J. Cell. Mol. Med. 2020, 24, 14525–14538. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gogoi, G.; Saikia, S.; Sharma, A.; Kalita, D.; Sarma, A.; Limaye, A.M.; Gaur, M.; Bhattacharyya, J.; Jaganathan, B.G. BMP4 Enhances Anoikis Resistance and Chemoresistance of Breast Cancer Cells through Ca-nonical BMP Signaling. J. Cell Commun. Signal. 2021, 16, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Kalagasidou, S.; Pouliliou, S.; Anthoulaki, X.; Georgiou, N.; Papamanolis, V.; Fasoulakis, Z.N. The Notch Pathway in Breast Cancer Progression. Sci. World J. 2018, 2018, 2415489. [Google Scholar] [CrossRef] [PubMed]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; de Sampaio e Spohr, T.C.L. A Highlight on Sonic Hedgehog Pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, A.; McGarry, S.; Han, X.; Liu, S.; Wang, L. CSCs in Breast Cancer—One Size Does Not Fit All: Therapeutic Advances in Targeting Heterogeneous Epithelial and Mesenchymal CSCs. Cancers 2019, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, D.; Ma, W.; Gao, S.; Wen, G.; Zhong, J. Wnt Signaling in Triple-Negative Breast Cancers: Its Roles in Molecular Subtyping and Cancer Cell Stemness and Its Crosstalk with Non-Coding RNAs. Life Sci. 2022, 300, 120565. [Google Scholar] [CrossRef] [PubMed]
- Pupa, S.M.; Ligorio, F.; Cancila, V.; Franceschini, A.; Mannucci, P.M.; Vernieri, C.; Castagnoli, L. HER2 Signaling and Breast Cancer Stem Cells: The Bridge behind HER2-Positive Breast Cancer Aggressiveness and Therapy Refractoriness. Cancers 2021, 13, 4778. [Google Scholar] [CrossRef] [PubMed]
- WWang, L.; Zeng, J.; Master, R.P.; Maharjan, C.K.; Carelock, M.E.; Reccoppa, T.B.A.; Kim, M.; Kolb, R.; Zhang, W. Breast Cancer Stem Cells: Signaling Pathways, Cellular Interactions, and Therapeutic Implications. Cancers 2022, 14, 3287. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, N.; Yamaguchi, Y.; Tazawa, C.; Moriya, T.; Hirakawa, H.; Hayashi, S. Differences in Stemness Properties Associated with the Heterogeneity of Luminal-Type Breast Cancer. Clin. Breast Cancer 2015, 15, e93–e103. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast Cancer Stem Cells, Heterogeneity, Targeting Therapies and Therapeutic Implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Guo, Q.; Li, X.; Yang, X.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H.; Xing, Y.; Xi, T.; et al. MiR-873/PD-L1 Axis Regulates the Stemness of Breast Cancer Cells. EBioMedicine 2019, 41, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, L.; Cancila, V.; Cordoba-Romero, S.L.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.V.; Milani, M.; Volpari, T.; Chiodoni, C.; et al. WNT Signaling Modulates PD-L1 Expression in the Stem Cell Compartment of Triple-Negative Breast Cancer. Oncogene 2019, 38, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, M.; Wu, P.; Chen, C.; Xu, Z.P.; Gu, W. Increased PD-L1 Expression in Breast and Colon Cancer Stem Cells. Clin. Exp. Pharmacol. Physiol. 2017, 44, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.; Finotello, F.; Rieder, D.; Trajanoski, Z. Neoantigens Generated by Individual Mutations and Their Role in Cancer Immunity and Immunotherapy. Front. Immunol. 2017, 8, 1679. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non–Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Chowell, D.; Morris, L.G.T.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N.; et al. Patient HLA Class I Genotype Influences Cancer Response to Checkpoint Blockade Immunotherapy. Science 2018, 359, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Shirazi, M.; Banchereau, J.; Bell, D.; Burkeholder, S.; Kraus, E.T.; Davoust, J.; Palucka, K.A. Dendritic Cells Capture Killed Tumor Cells and Present Their Antigens to Elicit Tumor-Specific Immune Responses. J. Immunol. 2000, 165, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Nussenzweig, M.C. Dendritic Cells: Features and Functions. Immunol. Rev. 1980, 53, 127–147. [Google Scholar] [CrossRef]
