How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology
Abstract
:1. Introduction
2. Materials and Methods
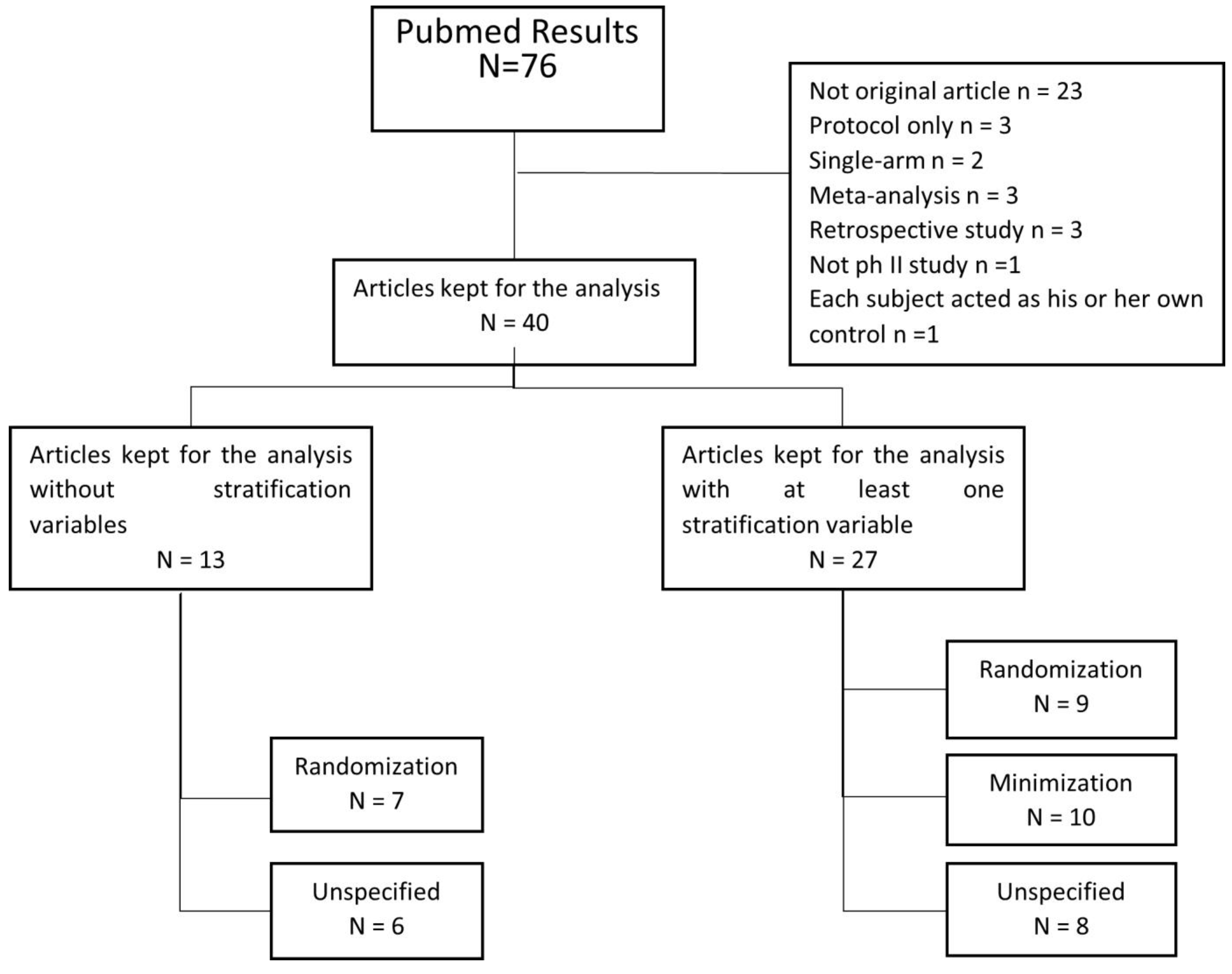
2.1. Literature Search Strategy and Selection Criteria
2.2. Real Clinical Study Applications
2.2.1. Simulating Arm Allocation
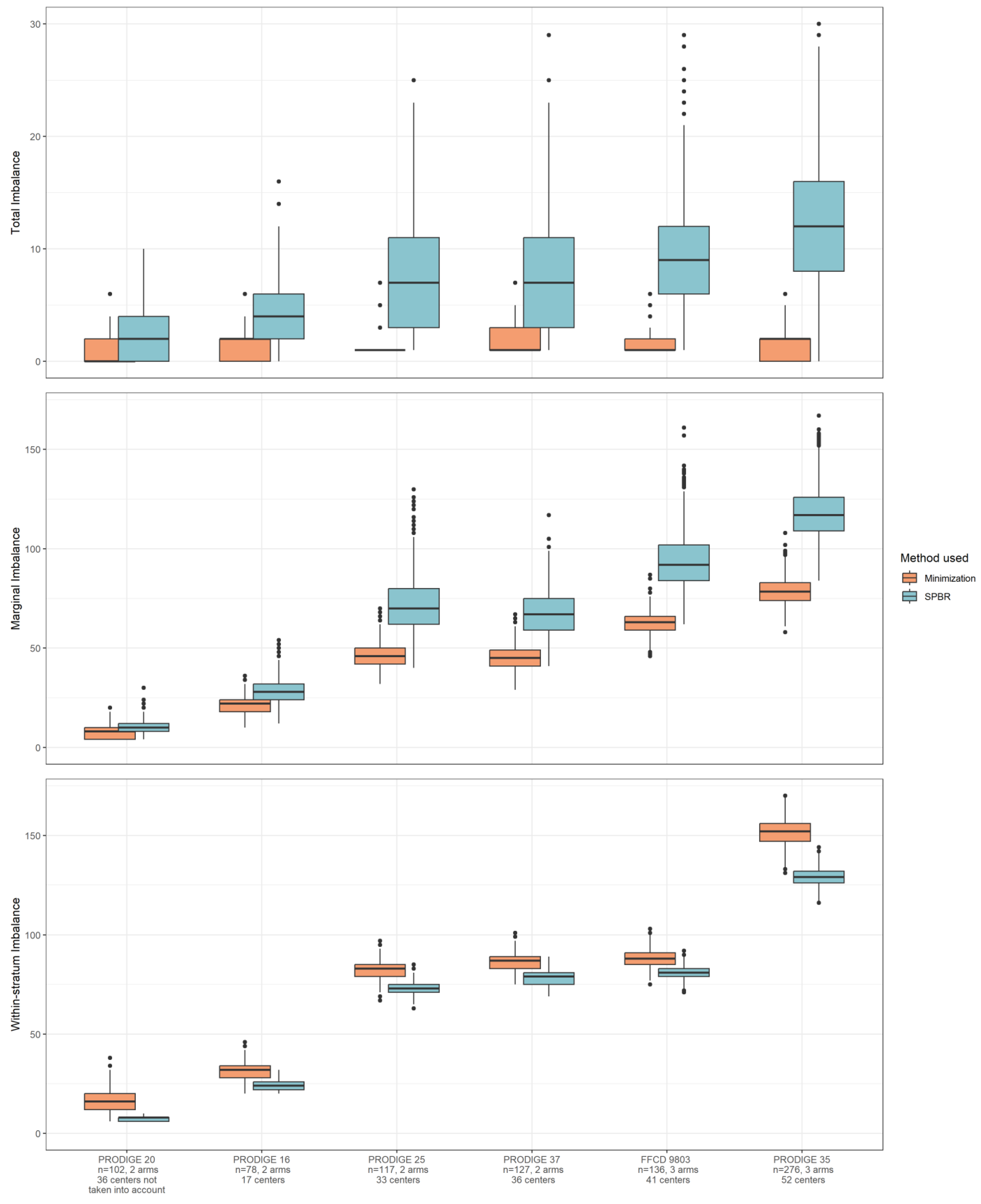
2.2.2. Imbalance Measurements
- Total imbalance is the difference measured between arms, calculated using the total number of patients assigned to each arm, 0 in our example.
- Marginal imbalance (or covariable margin imbalance) is calculated as the sum of the differences between arms for each modality of the variables, 2 in our example.
- Within-stratum imbalance is calculated as the total differences between treatment arms for each combination of stratification variables, 16 in our example.
2.2.3. Simulation of Endpoints
2.3. Software
3. Results
3.1. Review of Our Selected Articles
3.2. Imbalance in Real Clinical Trial Data
3.3. Impact on Endpoint Evaluation in Real Clinical Trial Data
4. Discussion
4.1. Literature Review (Main Finding)
4.2. Imbalance in Real Clinical Trial Data
4.3. Center as a Stratification Variable
4.4. Other Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Rubinstein, L.V.; Korn, E.L.; Freidlin, B.; Hunsberger, S.; Ivy, S.P.; Smith, M.A. Design Issues of Randomized Phase II Trials and a Proposal for Phase II Screening Trials. J. Clin. Oncol. 2005, 23, 7199–7206. [Google Scholar] [CrossRef] [PubMed]
- Phase II Trials in the EORTC. The Protocol Review Committee, the Data Center, the Research and Treatment Division, and the New Drug Development Office. European Organization for Research and Treatment of Cancer. Eur. J. Cancer 1997, 33, 1361–1363.
