Durable Objective Response to Lurbinectedin in Small Cell Bladder Cancer with TP53 Mutation: A Molecular-Directed Strategy
Abstract
1. Introduction
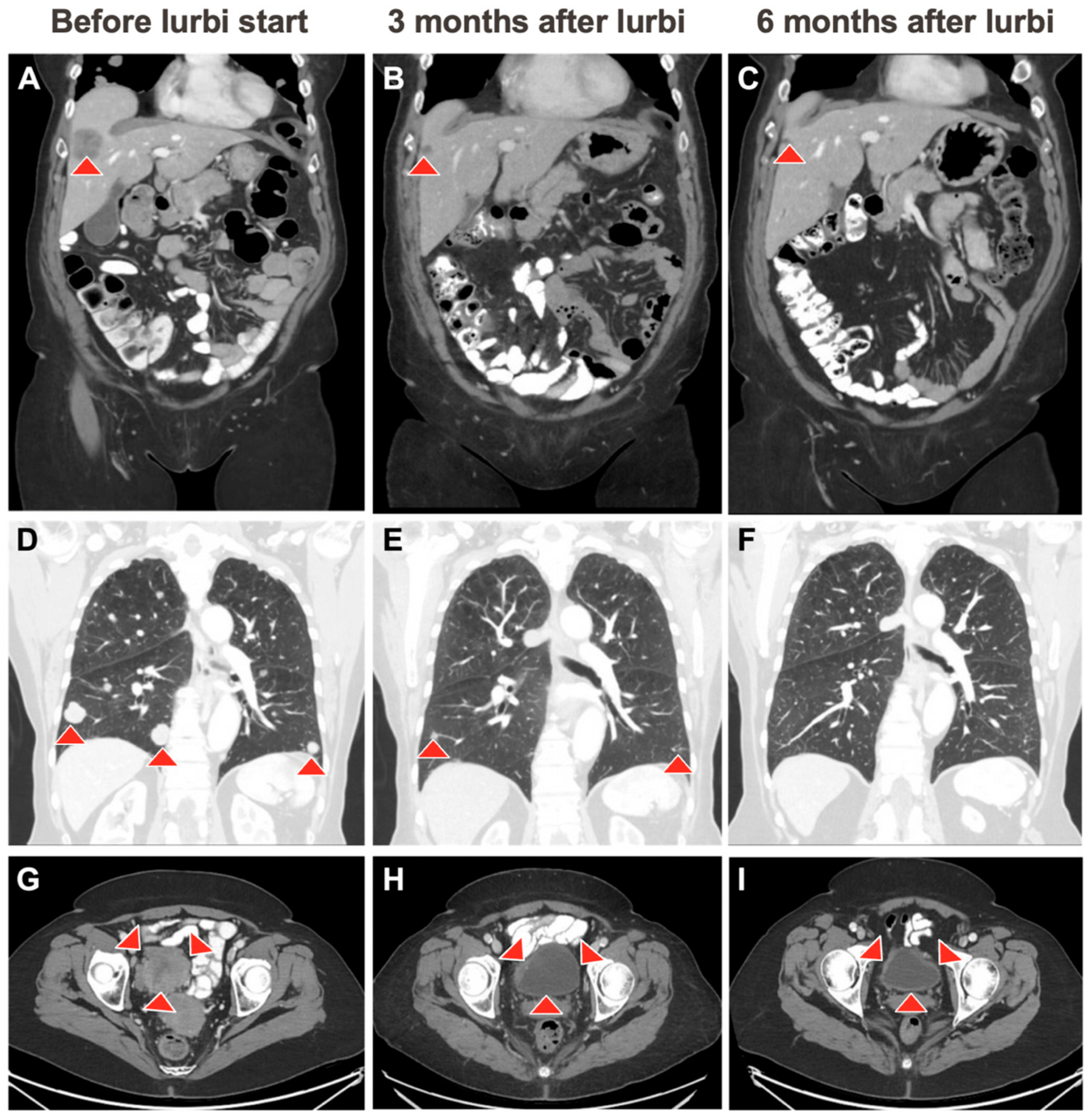
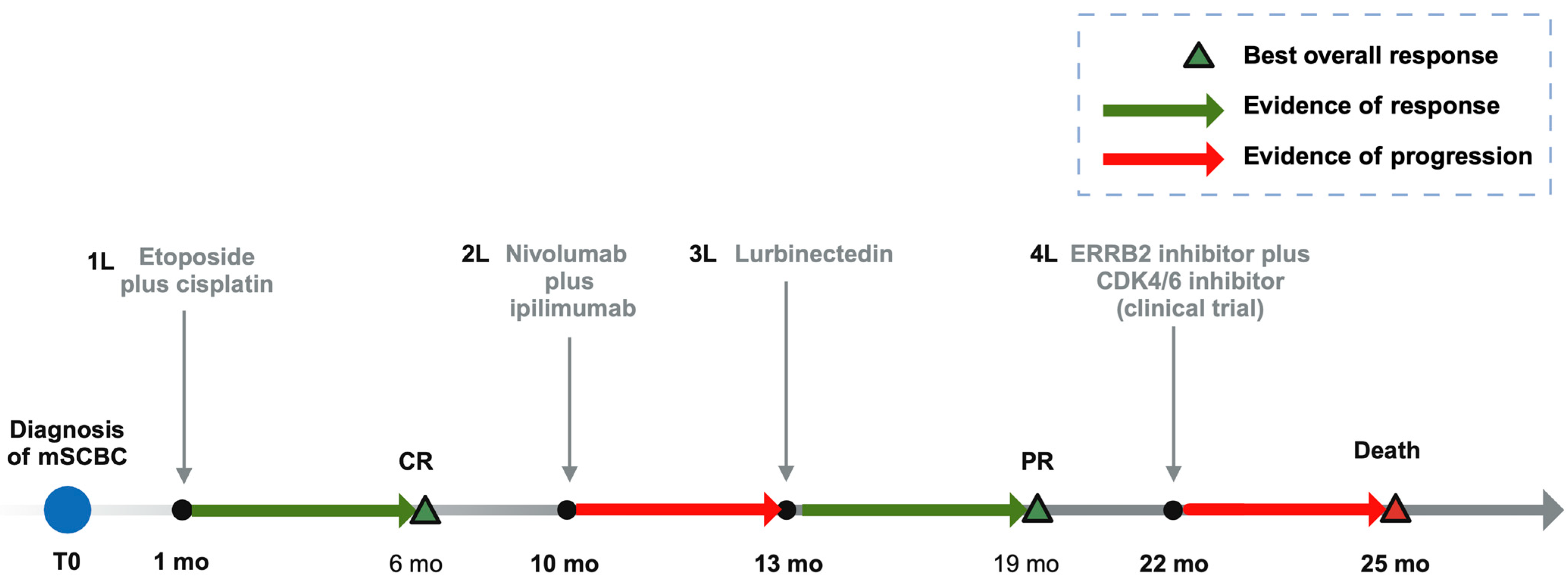
2. Case Description
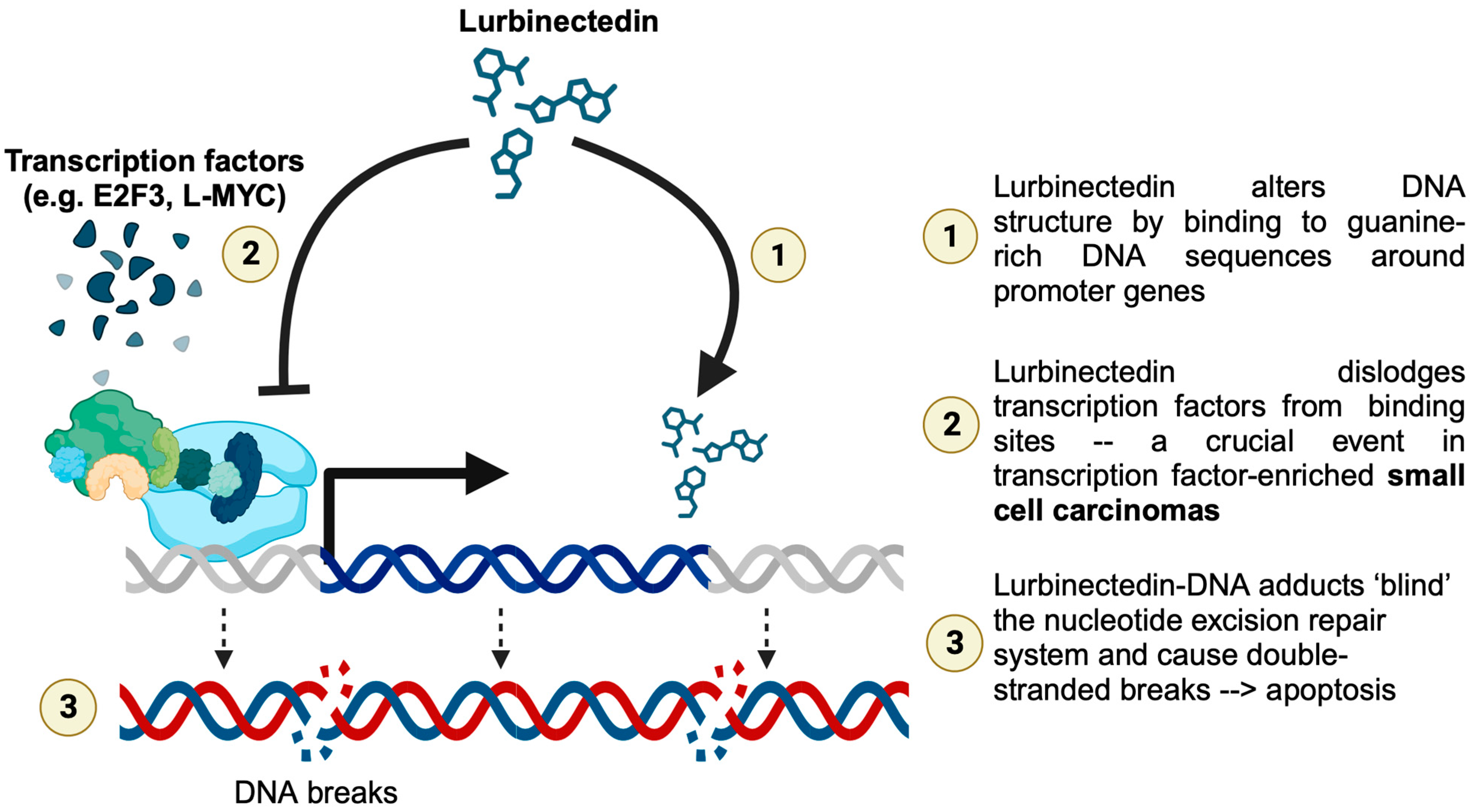
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, Q.; Wang, J.; Tong, M.; Xing, H.; Xue, Y.; Pan, H.; Hunag, C.; Li, D. Small cell carcinoma of the bladder: The characteristics of molecular alterations, treatment, and follow-up. Med. Oncol. 2019, 36, 98. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.J.; Campbell, M.T.; Alhalabi, O. Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand? Biomedicines 2024, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Alhalabi, O.; Wilson, N.; Xiao, L.; Lin, Y.; Khandelwal, J.; Moussa, M.J.; Msaouel, P.; Navai, N.; Gao, J.; Kamat, A.M.; et al. Comparative Effectiveness Analysis of Treatment Strategies for Surgically Resectable Neuroendocrine Carcinoma of the Urinary Tract. Eur. Urol. Oncol. 2023, 6, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Siefker-Radtke, A.O.; Kamat, A.M.; Grossman, H.B.; Williams, D.L.; Qiao, W.; Thall, P.F.; Dinney, C.P.; Millikan, R.E. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J. Clin. Oncol. 2009, 27, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jaigirdar, A.A.; Mulkey, F.; Cheng, J.; Hamed, S.S.; Li, Y.; Liu, J.; Zhao, H.; Goheer, A.; Helms, W.S.; et al. FDA approval summary: Lurbinectedin for the treatment of metastatic small cell lung cancer. Clin. Cancer Res. 2021, 27, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Padda, S.K.; Weiss, J.; Owonikoko, T.K. Advances in Treatment of Recurrent Small Cell Lung Cancer (SCLC): Insights for Optimizing Patient Outcomes from an Expert Roundtable Discussion. Adv. Ther. 2021, 38, 5431–5451. [Google Scholar] [CrossRef] [PubMed]
- Zepzelca. Prices, Coupons, Copay & Patient Assistance—Drugs.com. Available online: https://www.drugs.com/price-guide/zepzelca# (accessed on 7 June 2024).