- Lei, M.M.L.; Lee, T.K.W. Cancer Stem Cells: Emerging Key Players in Immune Evasion of Cancers. Front. Cell Dev. Biol. 2021, 9, 692940. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M. Transforming Growth Factor-β and Breast Cancer: Transforming Growth Factor-β/SMAD Signaling Defects and Cancer. Breast Cancer Res. 2000, 2, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-β Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Zhang, J.; Hu, Y.Y.; Najafabadi, A.H.; Moon, J.C.; Wicha, M.S.; Kaspo, B.; Whitfield, J.; Chang, A.E.; Li, Q. Efficacy of an ALDH Peptide-Based Dendritic Cell Vaccine Targeting Cancer Stem Cells. Cancer Immunol. Immunother. 2022, 71, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, S.J.; Park, Y.S.; Park, H.S.; Yang, K.M.; Heo, K. EpCAM Peptide-Primed Dendritic Cell Vaccination Confers Significant Anti-Tumor Immunity in Hepatocellular Carcinoma Cells. PLoS ONE 2018, 13, e0190638. [Google Scholar] [CrossRef] [PubMed]
- Sumransub, N.; Jirapongwattana, N.; Jamjuntra, P.; Thongchot, S.; Chieochansin, T.; Yenchitsomanus, P.; Thuwajit, P.; Warnnissorn, M.; O-Charoenrat, P.; Thuwajit, C. Breast Cancer Stem Cell RNA-Pulsed Dendritic Cells Enhance Tumor Cell Killing by Effector T-Cells. Oncol. Lett. 2020, 19, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Nnv, R.; Kundu, G.C. PO-285 Role of Tumour Associated Macrophages (TAMs) in Regulation of Cancer Stem Cell (CSCs) Enrichment in Breast Cancer. ESMO Open 2018, 3, A132. [Google Scholar] [CrossRef]
- Luo, S.; Yang, G.; Ye, P.; Cao, N.; Chi, X.; Yang, W.-H.; Yan, X. Macrophages Are a Double-Edged Sword: Molecular Crosstalk between Tumor-Associated Macrophages and Cancer Stem Cells. Biomolecules 2022, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Mousa, H.S.; Correnti, M.; Sica, A.; Invernizzi, P. Cancer Stem Cells and Tumor-Associated Macrophages: A Roadmap for Multitargeting Strategies. Oncogene 2015, 35, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK Cells Are Never Alone: Crosstalk and Communication in Tumour Microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Chen, M.; Smith, R.C.; Hagino, T.; Perez-Cunningham, J.; Sckisel, G.D.; Urayama, S.; et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J. Immunol. 2015, 195, 4010–4019. [Google Scholar] [CrossRef]
- Wu, B.; Shi, X.; Jiang, M.; Liu, H. Cross-Talk between Cancer Stem Cells and Immune Cells: Potential Therapeutic Targets in the Tumor Immune Microenvironment. Mol. Cancer 2023, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kim, H.J. NK Cells Lose Their Cytotoxicity Function against Cancer Stem Cell-Rich Radiotherapy-Resistant Breast Cancer Cell Populations. Int. J. Mol. Sci. 2021, 22, 9639. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef] [PubMed]
- Sipos, F.; Műzes, G. Cancer Stem Cell Relationship with Pro-Tumoral Inflammatory Microenvironment. Biomedicines 2023, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Nengroo, M.A.; Verma, A.; Datta, D. Cytokine Chemokine Network in Tumor Microenvironment: Impact on CSC Properties and Therapeutic Applications. Cytokine 2022, 156, 155916. [Google Scholar] [CrossRef]
- Chen, W.; Qin, Y.; Liu, S. Cytokines, Breast Cancer Stem Cells (BCSCs) and Chemoresistance. Clin. Transl. Med. 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Wang, S.E. Cytokines Driving Breast Cancer Stemness. Mol. Cell. Endocrinol. 2014, 382, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Maccalli, C.; De Maria, R. Cancer Stem Cells: Perspectives for Therapeutic Targeting. Cancer Immunol. Immunother. 2014, 64, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.T.; Mallet, F.; Gaugler, B.; Sainty, D.; Arnoulet, C.; Gastaut, J.A.; Olive, D. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000, 60, 4403–4411. [Google Scholar] [PubMed]