- ICH Harmonised Tripartite Guideline. Statistical Principles for Clinical Trials. International Conference on Harmonisation E9 Expert Working Group. Stat. Med. 1999, 18, 1905–1942.
- Guyatt, G.H.; Alexander, P.E. Randomization. In Important Considerations for Clinical Trial Methodologies; Future Science Book Series; Future Science Ltd.: London, UK, 2013; pp. 40–60. [Google Scholar]
- Chia, K.S. Randomisation: Magical Cure for Bias? Ann. Acad. Med. Singap. 2000, 29, 563–564. [Google Scholar] [PubMed]
- Sedgwick, P. Treatment Allocation in Trials: Stratified Randomisation. BMJ 2015, 350, h978. [Google Scholar] [CrossRef] [PubMed]
- Matts, J.P.; Lachin, J.M. Properties of Permuted-Block Randomization in Clinical Trials. Control Clin. Trials 1988, 9, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; MacRae, K.D. Minimisation: The Platinum Standard for Trials?: Randomisation Doesn’t Guarantee Similarity of Groups; Minimisation Does. BMJ 1998, 317, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Taves, D.R. Minimization: A New Method of Assigning Patients to Treatment and Control Groups. Clin. Pharmacol. Ther. 1974, 15, 443–453. [Google Scholar] [CrossRef]
- Pocock, S.J.; Simon, R. Sequential Treatment Assignment with Balancing for Prognostic Factors in the Controlled Clinical Trial. Biometrics 1975, 31, 103–115. [Google Scholar] [CrossRef]
- Altman, D.G.; Schulz, K.F.; Moher, D.; Egger, M.; Davidoff, F.; Elbourne, D.; Gøtzsche, P.C.; Lang, T.; CONSORT GROUP (Consolidated Standards of Reporting Trials). The Revised CONSORT Statement for Reporting Randomized Trials: Explanation and Elaboration. Ann. Intern. Med. 2001, 134, 663–694. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall/CRC: New York, NY, USA, 1990; ISBN 978-0-429-25858-9. [Google Scholar]
- Altman, D.G.; Bland, J.M. Treatment Allocation by Minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Thorpe, H.; Hawkins, K.; Brown, J. Minimization—Reducing Predictability for Multi-Centre Trials Whilst Retaining Balance within Centre. Stat. Med. 2005, 24, 3715–3727. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Ye, X.; Ma, W.; Hu, F. Carat: Covariate-Adaptive Randomization for Clinical Trials. J. Stat. Softw. 2023, 107, 1–47. [Google Scholar]
- European Medicines Agency. Guideline on Adjustment for Baseline Covariates in Clinical Trials; European Medicines Agency: Amsterdam, The Netherlands, 2015; p. 11. [Google Scholar]
- Bouché, O.; Raoul, J.L.; Bonnetain, F.; Giovannini, M.; Etienne, P.L.; Lledo, G.; Arsène, D.; Paitel, J.F.; Guérin-Meyer, V.; Mitry, E.; et al. Randomized Multicenter Phase II Trial of a Biweekly Regimen of Fluorouracil and Leucovorin (LV5FU2), LV5FU2 Plus Cisplatin, or LV5FU2 Plus Irinotecan in Patients with Previously Untreated Metastatic Gastric Cancer: A Fédération Francophone de Cancérologie Digestive Group Study—FFCD 9803. JCO 2004, 22, 4319–4328. [Google Scholar] [CrossRef]
- Turpin, A.; de Baere, T.; Heurgué, A.; Le Malicot, K.; Ollivier-Hourmand, I.; Lecomte, T.; Perrier, H.; Vergniol, J.; Sefrioui, D.; Rinaldi, Y.; et al. Liver Transarterial Chemoembolization and Sunitinib for Unresectable Hepatocellular Carcinoma: Results of the PRODIGE 16 Study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101464. [Google Scholar] [CrossRef]