- 2369P Phase II Study of Nivolumab (Nivo) and Ipilimumab (Ipi) for Advanced Bladder Cancer with Variant Histologies (BCVH)—Annals of Oncology. Available online: https://www.annalsofoncology.org/article/S0923-7534(23)01854-9/fulltext (accessed on 2 May 2024).
- Nizam, A.; Jindal, T.; Jiang, C.Y.; Alhalabi, O.; Davidsohn, M.P.; Bakaloudi, D.R.; Talukder, R.; Nguyen, C.B.; Oh, E.; Taylor, A.K.; et al. Outcomes with sacituzumab govitecan (SG) in patients (pts) with advanced urothelial carcinoma (aUC) and variant histologies (VH): Analysis of the UNITE study. J. Clin. Oncol. 2024, 42 (Suppl. S4), 667. [Google Scholar] [CrossRef]
- Jindal, T.; Jiang, C.Y.; Alhalabi, O.; Nguyen, C.B.; Nizam, A.; Basu, A.; Asim, M.A.; Zakharia, Y.; Milowsky, M.I.; Brown, J.R.; et al. Outcomes with enfortumab vedotin in patients with advanced urothelial carcinoma and histology variants: Analysis of the UNITE study. J. Clin. Oncol. 2024, 42 (Suppl. S4), 652. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Ghali, F.; Vakar-Lopez, F.; Roudier, M.P.; Garcia, J.; Arora, S.; Cheng, H.H.; Schweizer, M.T.; Haffner, M.C.; Lee, J.K.; Yu, E.Y.; et al. Metastatic Bladder Cancer Expression and Subcellular Localization of Nectin-4 and Trop-2 in Variant Histology: A Rapid Autopsy Study. Clin. Genitourin. Cancer 2023, 21, 669–678. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S. Trabectedin and lurbinectedin: Mechanisms of action, clinical impact, and future perspectives in uterine and soft tissue sarcoma, ovarian carcinoma, and endometrial carcinoma. Front. Oncol. 2022, 12, 914342. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Braña, I.; Longhi, A.; Boni, V.; Delord, J.P.; Awada, A.; Boudou-Rouquette, P.; Sarantopoulos, J.; Shapiro, G.I.; Elias, A.; et al. Antitumor Activity of Lurbinectedin, a Selective Inhibitor of Oncogene Transcription, in Patients with Relapsed Ewing Sarcoma: Results of a Basket Phase II Study. Clin. Cancer Res. 2022, 28, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Boudin, L.; Patient, M.; Romeo, E.; Bladé, J.S.; de Lesquen, H. Lurbinectedin for metastatic small-cell bladder carcinoma. Eur. J. Cancer 2021, 151, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.; Oaknin, A.; Trigo, J.M.; Moreno, V.; Delord, J.P.; Boni, V.; Braña, I.; Fernández, C.; Kahatt, C.; Nieto, A.; et al. Pooled Safety Analysis of Single-Agent Lurbinectedin in Patients With Advanced Solid Tumours. Eur. J. Cancer 2023, 192, 113259. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Oaknin, A.; Ray-Coquard, I.; Vergote, I.; Scambia, G.; Colombo, N.; Fernandez, C.; Alfaro, V.; Kahatt, C.; Nieto, A.; et al. Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: A multicenter, randomized, controlled, open-label phase 3 study (CORAIL). Gynecol. Oncol. 2021, 163, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Azaiza, M.; Yallapragada, S.; Siegert, J.; Andros, G. A case of metastatic lung adenocarcinoma of the bladder in a patient with no documented history of lung cancer. Urol. Case Rep. 2023, 49, 102441. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Ingelmo-Torres, M.; Gómez, A.; Roldán, F.L.; Segura, N.; Ribal, M.J.; Alcaraz, A.; Izquierdo, L.; Mengual, L. Prognostic implication of TERT promoter mutation and circulating tumor cells in muscle-invasive bladder cancer. World J. Urol. 2022, 40, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Oeggerli, M.; Tomovska, S.; Schraml, P.; Calvano-Forte, D.; Schafroth, S.; Simon, R.; Gasser, T.; Mihatsch, M.J.; Sauter, G. E2F3 amplification and overexpression is associated with invasive tumor growth and rapid tumor cell proliferation in urinary bladder cancer. Oncogene 2004, 23, 5616–5623. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Isharwal, S.; Arcila, M.E.; Funt, S.; Rosenberg, J.E.; Barjorin, D.F.; Bochner, B.H.; Berger, M.F.; Al-Ahmadie, H.; Solit, D.B.; et al. Targeting ERBB2 mutations in urothelial carcinoma. J. Clin. Oncol. 2017, 35 (Suppl. S6), 354. [Google Scholar] [CrossRef]
- My Cancer Genome. ERBB2 S310F. Available online: https://www.mycancergenome.org/content/alteration/erbb2-s310f/ (accessed on 28 April 2024).
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Aix, S.P.; Ciuleanu, T.E.; Navarro, A.; Cousin, S.; Bonanno, L.; Smit, E.F.; Chiappori, A.; Olmedo, M.E.; Horvath, I.; Grohé, C.; et al. Combination lurbinectedin and doxorubicin versus physician’s choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): A multicentre, randomised, open-label, phase 3 trial. Lancet Respir. Med. 2023, 11, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, C.; Resetca, D.; Redel, C.; Lin, P.; MacDonald, A.S.; Ciaccio, R.; Kenny, T.M.G.; Wei, Y.; Andrews, S.W.; Sunnerhagen, M.; et al. MYC protein interactors in gene transcription and cancer. Nat. Rev. Cancer 2021, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barrretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Al-Ahmadie, H.; Schultz, N.; Hanrahan, A.J.; Osyrovnaya, I.; Balar, A.V.; Kim, P.H.; Lin, O.; Weinhold, N.; Sander, C.; et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J. Clin. Oncol. 2013, 31, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.J.; Naqvi, S.A.A.; Khandelwal, J.; Campbell, M.T.; Aparicio, A.; Siddiqui, B.A.; Bryce, A.H.; Singh, P.; Alhalabi, O. Clinical efficacy of lurbinectedin in metastatic neuroendocrine carcinomas of the genitourinary tract: Multi-institutional real-world experience. J. Clin. Oncol. 2024, 42 (Suppl. S4), 601. [Google Scholar] [CrossRef]
- Meyer, H.M.; Sunkara, R.; Beltran, H.; Rothmann, E.; Courtney, K.D.; Armstrong, A.J.; Lipucci, A.; Stanton, M.L.; Bryce, A.H. Lurbinectedin in prostatic small cell and neuroendocrine carcinoma. J. Clin. Oncol. 2024, 42 (Suppl. S4), 164. [Google Scholar] [CrossRef]
- Study Details. Lurbinectedin Monotherapy in Participants with Advanced or Metastatic Solid Tumors. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05126433 (accessed on 11 December 2023).