- Ishikawa, F.; Yoshida, S.; Saito, Y.; Hijikata, A.; Kitamura, H.; Tanaka, S.; Nakamura, R.; Tanaka, T.; Tomiyama, H.; Saito, N.; et al. Chemotherapy-Resistant Human AML Stem Cells Home to and Engraft within the Bone-Marrow Endosteal Region. Nat. Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, W.; Prins, A.; Ploemacher, R.; Wognum, B.; Wagemaker, G.; Lowenberg, B.; Wielenga, J. Long-Term Leukemia-Initiating Capacity of a CD34-Subpopulation of Acute Myeloid Leukemia. Blood 1996, 87, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological Hallmarks of Stromal Cells in the Tumour Microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the Tumor Microenvironment: Removing Obstruction to Anticancer Immune Responses and Immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Komorowski, M.P.; Seshadri, M.; Rokita, H.; McGray, A.J.R.; Opyrchal, M.; Odunsi, K.O.; Kozbor, D. CXCL12/CXCR4 Blockade by Oncolytic Virotherapy Inhibits Ovarian Cancer Growth by Decreasing Immunosuppression and Targeting Cancer-Initiating Cells. J. Immunol. 2014, 193, 5327–5337. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor Endothelium FasL Establishes a Selective Immune Barrier Promoting Tolerance in Tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, D.G.; Rycaj, K. Cancer Stem Cells: Regulation Programs, Immunological Properties and Immunotherapy. Semin. Cancer Biol. 2018, 52, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.X.; Kryczek, I.; Zhao, L.; Zhao, E.; Kuick, R.; Roh, M.H.; Vatan, L.; Szeliga, W.; Mao, Y.; Thomas, D.G.; et al. Myeloid-Derived Suppressor Cells Enhance Stemness of Cancer Cells by Inducing MicroRNA101 and Suppressing the Corepressor CtBP2. Immunity 2013, 39, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Di Tomaso, T.; Mazzoleni, S.; Wang, E.; Sovena, G.; Clavenna, D.; Franzin, A.; Mortini, P.; Ferrone, S.; Doglioni, C.; Marincola, F.M. Immunobiological Characterization of Cancer Stem Cells Isolated from Glioblastoma Patients. Clin. Cancer Res. 2010, 16, 800–813. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Niu, X.; Chen, X.; Li, J.; Zhou, M.; He, Y.; Le, Y.; Guo, K. Resistance of Leukemic Stem-like Cells in AML Cell Line KG1a to Natural Killer Cell-Mediated Cytotoxicity. Cancer Lett. 2012, 318, 173–179. [Google Scholar] [CrossRef]
- Paczulla, A.M.; Rothfelder, K.; Raffel, S.; Konantz, M.; Steinbacher, J.; Wang, H.; Tandler, C.; Mbarga, M.; Schaefer, T.; Falcone, M.; et al. Absence of NKG2D Ligands Defines Leukaemia Stem Cells and Mediates Their Immune Evasion. Nature 2019, 572, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Hagel, K.R.; Hawk, M.A.; Schafer, Z.T. Metabolism during ECM Detachment: Achilles Heel of Cancer Cells? Trends Cancer 2017, 3, 475–481. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, J.H.; Longmire, M.; Wang, H.; Kohrt, H.E.; Chang, H.Y.; Sunwoo, J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell–Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin. Cancer Res. 2016, 22, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Pal, S.K.; Reckamp, K.L.; Figlin, R.A.; Yu, H. STAT3: A Target to Enhance Antitumor Immune Response. Cancer Immunology and Immunotherapy. Curr. Top. Microbiol. Immunol. 2010, 344, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Almozyan, S.; Colak, D.; Mansour, F.; Alaiya, A.; Al-Harazi, O.; Qattan, A.; Al-Mohanna, F.; Al-Alwan, M.; Ghebeh, H. PD-L1 Promotes OCT4 and Nanog Expression in Breast Cancer Stem Cells by Sustaining PI3K/AKT Pathway Activation. Int. J. Cancer 2017, 141, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Codd, A.S.; Kanaseki, T.; Torigo, T.; Tabi, Z. Cancer Stem Cells as Targets for Immunotherapy. Immunology 2017, 153, 304–314. [Google Scholar] [CrossRef]
