Abstract
Women with left-sided breast cancer receiving adjuvant radiotherapy have increased incidence of cardiac mortality due to ischemic heart disease; to date, no threshold dose for late cardiac/pulmonary morbidity or mortality has been established. We investigated the likelihood of cardiac death and radiation pneumonitis in women with left-sided breast cancer who received comprehensive lymph node irradiation. The differences in dosimetric parameters between free-breathing (FB) and deep inspiration breath hold (DIBH) techniques were also addressed. Based on NTCP calculations, the probability of cardiac death was significantly reduced with the DIBH compared to the FB technique (p < 0.001). The risk of radiation pneumonitis was not clinically significant. There was no difference in coverage between FB and DIBH plans. Doses to healthy structures were significantly lower in DIBH plan than in FB plan for V20, V30, and ipsilateral total lung volume. Inspiratory gating reduces the dose absorbed by the heart without compromising the target range, thus reducing the likelihood of cardiac death.
1. Introduction
Breast cancer (BC) is one of the leading causes of death in women worldwide [1]. When indicated either after conservative breast surgery or after mastectomy, radiation therapy improves local control, disease-free, and overall survival by preventing local recurrence and metastatic disease [2]. Currently, the multimodality approach used to treat BC has been associated with elevated cardiac mortality since anthracycline-based chemotherapy, ERBB2 antagonists, endocrine therapy, and breast irradiation are linked to an elevated risk of lung disease, coronary artery disease, and a broad spectrum of cardiac anomalies like cardiac ischemia, heart failure, valvular heart disease, QT interval prolongation, and arrhythmias [3]. Cardiac-protective radiotherapy techniques like deep inspiration breath hold (DIBH) have evolved through time to decrease the incidence of cardiac death and coronary events, therefore allowing moderate hypofractionation and ultra-short radiotherapy schemes as a more convenient technique for patients and health institutions [4].
To date, no accurate threshold doses for late cardiac/lung morbidity or mortality have been established. The close interaction between radiobiological response models and clinical practice has yielded some evaluation tools, such as tumor control probability (TCP) and normal tissue complication probability (NTCP) models, which approximate the clinically observed treatment outcomes and complications [5]. The two best-known models are the Lyman Kutcher Burman and the relative seriality model [6,7]. The present study intended to analyze the radiobiological implication, in the form of TCP and NTCP, of the dose difference resulting from deep inspiration breath hold (DIBH) and free-breathing (FB) techniques on cardiac and lung doses during left-sided breast irradiation since the benefits of this technique as showed by radiobiological models are lacking in the medical literature.
2. Materials and Methods
After institutional ethical committee approval (HEM-2977-19-20-1), a sample of 22 patients referred and treated with adjuvant radiotherapy between 2015 and 2019 was randomly selected. Women aged over 18 years with left-breast-sided cancer, a histopathological diagnosis of breast carcinoma, and postoperative indication for adjuvant radiotherapy to chest wall/breast and regional nodes, including internal mammary chain, who also signed an informed consent form for both voluntary DIBH and FB planning computed tomography (CT) scan simulations were included.
2.1. Simulation Procedure
As a routine procedure in the Radiation Oncology and Medical Physics Department, one week before the virtual CT simulation is performed, patients undergoing respiratory gating techniques are instructed to practice deep inspiration and breath-hold for 10 to 20 s at home. The RPM respiratory gating system (Varian Medical Systems, Palo Alto, California, USA) was used for breathing tracking. The curves generated by deep inspiration breath hold and gating window in the CT scanning were recorded and used as references for daily treatment delivery. A 16-slice CT simulation for all 22 patients was performed in the supine position using a standard breast board in free-breathing (FB) and DIBH covering the volume from the mid-neck to the upper abdomen during FB and DIBH and a slice thickness of 2.5 mm.