- Aparicio, T.; Bouché, O.; Taieb, J.; Maillard, E.; Kirscher, S.; Etienne, P.-L.; Faroux, R.; Khemissa Akouz, F.; El Hajbi, F.; Locher, C.; et al. Bevacizumab+chemotherapy versus Chemotherapy Alone in Elderly Patients with Untreated Metastatic Colorectal Cancer: A Randomized Phase II Trial—PRODIGE 20 Study Results. Ann. Oncol. 2018, 29, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Legoux, J.L.; Faroux, R.; Barriere, N.; Le Malicot, K.; Tougeron, D.; Lorgis, V.; Guérin-Meyer, V.; Bourgeois, V.; Malka, D.; Aparicio, T.; et al. 444P PRODIGE 25 (FFCD 11-01)-FOLFA: A Randomized Phase II Trial Evaluating Aflibercept Associated with LV5FU2 Regimen as First-Line Treatment of Non-Resectable Metastatic Colorectal Cancers. Ann. Oncol. 2020, 31, S430–S431. [Google Scholar] [CrossRef]
- Dahan, L.; Williet, N.; Le Malicot, K.; Phelip, J.-M.; Desrame, J.; Bouché, O.; Petorin, C.; Malka, D.; Rebischung, C.; Aparicio, T.; et al. Randomized Phase II Trial Evaluating Two Sequential Treatments in First Line of Metastatic Pancreatic Cancer: Results of the PANOPTIMOX-PRODIGE 35 Trial. J. Clin. Oncol. 2021, 39, 3242–3250. [Google Scholar] [CrossRef]
- Rinaldi, Y.; Pointet, A.L.; Khemissa Akouz, F.; Le Malicot, K.; Wahiba, B.; Louafi, S.; Gratet, A.; Miglianico, L.; Laharie, H.; Bouhier Lepoirrier, K.; et al. Gemcitabine plus Nab-Paclitaxel until Progression or Alternating with FOLFIRI.3, as First-Line Treatment for Patients with Metastatic Pancreatic Adenocarcinoma: The Federation Francophone de CancErologie Digestive-PRODIGE 37 Randomised Phase II Study (FIRGEMAX). Eur. J. Cancer 2020, 10, 25–34. [Google Scholar] [CrossRef]
- Jin, M.; Polis, A.; Hartzel, J. Algorithms for minimization randomization and the implementation with a R package. Commun. Stat. Simul. Comput. 2021, 50, 3077–3087. [Google Scholar] [CrossRef]
- Snow, G. Blockrand: Randomization for Block Random Clinical Trials. version 1.5. 2022. Available online: https://cran.r-project.org/web/packages/blockrand/index.html (accessed on 25 September 2023).
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA 2019, 321, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Shi, Q.; Bhadkamkar, N.A.; Cleeland, C.S.; Garcia-Gonzalez, A.; Aguilar, J.R.; Heijnen, C.; Eng, C. Minocycline for Symptom Reduction During Oxaliplatin-Based Chemotherapy for Colorectal Cancer: A Phase II Randomized Clinical Trial. J. Pain Symptom Manag. 2019, 58, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, O.S.; Fristrup, C.W.; Pfeiffer, P.; Lundell, L.; Mortensen, M.B. Phase II Randomized Clinical Trial of Endosonography and PET/CT versus Clinical Assessment Only for Follow-up after Surgery for Upper Gastrointestinal Cancer (EUFURO Study). Br. J. Surg. 2019, 106, 1761–1768. [Google Scholar] [CrossRef]
- Bednarski, B.K.; Nickerson, T.P.; You, Y.N.; Messick, C.A.; Speer, B.; Gottumukkala, V.; Manandhar, M.; Weldon, M.; Dean, E.M.; Qiao, W.; et al. Randomized Clinical Trial of Accelerated Enhanced Recovery after Minimally Invasive Colorectal Cancer Surgery (RecoverMI Trial). Br. J. Surg. 2019, 106, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamada, A.; Hirata, S.; Tanaka, H.; Sakuratani, T.; Matsuhashi, N.; Yamaguchi, K.; Shimokawa, T.; Yoshida, K. Efficacy and Safety of Enoxaparin for Prophylaxis of Postoperative Venous Thromboembolism After Esophagectomy: A Single-Center Prospective Randomized Controlled Phase II Study. Anticancer Res. 2019, 39, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh Kermani, A.; Hosseini, S.; Fanipakdel, A.; Joudi Mashhad, M.; Akhavan Rezayat, K.; Zardadi, M.; Gholami, A.; Javadinia, S.A.; Ferns, G.A.; Avan, A. A Randomized Clinical Trial on the Antitumoral Effects of Low Molecular Weight Heparin in the Treatment of Esophageal Cancer. J. Cell. Physiol. 