- Espinoza, I.; Miele, L. Deadly Crosstalk: Notch Signaling at the Intersection of EMT and Cancer Stem Cells. Cancer Lett. 2013, 341, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Okamoto, I.; Maehama, T.; Mimori, K.; Suzuki, A. Capturing the Mammalian Hippo: Elucidating Its Role in Cancer. Cancer Sci. 2013, 104, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Pelullo, M.; Zema, S.; Nardozza, F.; Checquolo, S.; Screpanti, I.; Bellavia, D. Wnt, Notch, and TGF-β Pathways Impinge on Hedgehog Signaling Complexity: An Open Window on Cancer. Front. Genet. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Das, B.; Lin, T.L.; Grimes, C.; Zhang, X.; Lavezzi, T.E.; Huang, L.; Cole, J.W.; Yau, L.C.; Li, L. A Rare Fraction of Drug-Resistant Follicular Lymphoma Cancer Stem Cells Interacts with Follicular Dendritic Cells to Maintain Tumourigenic Potential. Br. J. Haematol. 2012, 158, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, B.; Guan, C.; Wu, B.; Cai, C.; Wang, M.; Zhang, B.; Liu, T.; Yang, P. Foxp3+ IL-17+ T Cells Promote De-velopment of Cancer-Initiating Cells in Colorectal Cancer. J. Leukoc. Biol. 2010, 89, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Q.; Wang, Z.; Jiang, J.; Yu, S.-C.; Ping, Y.-F.; Yang, J.; Xu, S.-L.; Ye, X.-Z.; Xu, C.; et al. Metastatic Consequences of Immune Escape from NK Cell Cytotoxicity by Human Breast Cancer Stem Cells. Clin. Cancer Res. 2014, 74, 5746–5757. [Google Scholar] [CrossRef]
- Chen, H.C.; Joalland, N.; Bridgeman, J.; Alchami, F.S.; Jarry, U.; Khan, M.S.; Piggott, L.; Shanneik, Y.; Li, J.; Herold, M.J.; et al. Synergistic Targeting of Breast Cancer Stem-like Cells by Human γδ T Cells and CD8 + T Cells. Immunol. Cell Biol. 2017, 95, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Restifo, N.P. Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Narayanasamy, B.; Heo, J. Viruses as Nanomedicine for Cancer. Int. J. Nanomed. 2016, 11, 4835–4847. [Google Scholar] [CrossRef] [PubMed]
- Teitz-Tennenbaum, S.; Wicha, M.S.; Chang, A.E.; Li, Q. Targeting Cancer Stem Cells via Dendritic-Cell Vaccination. OncoImmunology 2012, 1, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Klampatsa, A.; Dimou, V.; Albelda, S.M. Mesothelin-Targeted CAR-T Cell Therapy for Solid Tumors. Expert. Opin. Biol. Ther. 2020, 21, 473–486. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Liu, W.; Li, X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. BioMed Res. Int. 2020, 2020, 4795171. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Qian, M.; Ho, M. The Role of Mesothelin in Tumor Progression and Targeted Therapy. Anti-Cancer Agents Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef]
- June, C.H.; Riddell, S.R.; Schumacher, T.N. Adoptive Cellular Therapy: A Race to the Finish Line. Sci. Transl. Med. 2015, 7, 280ps7. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.S.; Rosenberg, S.A. Exploiting the Curative Potential of Adoptive T-Cell Therapy for Cancer. Immunol. Rev. 2013, 257, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sun, B.; Dai, H.; Li, W.; Shi, L.; Zhang, P.; Li, S.; Zhao, X. T Cells Expressing NKG2D Chimeric Antigen Receptors Efficiently Eliminate Glioblastoma and Cancer Stem Cells. J. Immunother. Cancer 2019, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A.; Platten, M.; Lutz, S.Z.; Naumann, U.; Aulwurm, S.; Bischo, F.; Bühring, H.J.; Dichgans, J.; Rammensee, H.G.; Steinle, A.; et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003, 63, 8996–9006. [Google Scholar] [PubMed]
- Salih, H.R.; Antropius, H.; Gieseke, F.; Lutz, S.Z.; Kanz, L.; Rammensee, H.G.; Steinle, A. Functional Expression and Release of Ligands for the Activating Immunoreceptor NKG2D in Leukemia. Blood 2003, 102, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Salih, H.R.; Rammensee, H.-G.; Steinle, A. Cutting Edge: Down-Regulation of MICA on Human Tumors by Proteolytic Shedding. J. Immunol. 2002, 169, 4098–4102. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-Derived Soluble MIC Ligands Impair Expression of NKG2D and T-Cell Activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Prigione, I.; Airoldi, I.; Camoriano, M.; Levreri, I.; Gambini, C.; Pende, D.; Steinle, A.; Ferrone, S.; Pistoia, V. Downregulation And/or Release of NKG2D Ligands as Immune Evasion Strategy of Human Neuroblastoma. Neoplasia 2004, 6, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, S.; Stieber, P.; Peterfi, A.; Nagel, D.; Steinle, A.; Salih, H.R. Soluble MICA in Malignant Diseases. Int. J. Cancer 2006, 118, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Prasad, S.; Gaedicke, S.; Hettich, M.; Firat, E.; Niedermann, G. Patient-Derived Glioblastoma Stem Cells Are Killed by CD133-Specific CAR T Cells but Induce the T Cell Aging Marker CD57. Oncotarget 2014, 6, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, Y.; Ma, W.; Zhang, S.; Zhang, Y.