2.2. Contouring Process
For locoregional treatment after lumpectomy, the planning target volume (PTV) was the clinical limit of the breast, ipsilateral axillary lymph nodes level I–III, supraclavicular and infraclavicular fossa, and internal mammary chain. For postmastectomy radiotherapy, the PTV was defined as the part of the thoracic wall where the breast had been located and, using the contralateral breast as a reference, the ipsilateral axillary lymph nodes level I–III, supraclavicular and infraclavicular fossa, and internal mammary chain. The delineated organs at risk (OARs) were the heart, left anterior descending coronary artery (LAD), both lungs (contoured separately), thyroid, and right breast. The radiation oncologist manually delineated the heart and LAD, whereas the treatment planning system automatically delineated the lungs (Eclipse, Version 16.0, Varian Medical Systems, Palo Alto, California, USA). The heart was defined as the entire myocardium and pericardium, starting superiorly at the beginning of the pulmonary trunk and aorta. LAD was delineated from the exit of the left coronary artery from the aorta, continuing in the anterior interventricular sulcus as visualized. Regarding the treatment beams’ arrangement, identical plans were created in the DIBH and FB CT scans.
2.3. Radiotherapy Planning and Dosimetric Evaluation
The mono isocentric technique with supraclavicular, nondivergent tangential fields and compensating fields (field-in-field technique) was used on both CTs. We prescribed 50 Gy in 25 fractions to the mean PTV using 6, 10, or 15 MV photons. Patients with breast-conserving surgery requiring a boost to the surgical bed received a total dose of 10 Gy in 5 fractions. Dose coverage of the PTV was prioritized over the absorbed dose to the OARs and, for risk calculation, dose–volume histograms (DVHs) were revised. A TrueBeam V2.5 linear accelerator was utilized for radiation therapy and the calculation algorithm used was the Anisotropic Analytic Algorithm (AAA) version 16.1 [8].
Dosimetric goals of the treatment plans were that 95% of the PTV volume should be covered by 95% of the prescribed dose (V95%, PTV = 95%) and an acceptable value of 90% of the PTV volume should be covered by 90% of the prescribed dose (V90%, PTV = 90%). The maximum doses represented by the doses received by 2% of the target volumes (D2%), the minimum doses represented by 98% of the target volumes (D98%), and the mean doses were reported. At the same time, the absorbed dose of the OARs was kept as low as possible. The plans’ target conformity degrees were evaluated using the conformity index. The target dose homogeneity (HI) was expressed in terms of the ratio (D2%-D98%)/D50%, where D50% was the minimum dose represented by 50% of the target volumes [9]. The parameters obtained from the DVHs were mean and maximum dose to the left lung and relative volumes of the left lung receiving 5 Gy (V5), 10 Gy (V10), 20 Gy (V20), and 30 Gy (V30). Additional parameters were mean and maximum dose to the heart, relative volumes of the heart receiving at least 5 Gy (V5), 10 Gy (V10), 15 Gy (V15), 20 Gy (V20), 25 Gy (V25), 30 Gy (V30), 40 Gy (V40), and 50 Gy (V50). In addition, we also used mean and maximum dose to the LAD, and relative volumes of LAD receiving 5 Gy (V5), 10 Gy (V10), and 25 Gy (V25) [10].
2.4. Radiobiological Assessment
The software used for TCP and NTCP calculations was the Biological Evaluation Application™ V.1.6.1.4, developed by RaySearch Laboratories, Stockholm, Sweden. This software evaluates the estimated probabilities of a clinical outcome in terms of TCP Poisson-LQ for targets and NTCP Poisson-LQ for the OARs. First, each homogeneous dose level of the differential DVHs was converted to a total dose delivered in 2 Gy fractions with the biologically effective dose method, with α/β = 4.6 Gy [11]. Next, the TCP was calculated using the Poisson model for inhomogeneous dose distribution [12]. The NTCP for cardiac death risk and radiation pneumonitis were calculated using the relative seriality model [13]. Input data for the NTCP calculations with cardiac death risk as an endpoint were taken from Gagliardi et al. for the entire heart volume [14]. For radiation pneumonitis, input data were taken from the work published by Gagliardi et al. and then corrected for the AAA by the use of algorithm-specific NTCP parameters determined by Hedin et al. [15,16]. For input data see Table 1.