2019, 234, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.J.; Conti, J.A.; Dalton, R.; Rockall, T.A.; Allison, A.S.; Ockrim, J.B.; Jourdan, I.C.; Torkington, J.; Phillips, S.; Allison, J.; et al. 2D versus 3D Laparoscopic Total Mesorectal Excision: A Developmental Multicentre Randomised Controlled Trial. Surg. Endosc. 2019, 33, 3370–3383. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, R.; Miehlke, S.; Thiede, C.; Brueckner, S.; Dawel, M.; Kuhn, M.; Ruskoné-Formestraux, A.; Stolte, M.; Jentsch, C.; Hampe, J.; et al. Sequential H. Pylori Eradication and Radiation Therapy with Reduced Dose Compared to Standard Dose for Gastric MALT Lymphoma Stages IE & II1E: A Prospective Randomized Trial. J. Gastroenterol. 2019, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Boku, N.; Ryu, M.-H.; Kato, K.; Chung, H.C.; Minashi, K.; Lee, K.-W.; Cho, H.; Kang, W.K.; Komatsu, Y.; Tsuda, M.; et al. Safety and Efficacy of Nivolumab in Combination with S-1/Capecitabine plus Oxaliplatin in Patients with Previously Untreated, Unresectable, Advanced, or Recurrent Gastric/Gastroesophageal Junction Cancer: Interim Results of a Randomized, Phase II Trial (ATTRACTION-4). Ann. Oncol. 2019, 30, 250–258. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Vincent, J.; Bengrine, L.; Borg, C.; Jouve, J.L.; Loffroy, R.; Guiu, B.; Blanc, J.; Bertaut, A. Hepatic Arterial Chemotherapy with Raltitrexed and Oxaliplatin versus Standard Chemotherapy in Unresectable Liver Metastases from Colorectal Cancer after Conventional Chemotherapy Failure (HEARTO): A Randomized Phase-II Study. J. Cancer Res. Clin. Oncol. 2019, 145, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Marmorino, F.; Bergamo, F.; Aprile, G.; Salvatore, L.; Masi, G.; Dell’Aquila, E.; Antoniotti, C.; Murgioni, S.; Allegrini, G.; et al. Phase II Randomised Study of Maintenance Treatment with Bevacizumab or Bevacizumab plus Metronomic Chemotherapy after First-Line Induction with FOLFOXIRI plus Bevacizumab for Metastatic Colorectal Cancer Patients: The MOMA Trial. Eur. J. Cancer 2019, 109, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guan, Y.; Gu, W.; Yan, S.; Zhou, J.; Huang, D.; Tong, T.; Li, C.; Cai, S.; Zhang, Z.; et al. Long-Course Neoadjuvant Chemoradiotherapy with versus without a Concomitant Boost in Locally Advanced Rectal Cancer: A Randomized, Multicenter, Phase II Trial (FDRT-002). Radiat. Oncol. 2019, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Du, Y.; Xu, Z.; Huang, L.; Cheng, X. Comparison of D2 and D2 plus Radical Surgery for Advanced Distal Gastric Cancer: A Randomized Controlled Study. World J. Surg. Oncol. 2019, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Ou, F.-S.; Ahn, D.H.; Boland, P.M.; Ciombor, K.K.; Heying, E.N.; Dockter, T.J.; Jacobs, N.L.; Pasche, B.C.; Cleary, J.M.; et al. Regorafenib Dose-Optimisation in Patients with Refractory Metastatic Colorectal Cancer (ReDOS): A Randomised, Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2019, 20, 1070–1082. [Google Scholar] [CrossRef]