-Q. Adoptive T-Cell Therapy of Prostate Cancer Targeting the Cancer Stem Cell Antigen EpCAM. BMC Immunol. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, Q.; Liu, S.; Ning, N.; Zhang, X.; Xu, Y.; Chang, A.E.; Wicha, M.S. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells 2015, 33, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Kisselbach, L.; Merges, M.; Bossie, A.; Boyd, A. CD90 Expression on Human Primary Cells and Elimination of Contaminating Fibroblasts from Cell Cultures. Cytotechnology 2009, 59, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.K.; Lam, C.T.; Poon, R.T.P.; Fan, S.T. Significance of CD90+ Cancer Stem Cells in Human Liver Cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Rege, T.A.; Hagood, J.S. Thy-1 as a Regulator of Cell-Cell and Cell-Matrix Interactions in Axon Regeneration, Apoptosis, Adhesion, Migration, Cancer, and Fibrosis. FASEB J. 2006, 20, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.E.; Esterly, K.; Awadallah, A.; Parrish, C.R.; Poynter, G.M.; Goltry, K.L. Clinical-Scale Expansion of a Mixed Population of Bone Marrow-Derived Stem and Progenitor Cells for Potential Use in Bone Tissue Regeneration. Stem Cells 2007, 25, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, C. Establishment, Characterization, and Long-Term Maintenance of Cultures of Human Fetal Hepatocytes. Hepatology 2003, 38, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.B.; Bruno, S.; Buttiglieri, S.; Tetta, C.; Gatti, S.; Deregibus, M.C.; Bussolati, B.; Camussi, G. Isolation and Characterization of a Stem Cell Population from Adult Human Liver. Stem Cells 2006, 24, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.Y.; Riehle, K.J.; Lazaro, C.; Teoh, N.; Haque, J.; Campbell, J.S.; Fausto, N. Isolation of Multipotent Progenitor Cells from Human Fetal Liver Capable of Differentiating into Liver and Mesenchymal Lineages. Proc. Natl. Acad. Sci. USA 2006, 103, 9912–9917. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.W.; Wang, X.; Diehn, M.; Shedden, K.; Chen, G.Y.; Sherlock, G.; Gurney, A.; Lewicki, J.; Clarke, M.F. Isolation and Molecular Characterization of Cancer Stem Cells in MMTV-Wnt-1Murine Breast Tumors. Stem Cells 2008, 26, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, J.; Jiang, Y.; Yu, C.; Ma, Z. Predictive Value of CD44 and CD24 for Prognosis and Chemotherapy Response in Invasive Breast Ductal Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 11287–11295. [Google Scholar] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Visus, C.; Wang, Y.; Lozano-Leon, A.; Ferris, R.L.; Silver, S.; Szczepanski, M.J.; Brand, R.E.; Ferrone, C.R.; Whiteside, T.L.; Ferrone, S.; et al. Targeting ALDH bright Human Carcinoma–Initiating Cells with ALDH1A1-Specific CD8+ T Cells. Clin. Cancer Res. 2011, 17, 6174–6184. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Baeuerle, P.A.; Gires, O. The Emerging Role of EpCAM in Cancer and Stem Cell Signaling. Cancer Res. 2009, 69, 5627–5629. [Google Scholar] [CrossRef]
- Gires, O.; Klein, C.A.; Baeuerle, P.A. On the Abundance of EpCAM on Cancer Stem Cells. Nat. Rev. Cancer 2009, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM Is Overexpressed in Breast Cancer and Is a Potential Target for Breast Cancer Gene Therapy. Cancer Res. 2004, 64, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- Gastl, G.; Spizzo, G.; Obrist, P.; Dünser, M.; Mikuz, G. Ep-CAM Overexpression in Breast Cancer as a Predictor of Survival. Lancet 2000, 356, 1981–1982. [Google Scholar] [CrossRef] [PubMed]
- Multi-4SCAR-T Therapy Targeting Breast Cancer. 2020. Available online: https://clinicaltrials.gov (accessed on 3 July 2023).