Table 1.
Input parameters used in the biological evaluation application software.
2.5. Statistical Analysis
The Wilcoxon signed-rank test was used for continuous variables to evaluate FB vs. DIBH within each cohort. Values of p > 0.01 were considered statistically significant. The analysis was performed using STATA Version 18.
3. Results
This study analyzed several dose parameters of the target volume, left lung, heart, and LAD using two different breathing methods. All patients in our cohort could undergo the training required to use the DIBH technique. The patients’ median age in our cohort was 50 years (range 35 to 80 years), with 63.6% being postmenopausal by the time of adjuvant radiotherapy (see Table 2).

Table 2.
Baseline demographics for left-sided breast cancer patients in the study.
3.1. Plan Comparison and DVH Evaluation
All dosimetry results are shown in Table 3. There was no difference in target coverage for all patients between FB and DIBH plans. Dose to normal structures was significantly lower in DIBH plans than in the FB plans for V20 and V30. Of note is that the total ipsilateral lung volume is bigger in DIBH plans. The largest dose reductions were seen for the heart and LAD. Overall, DIBH reduced Dmean to the heart by 55.8% and the LAD by 73.3% compared to the FB plan. When DIBH was compared to FB, Dmean was 2.4 Gy versus 4.3 Gy for the heart and 6.2 Gy vs. 23.3 Gy for the LAD (p < 0.001), respectively. Equally significant were differences in V5, V10, V15, V20, V25, V30, V40, and V50 for the heart and V5, V10, and V25 for the LAD (p < 0.001).

Table 3.
Treatment planning data for target and organs at risk for FB and enhanced DIBH for locoregional treatment presented as median values with standard deviation and p-values for paired Wilcoxon tests.
3.2. TCP and NTCP Evaluation
Figure 1 illustrates the DVHs for FB and DIBH radiation techniques. Figure 2a,b compare the TCPs and NTCPs for target volume and healthy tissues, respectively, among techniques. The TCP values for the chest wall/breast and supraclavicular, axillary, and internal mammary nodes were 91.2% in FB and 93.5% in DIBH (p = 0.006). Based on NTCP calculations, the risk of cardiac death was significantly decreased for DIBH compared to FB (1 vs. 0.45), suggesting that using DIBH for patients with left-sided breast cancer and locoregional treatment is beneficial (p < 0.001). The risk of radiation pneumonitis did not achieve clinical significance for FB and DIBH techniques (1.2 vs. 1.1, p = 0.237; Table 4).