- Simon, N.I.; Chandran, E.; Niglio, S.A.; Kydd, A.R.; Atiq, S.O.; Ley, L.; Wang, T.; Boudjadi, S.; Smith, E.; Akbulut, D.; et al. A phase II study of lurbinectedin with or without avelumab in small cell carcinoma of the bladder (LASER). J. Clin. Oncol. 2024, 42 (Suppl. S16), TPS4629. [Google Scholar] [CrossRef]
| Genes, Prior to Lurbinectedin Treatment | Genes, after Lurbinectedin Treatment | Finding | Cytoband |
|---|---|---|---|
| E2F3 | E2F3 | Amplification | 6p22.3 |
| ERG | ERG | Amplification | 21q22.2 |
| FOXA1 | - | Amplification | 14q21.1 |
| MYCL | MYCL | Amplification | 1p34.2 |
| SDHC | SDHC | Amplification | 1q23.3 |
| - | SOX10 | Amplification | 22q13.1 |
| Genes, Prior to Lurbinectedin Treatment | Genes, after Lurbinectedin Treatment | DNA | Protein | Location | VAF, Prior to Lurbinectedin Treatment | VAF, after Lurbinectedin Treatment | Type |
|---|---|---|---|---|---|---|---|
| MGA | MGA | c 8014C>T | p. P2672S | Exon 24 | 23% | 31% | SNV—Missense |
| STAT5B | STAT5B | c 1165C>T | p. R389C | Exon 9 | 17% | 42% | SNV—Missense |
| STK19 | STK19 | c 97G>T | p. E33 | Exon 1 | 28% | 20% | SNV—Nonsense |
| TERT | TERT | c 124C>T | - | UTR5 | 50% | 42% | SNV |
| TP53 | TP53 | c 192del | p. R65fs*58 | Exon 4 | 76% | 84% | Deletion—Frameshift |
| - | AMER1 | c 533G>A | p. R176H | Exon 2 | - | 27% | SNV—Missense |
| - | ELF3 | c 218T>C | p. L73P | Exon 3 | - | 21% | SNV—Missense |
| - | ERBB2 | c. 929C>T | p. S310F | Exon 8 | - | 21% | SNV—Missense |
| - | FLT1 | c 2306C>T | p. A769V | Exon 16 | - | 23% | SNV—Missense |
| - | IRS2 | C 994C>A | p. P332T | Exon 1 | - | 22% | SNV—Missense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, M.J.; Khandelwal, J.; Wilson, N.R.; Naik, S.A.; Subbiah, V.; Campbell, M.T.; Msaouel, P.; Singh, P.; Alhalabi, O. Durable Objective Response to Lurbinectedin in Small Cell Bladder Cancer with TP53 Mutation: A Molecular-Directed Strategy. Curr. Oncol. 2024, 31, 3342-3349. https://doi.org/10.3390/curroncol31060254
Moussa MJ, Khandelwal J, Wilson NR, Naik SA, Subbiah V, Campbell MT, Msaouel P, Singh P, Alhalabi O. Durable Objective Response to Lurbinectedin in Small Cell Bladder Cancer with TP53 Mutation: A Molecular-Directed Strategy. Current Oncology. 2024; 31(6):3342-3349. https://doi.org/10.3390/curroncol31060254
Chicago/Turabian StyleMoussa, Mohammad Jad, Jaanki Khandelwal, Nathaniel R. Wilson, Sagar A. Naik, Vivek Subbiah, Matthew T. Campbell, Pavlos Msaouel, Parminder Singh, and Omar Alhalabi. 2024. "Durable Objective Response to Lurbinectedin in Small Cell Bladder Cancer with TP53 Mutation: A Molecular-Directed Strategy" Current Oncology 31, no. 6: 3342-3349. https://doi.org/10.3390/curroncol31060254
APA StyleMoussa, M. J., Khandelwal, J., Wilson, N. R., Naik, S. A., Subbiah, V., Campbell, M. T., Msaouel, P., Singh, P., & Alhalabi, O. (2024). Durable Objective Response to Lurbinectedin in Small Cell Bladder Cancer with TP53 Mutation: A Molecular-Directed Strategy. Current Oncology, 31(6), 3342-3349. https://doi.org/10.3390/curroncol31060254