- EpCAM CAR-T for Treatment of Advanced Solid Tumors. 2016. Available online: https://clinicaltrials.gov (accessed on 3 July 2023).
- Chiocca, E.A.; Rabkin, S.D. Oncolytic Viruses and Their Application to Cancer Immunotherapy. Cancer Immunol. Res. 2014, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; McFadden, G. Viruses for Tumor Therapy. Cell Host Microbe 2014, 15, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Shafren, D.R.; Dorahy, D.J.; Ingham, R.A.; Burns, G.F.; Barry, R.D. Coxsackievirus A21 Binds to Decay-Accelerating Factor but Requires Intercellular Adhesion Molecule 1 for Cell Entry. J. Virol. 1997, 71, 4736–4743. [Google Scholar] [CrossRef]
- Gholami, S.; Chen, C.-H.; Lou, E.; Brot, M.D.; Fujisawa, S.; Chen, N.; Szalay, A.; Fong, Y. Vaccinia Virus GLV-1h153 Is Effective in Treating and Preventing Metastatic Triple-Negative Breast Cancer. Ann. Surg. 2012, 256, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Zeh, H.J.; Downs-Canner, S.; McCart, J.A.; Guo, Z.S.; Rao, U.N.M.; Ramalingam, L.; Thorne, S.H.; Jones, H.L.; Kalinski, P.; Wieckowski, E.; et al. First-In-Man Study of Western Reserve Strain Oncolytic Vaccinia Virus: Safety, Systemic Spread, and Antitumor Activity. Mol. Ther. 2015, 23, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, N.G.; Minev, B.R.; Szalay, A.A. Oncolytic Vaccinia Virus GLV-1h68 Strain Shows Enhanced Replication in Human Breast Cancer Stem-like Cells in Comparison to Breast Cancer Cells. J. Transl. Med. 2012, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, G.; Yuan, X.; Xu, M.; Wang, H.; Ji, J.; Konda, B.; Black, K.L.; Yu, J.S. Antigen-Specific T-Cell Response from Dendritic Cell Vaccination Using Cancer Stem-like Cell-Associated Antigens. Stem Cells 2009, 27, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Y.; Zhou, P.; Ma, H.; Zhao, X.; He, X.; Wang, T.; Zhang, J.; Liu, Y.; Zhang, T. Targeting CD133high Colorectal Cancer Cells in Vitro and in Vivo with an Asymmetric Bispecific Antibody. J. Immunother. 2015, 38, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.; Nguyen, S.; Pham, V.; Phan, N.; Nguyen, H.; Tran, C.; Nguyen, G. Targeting Specificity of Dendritic Cells on Breast Cancer Stem Cells: In Vitro and in Vivo Evaluations. OncoTargets Ther. 2015, 8, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Le, H.T.; Vu, B.T.; Pham, V.Q.; Le, P.M.; Phan, N.L.-C.; Trinh, N.V.; Nguyen, H.T.-L.; Nguyen, S.T.; Nguyen, T.L.; et al. Targeting Breast Cancer Stem Cells by Dendritic Cell Vaccination in Humanized Mice with Breast Tumor: Preliminary Results. OncoTargets Ther. 2016, 9, 4441–4451. [Google Scholar] [CrossRef] [PubMed]
- Safety Study of Cancer Stem Cell Vaccine to Treat Breast Cancer (CSC). 2014. Available online: https://clinicaltrials.gov (accessed on 3 July 2023).