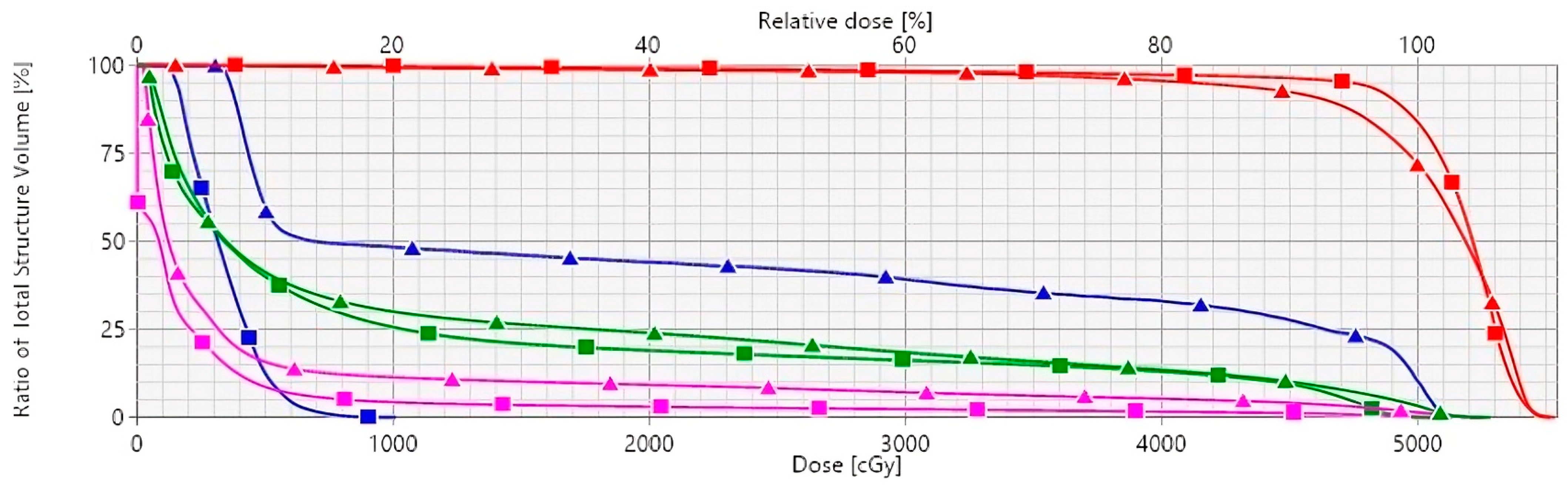
Figure 1.
Mean dose–volume histograms comparing DIBH (square lines) and FB (triangle lines) for LAD (blue), heart (magenta), ipsilateral lung (green), and PTV (red). Abbreviations: DIBH, deep inspiration breath hold; FB, free-breathing; LAD, left anterior descending coronary artery; PTV, planning target volume.
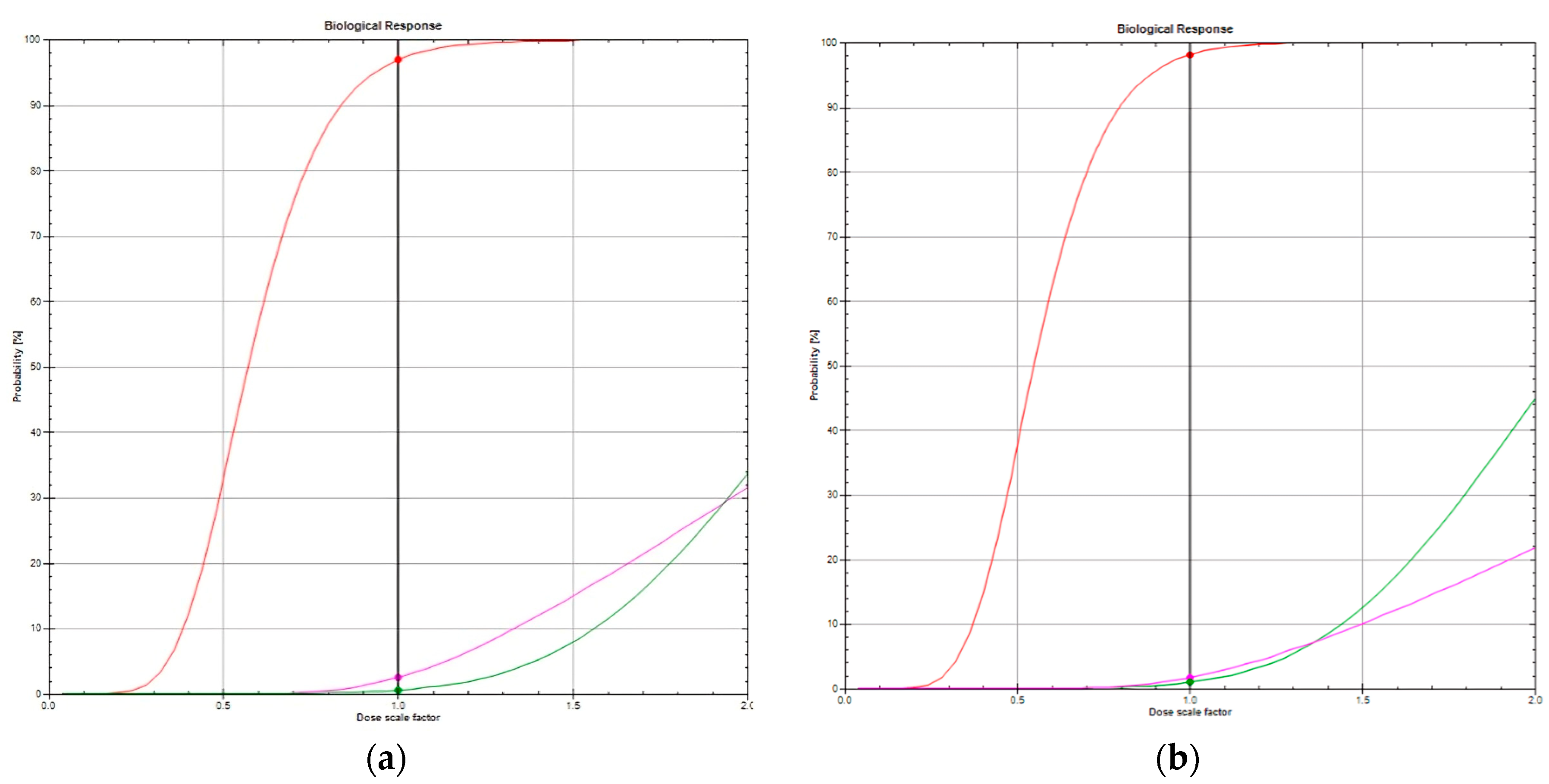
Figure 2.
(a) TCP of 96.9% (red line), risk of cardiac death of 2.5% (magenta), risk of radiation pneumonitis of 0.55% (green) in a patient with FB technique. (b) TCP of 98.2% (red line), risk of cardiac death of 1.8% mMagenta), risk of radiation pneumonitis of 0.98% (green) in the same patient irradiated with DIBH technique. Abbreviations: DIBH, deep inspiration breath hold; FB, free-breathing; TCP, tumor control probability.

Table 4.
Tumor control probability, cardiac death risk, risk of radiation pneumonitis in percent for locoregional treatment, presented as median values, range in brackets and p-values for paired Wilcoxon tests.
4. Discussion
Breast cancer is one of the most frequent tumors in women worldwide. Even when survival for patients with this disease is longer due in part to the use of novel systemic drugs, advances in surgical approaches, and novel radiotherapy techniques, physicians are now dealing with the long-term effects of cancer treatments for which they have to be aware [16]. Physicians caring for cancer survivors have expressed concern over the potential heart and lung toxicities caused by breast radiation therapy, in addition to the side effects of chemotherapy and endocrine therapy. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) published one of the earliest reports on the cardiopulmonary toxicity associated with breast radiotherapy in a meta-analysis. The report demonstrated a hazard ratio of 1.27 for heart disease, although it included studies that used outdated radiation techniques [17]. Women with left-breast cancer receiving radiotherapy have an increased incidence of major coronary events compared to women with right-sided cancer due to ischemic heart disease, with 7.4% per Gy of radiation received to the heart [18]. In addition to primarily mild side effects, including erythema, edema, and inflammation, that happen in the short- and mid-term, clinicians must be aware of long-term effects on the heart and lungs since most patients with BC reach long survival rates, thus embracing the long-term consequences of systemic therapies and radiotherapy [19]. Another adverse effect contributing to decreased quality of life in patients receiving thoracic radiotherapy is radiation-induced lung toxicity ultimately causing lung fibrosis [20]. The CANTO-RT trial was a prospective longitudinal cohort that recorded adverse events in patients receiving breast radiotherapy. With a 60-month follow-up, the incidence of radiation-induced lung toxicity was about 2.4% for the entire group, with pulmonary medical history, chemotherapy use, and nodal irradiation being risk factors for its occurrence [21].
Since then, much has been done to lower the risk of cardiac and lung toxicity in patients receiving radiotherapy for left-breast cancer, and the breath hold technique is one of the available methods developed to lessen this risk. A recent study published by Mahmoud et al. compared the incidence of cardiac events in patients with breast cancer who received FB (free-breathing) vs. DIBH (deep inspiration breath hold) techniques in a large retrospective cohort. Although the incidence of cardiac events was higher in the FB arm than in the DIBH arm, it did not reach statistical significance. However, the study found that having hypertension, smoking, and a high heart mean dose were independent risk factors for the occurrence of cardiac events [22]. Our study evaluated the dosimetric parameters of DIBH compared to the FB RT technique, finding a notable reduction in heart and lung volumes for DIBH. Therefore, we showed that DIBH not only reduces the Dmean and Dmax to the heart and LAD but also the risk of lung and heart toxicity using radiobiological models.
TCP and NTCP models have been developed to determine the success rate of a given RT treatment while minimizing the risks of tissue toxicity. These models combine clinical outcomes with dosimetric information regarding dose–volume histograms (DVHs). Mathematical calculations are used to derive model parameters that factor in clinical outcomes to estimate the risk of tumor relapse or toxicity. Both models condense all patient dosimetric data into DVHs, which may limit their descriptive and predictive power [23]. The study conducted by Utehina et al. highlights the potential benefits of using respiratory-gated techniques in postoperative radiation therapy for early-stage left-sided breast cancer. The results indicate that the use of respiratory-gated techniques significantly reduces the risks of pneumonitis and cardiac mortality as compared to the control group. This is a significant finding that could have a positive impact on the clinical management of breast cancer patients. The risks of pneumonitis and cardiac mortality were reduced from 0.6% to 0.3% and from 1.3% to 0.2%, respectively. This study underscores the importance of respiratory-gated techniques in reducing the risk of side effects in patients undergoing radiation therapy for breast cancer [24].
This research delves into the most suitable radiation therapy techniques for treating left-sided breast cancer patients undergoing nodal irradiation. This study evaluated two techniques frequently employed in radiation therapy and provided valuable insights. By analyzing the differences in doses and volumes of the heart and lungs during radiation therapy, both radiation techniques helped to identify the most suitable breathing technique. This study’s key finding is that the TCPs and NTCPs varied between the two techniques, with the TCP of the DIBH being higher than the FB plans (2.3%). The risk of cardiac mortality is significantly reduced when DIBH is used. This research shows that DIBH is the preferred technique for patients with left-sided breast cancer when regional nodal irradiation is necessary, as it reduces the median cardiac death risk by 0.55 percentage points. The results of this study are significant, as they guide physicians and medical practitioners on the most suitable RT techniques for treating patients with left-sided breast cancer undergoing nodal irradiation. However, it is essential to note that the retrospective nature of this publication and its sample size may be two of the main limitations, suggesting the need for further investigation in this area.
Biological models can be valuable in clinical applications as they can predict radiation response in patients. However, these predictions have some limitations due to the uncertainty of the model parameters involved. NTCP models are often used in comparative planning studies, as they provide a correct qualitative description of the radiation response, and only the ranking of NTCP values is considered. However, this type of application has a significant drawback: the uncertainty of the predictions about the model parameters is not specified quantitatively, raising questions about the significance of differences in NTCP values for different treatment plans or techniques. TCP models face similar limitations, as parameters such as proliferation, oxygenation, and angiogenesis are much more varied for tumors than for normal tissues, and may change during radiotherapy, making the clinical application of TCP models challenging. Nonetheless, the models can still help improve the understanding of tumor response to radiation and its interaction with other influencing factors [25,26].