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK Cell-Based Immunotherapy for Malignant Diseases. Cell. Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human Cancer Cells with Stem Cell-like Phenotype Exhibit Enhanced Sensitivity to the Cytotoxicity of IL-2 and IL-15 Activated Natural Killer Cells. Cell. Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Smith, R.A.; Monjazeb, A.M.; Chen, M.; Murphy, W.J. Enhanced Targeting of Stem-like Solid Tumor Cells with Radiation and Natural Killer Cells. Oncoimmunology 2015, 4, e1036212. [Google Scholar] [CrossRef] [PubMed]
- Uyttendaele, H.; Soriano, J.V.; Montesano, R.; Kitajewski, J. Notch4 and Wnt-1 Proteins Function to Regulate Branching Morphogenesis of Mammary Epithelial Cells in an Opposing Fashion. Dev. Biol. 1998, 196, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Vaughan, C.; Mal, A.; De, A.; McCarthy, J. The γ-Secretase Protease Complexes in Neurodegeneration. Cancer Immun. 2017, 47–87. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Paulson, A.; Charafe-Jauffret, E.; Ginestier, C.; Brown, M.; Dutcher, J.; Clouthier, S.G.; Wicha, M.S. Regulation of Mammary Stem/Progenitor Cells by PTEN/Akt/β-Catenin Signaling. PLoS Biol. 2009, 7, e1000121. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Cam-pos-Olivas, R.; Graña, O.; et al. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.; Noto, A.; Pisanu, M.E.; De Vitis, C.; Maugeri-Saccà, M.; Ciliberto, G. Metabolic Features of Cancer Stem Cells: The Emerging Role of Lipid Metabolism. Oncogene 2018, 37, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Maggiolini, M.; Tanowitz, H.B.; Sotgia, F.; Lisanti, M.P. Targeting Hypoxic Cancer Stem Cells (CSCs) with Doxycycline: Implications for Optimizing Anti-Angiogenic Therapy. Oncotarget 2017, 8, 56126–56142. [Google Scholar] [CrossRef] [PubMed]
- El Hout, M.; Dos Santos, L.; Hamaï, A.; Mehrpour, M. A Promising New Approach to Cancer Therapy: Targeting Iron Metabolism in Cancer Stem Cells. Semin. Cancer Biol. 2018, 53, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Berkenblit, A. Antibody–Drug Conjugates for Cancer Treatment. Annu. Rev. Med. 2018, 69, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in Cancer: Cancer Stem Cells versus Clonal Evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef]
- Corbeil, D.; Marzesco, A.M.; Wilsch-Bräuninger, M.; Huttner, W.B. The Intriguing Links between Prominin-1 (CD133), Cholesterol-Based Membrane Microdomains, Remodeling of Apical Plasma Membrane Protrusions, Extracellular Mem-brane Particles, and (Neuro)Epithelial Cell Differentiation. FEBS Lett. 2010, 584, 1659–1664. [Google Scholar] [CrossRef]
- Kastan, M.; Schlaffer, E.; Russo, J.; Colvin, O.; Civin, C.; Hilton, J. Direct Demonstration of Elevated Aldehyde Dehy-drogenase in Human Hematopoietic Progenitor Cells. Blood 1990, 75, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, S.; Rahbarizadeh, F.; Ahmadvand, D.; Rasaee, M.J.; Pognonec, P. A Caspase 8-Based Suicide Switch Induces Apoptosis in Nanobody-Directed Chimeric Receptor Expressing T Cells. Int. J. Hematol. 2012, 95, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.E.; Berger, C.; Lin, Y.; Wang, J.; Lin, X.; Frayo, S.E.; Brouns, S.A.; Spencer, D.M.; Till, B.G.; Jensen, M.C.; et al. Combining a CD20 Chimeric Antigen Receptor and an Inducible Caspase 9 Suicide Switch to Improve the Efficacy and Safety of T Cell Adoptive Immunotherapy for Lymphoma. PLoS ONE 2013, 8, e82742. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ma, J.S.Y.; Yun, H.; Cao, Y.; Kim, J.Y.; Chi, V.; Wang, D.; Woods, A.; Sherwood, L.; Caballero, D.; et al. Redirection of Genetically Engineered CAR-T Cells Using Bifunctional Small Molecules. J. Am. Chem. Soc. 2015, 137, 2832–2835. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Roybal, K.T.; Puchner, E.M.; Onuffer, J.; Lim, W.A. Remote Control of Therapeutic T Cells through a Small Molecule–Gated Chimeric Receptor. Science 2015, 350, aab4077. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Morton, J.J.; Bird, G.; Refaeli, Y.; Jimeno, A. Humanized Mouse Xenograft Models: Narrowing the Tumor–Microenvironment Gap. Cancer Res. 2016, 76, 6153–6158. [Google Scholar] [CrossRef]