5. Conclusions
The enhanced inspiration-gating technique, a significant advancement in our research, has proven to significantly decrease the absorbed radiotherapy dose to the heart and LAD coronary artery without compromising the target coverage. This success story has resulted in a decreased cardiac mortality probability, a promising outcome. However, the reduction of the risk of pneumonitis between FB and DIBH could not be demonstrated. Several biological models have been developed. Although these models correctly describe the main characteristics of the radiation response, great caution must be taken if these models are to be applied to patients.
Author Contributions
C.H.F.-B. and D.M.U.-A. contributed equally to conceptualization, methodology, software, formal analysis, investigation, resources, data curation, writing—original draft preparation, review and editing, visualization, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán with the protocol code N°2019/2977 in the year 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The corresponding author can provide the database to the reader upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wheeler, S.B.; Rocque, G.; Basch, E. Benefits of Breast Cancer Screening and Treatment on Mortality. JAMA 2024, 331, 199–200. [Google Scholar] [CrossRef]
- Kolářová, I.; Melichar, B.; Sirák, I.; Vaňásek, J.; Petera, J.; Horáčková, K.; Pohanková, D.; Ďatelinka, F.; Šinkorová, Z.; Vošmik, M. The Role of Adjuvant Radiotherapy in the Treatment of Breast Cancer. Curr. Oncol. 2024, 31, 1207–1220. [Google Scholar] [CrossRef]
- Stefan, M.F.; Herghelegiu, C.G.; Magda, S.L. Accelerated Atherosclerosis and Cardiovascular Toxicity Induced by Radiotherapy in Breast Cancer. Life 2023, 13, 1631. [Google Scholar] [CrossRef]
- Stowe, H.B.; Andruska, N.D.; Reynoso, F.; Thomas, M.; Bergom, C. Heart Sparing Radiotherapy Techniques in Breast Cancer: A Focus on Deep Inspiration Breath Hold. Breast Cancer Targets Ther. 2022, 14, 175–186. [Google Scholar] [CrossRef]
- Fletcher, G.H. Keynote address: The scientific basis of the present and future practice of clinical radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1983, 9, 1073–1082. [Google Scholar] [CrossRef]
- Lyman, J.T. Complication probability as assessed from dose-volume histograms. Radiat. Res. 1985, 8, S13–S19. [Google Scholar] [CrossRef]
- Källman, P.; Agren, A.; Brahme, A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int. J. Radiat. Biol. 1992, 62, 249–262. [Google Scholar] [CrossRef]
- Edvardsson, A.; Nilsson, M.P.; Amptoulach, S.; Ceberg, S. Comparison of doses and NTCP to risk organs with enhanced inspiration gating and free breathing for left-sided breast cancer radiotherapy using the AAA algorithm. Radiat. Oncol. 2015, 10, 84. [Google Scholar] [CrossRef]
- Ko, H.; Chang, J.S.; Moon, J.Y.; Lee, W.H.; Shah, C.; Shim, J.S.A.; Han, M.C.; Baek, J.G.; Park, R.H.; Kim, Y.B.; et al. Dosimetric Comparison of Radiation Techniques for Comprehensive Regional Nodal Radiation Therapy for Left-Sided Breast Cancer: A Treatment Planning Study. Front. Oncol. 2021, 11, 645328. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Li, X.A. Lyman-Kutcher-Burman NTCP model parameters for radiation pneumonitis and xerostomia based on combined analysis of published clinical data. Phys. Med. Biol. 2008, 53, 737–755. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Warkentin, B.; Stavrev, P.; Stavreva, N.; Field, C.; Fallone, B.G. A TCP-NTCP estimation module using DVHs and known radiobiological models and parameter sets. J. Appl. Clin. Med. Phys. 2004, 5, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Morgan, D.; Niemierko, A.; Suit, H.D. Radiation dose-response of human tumors. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1227–1237. [Google Scholar] [CrossRef]
- Gagliardi, G.; Lax, I.; Ottolenghi, A.; Rutqvist, L.E. Long-term cardiac mortality after radiotherapy of breast cancer—Application of the relative seriality model. Br. J. Radiol. 1996, 69, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Hedin, E.; Bäck, A. Influence of different dose calculation algorithms on the estimate of NTCP for lung complications. J. Appl. Clin. Med. Phys. 2013, 14, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, D.; Arnold, C.; Neuman, D.; Fink, K.; Popovscaia, M.; Kvitsaridze, I.; Nevinny-Stickel, M.; Glatzer, M.; Lukas, P.; Seppi, T. Occurrence of pneumonitis following radiotherapy of breast cancer—A prospective study. Häufigkeit einer Pneumonitis nach Bestrahlung bei Brustkrebs –Eine prospektive Studie. Strahlenther. Onkol. 2018, 194, 520–532. [Google Scholar] [CrossRef]
- Terry, M.B.; Colditz, G.A. Epidemiology and Risk Factors for Breast Cancer: 21st Century Advances, Gaps to Address through Interdisciplinary Science. Cold Spring Harb. Perspect. Med. 2023, 13, a041317. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Henson, K.E.; McGale, P.; Darby, S.C.; Parkin, M.; Wang, Y.; Taylor, C.W. Cardiac mortality after radiotherapy, chemotherapy and endocrine therapy for breast cancer: Cohort study of 2 million women from 57 cancer registries in 22 countries. Int. J. Cancer 2020, 147, 1437–1449. [Google Scholar] [CrossRef]
- Käsmann, L.; Dietrich, A.; Staab-Weijnitz, C.A.; Manapov, F.; Behr, J.; Rimner, A.; Jeremic, B.; Senan, S.; De Ruysscher, D.; Lauber, K.; et al. Radiation-induced lung toxicity—Cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat. Oncol. 2020, 15, 214. [Google Scholar] [CrossRef]
- Gueiderikh, A.; Sarrade, T.; Kirova, Y.; De La Lande, B.; De Vathaire, F.; Auzac, G.; Martin, A.L.; Everhard, S.; Meillan, N.; Bourgier, C.; et al. Radiation-induced lung injury after breast cancer treatment: Incidence in the CANTO-RT cohort and associated clinical and dosimetric risk factors. Front. Oncol. 2023, 13, 1199043. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; Sadaka, E.A.; Abouegylah, M.; Amin, S.A.; Elmansy, H.; Asal, M.F.; Köksal, M.A.; Gawish, A. Impact of breath-hold technique on incidence of cardiac events in adjuvant left breast cancer irradiation. J. Cancer Res. Clin. Oncol. 2023, 149, 5853–5859. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, F.; Nahum, A.; Cella, L. Increasing the power of tumour control and normal tissue complication probability modelling in radiotherapy: Recent trends and current issues. Transl. Cancer Res. 2017, 6 (Suppl. 5), S807–S821. [Google Scholar] [CrossRef]
- Utehina, O.; Popovs, S.; Purina, D.; Slosberga, I.; Vevere, I.; Emzins, D.; Berzins, J.; Valuckas, K.P.; Janulionis, E.; Miller, A. Analysis of cardiac and pulmonary complication probabilities after radiation therapy for patients with early-stage breast cancer. Medicina 2009, 45, 276–285. [Google Scholar] [CrossRef]
- Kargar, N.; Zeinali, A.; Molazadeh, M. Impact of Dose Calculation Algorithms and Radiobiological Parameters on Prediction of Cardiopulmonary Complications in Left Breast Radiation Therapy. J. Biomed. Phys. Eng. 2024, 14, 129–140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).