- Santisteban, M.; Solans, B.P.; Hato, L.; Urrizola, A.; Mejías, L.D.; Salgado, E.; Sánchez-Bayona, R.; Toledo, E.; Rodríguez-Spiteri, N.; Olartecoechea, B.; et al. Final Results Regarding the Addition of Dendritic Cell Vaccines to Neoadjuvant Chemotherapy in Early HER2-Negative Breast Cancer Patients: Clinical and Translational Analysis. Ther. Adv. Med. Oncol. 2021, 13, 17588359211064653. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. Cancer Testis Antigens as Immunogenic and Oncogenic Targets in Breast Cancer. Immunotherapy 2018, 10, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Gluck, W.L.; Hurst, D.; Yuen, A.; Levine, A.M.; Dayton, M.A.; Gockerman, J.P.; Lucas, J.; Denis-Mize, K.; Tong, B.; Navis, D.; et al. Phase I Studies of Interleukin (IL)-2 and Rituximab in B-Cell Non-Hodgkin’s Lymphoma. Clin. Cancer Res. 2004, 10, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Witzig, T.E.; Kurtin, P.J.; Sloan, J.A.; Jelinek, D.F.; Howell, K.G.; Markovic, S.N.; Habermann, T.M.; Klee, G.G.; Atherton, P.J.; et al. Phase 1 Study of Interleukin-12 in Combination with Rituximab in Patients with B-Cell Non-Hodgkin Lymphoma. Blood 2002, 99, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gennari, R. Pilot Study of the Mechanism of Action of Preoperative Trastuzumab in Patients with Primary Operable Breast Tumors Overexpressing HER2. Clin. Cancer Res. 2004, 10, 5650–5655. [Google Scholar] [CrossRef] [PubMed]
| Immunotherapy | Intervention | Therapeutic Effect |
|---|---|---|
| Cytokine-induced killer (CIK) cells, NK cells, CD8+ T cells, and γδ T-cells | Targeting CSCs | Ideal for BCSCs [90]; promotes specific elimination [91] |
| DC-based vaccines | Targeting CSCs | Specifically targets BCSCs [93]; promotes specific elimination |
| Adoptive T-cell therapy | TIL isolation, culturing, and reinfusion | Enhances antitumor immunity through T-cell activation [92] |
| Oncolytic virotherapy (OVT) | Immunogenic cell death and T-cell activation | Induces antitumor immunity via immunogenic cell death [92] |
| Combination with other immunotherapies | Various immunotherapies combined | Synergistic effect utilizing oncolytic viruses, DC-based vaccines, and checkpoint blockades |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiou, M.; Diamantoudis, S.C.; Tsianava, C.; Nguyen, N.P. Immunotherapeutic Strategies Targeting Breast Cancer Stem Cells. Curr. Oncol. 2024, 31, 3040-3063. https://doi.org/10.3390/curroncol31060232
Vasileiou M, Diamantoudis SC, Tsianava C, Nguyen NP. Immunotherapeutic Strategies Targeting Breast Cancer Stem Cells. Current Oncology. 2024; 31(6):3040-3063. https://doi.org/10.3390/curroncol31060232
Chicago/Turabian StyleVasileiou, Maria, Sotirios Charalampos Diamantoudis, Christina Tsianava, and Nam P. Nguyen. 2024. "Immunotherapeutic Strategies Targeting Breast Cancer Stem Cells" Current Oncology 31, no. 6: 3040-3063. https://doi.org/10.3390/curroncol31060232
APA StyleVasileiou, M., Diamantoudis, S. C., Tsianava, C., & Nguyen, N. P. (2024). Immunotherapeutic Strategies Targeting Breast Cancer Stem Cells. Current Oncology, 31(6), 3040-3063. https://doi.org/10.3390/curroncol31060232