- Bennouna, J.; Hiret, S.; Bertaut, A.; Bouché, O.; Deplanque, G.; Borel, C.; François, E.; Conroy, T.; Ghiringhelli, F.; des Guetz, G.; et al. Continuation of Bevacizumab vs Cetuximab Plus Chemotherapy After First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Yamanaka, T.; Denda, T.; Tsuji, Y.; Shinozaki, K.; Komatsu, Y.; Kobayashi, Y.; Furuse, J.; Okuda, H.; Asayama, M.; et al. REVERCE: A Randomized Phase II Study of Regorafenib Followed by Cetuximab versus the Reverse Sequence for Previously Treated Metastatic Colorectal Cancer Patients. Ann. Oncol. 2019, 30, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Lobefaro, R.; Antista, M.; Lonardi, S.; Raimondi, A.; Morano, F.; Mosconi, S.; Rimassa, L.; Murgioni, S.; Sartore-Bianchi, A.; et al. Capecitabine and Temozolomide versus FOLFIRI in RAS-Mutated, MGMT-Methylated Metastatic Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Oki, E.; Emi, Y.; Yamanaka, T.; Uetake, H.; Muro, K.; Takahashi, T.; Nagasaka, T.; Hatano, E.; Ojima, H.; Manaka, D.; et al. Randomised Phase II Trial of MFOLFOX6 plus Bevacizumab versus MFOLFOX6 plus Cetuximab as First-Line Treatment for Colorectal Liver Metastasis (ATOM Trial). Br. J. Cancer 2019, 121, 222–229. [Google Scholar] [CrossRef]
- Malka, D.; François, E.; Penault-Llorca, F.; Castan, F.; Bouché, O.; Bennouna, J.; Ghiringhelli, F.; de la Fouchardière, C.; Borg, C.; Samalin, E.; et al. FOLFOX Alone or Combined with Rilotumumab or Panitumumab as First-Line Treatment for Patients with Advanced Gastroesophageal Adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): A Randomised, Open-Label, Three-Arm Phase II Trial. Eur. J. Cancer 2019, 115, 97–106. [Google Scholar] [CrossRef]
- Adenis, A.; Bennouna, J.; Etienne, P.L.; Bogart, E.; Francois, E.; Galais, M.P.; Ben Abdelghani, M.; Michel, P.; Metges, J.P.; Dahan, L.; et al. Continuation versus Discontinuation of First-Line Chemotherapy in Patients with Metastatic Squamous Cell Oesophageal Cancer: A Randomised Phase II Trial (E-DIS). Eur. J. Cancer 2019, 111, 12–20. [Google Scholar] [CrossRef]
- Munemoto, Y.; Nakamura, M.; Takahashi, M.; Kotaka, M.; Kuroda, H.; Kato, T.; Minagawa, N.; Noura, S.; Fukunaga, M.; Kuramochi, H.; et al. SAPPHIRE: A Randomised Phase II Study of Planned Discontinuation or Continuous Treatment of Oxaliplatin after Six Cycles of Modified FOLFOX6 plus Panitumumab in Patients with Colorectal Cancer. Eur. J. Cancer 2019, 119, 158–167. [Google Scholar] [CrossRef]
- Kobayashi, H.; Uetake, H.; Yasuno, M.; Sugihara, K. Effectiveness of Wound-Edge Protectors for Preventing Surgical Site Infections after Open Surgery for Colorectal Disease: A Prospective Cohort Study with Two Parallel Study Groups. Dig. Surg. 2019, 36, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.I.; Tan, B.R.; Reeves, J.A.; Xiong, H.; Somer, B.; Lenz, H.-J.; Hochster, H.S.; Scappaticci, F.; Palma, J.F.; Price, R.; et al. Phase II Randomized Trial of Sequential or Concurrent FOLFOXIRI-Bevacizumab Versus FOLFOX-Bevacizumab for Metastatic Colorectal Cancer (STEAM). Oncologist 2019, 24, 921–932. [Google Scholar] [CrossRef]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Martens, U.M.; Riera-Knorrenschild, J.; Greeve, J.; Florschütz, A.; Wessendorf, S.; Ettrich, T.; Kanzler, S.; Nörenberg, D.; Ricke, J.; et al. FOLFOXIRI Plus Panitumumab As First-Line Treatment of RAS Wild-Type Metastatic Colorectal Cancer: The Randomized, Open-Label, Phase II VOLFI Study (AIO KRK0109). J. Clin. Oncol. 2019, 37, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Chon, H.J.; Kim, J.H.; Jung, M.; Nam, C.M.; Kim, H.S.; Kang, B.; Chung, H.C.; Rha, S.Y. Randomised Phase II Trial Comparing Four Front-Line Doublets in Asian Patients with Metastatic Gastric Cancer. Eur. J. Cancer 2019, 112, 20–28. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Muro, K.; Shitara, K.; Oh, D.-Y.; Kang, Y.-K.; Chung, H.C.; Kudo, T.; Chin, K.; Kadowaki, S.; Hamamoto, Y.; et al. Effect of First-Line S-1 Plus Oxaliplatin with or Without Ramucirumab Followed by Paclitaxel Plus Ramucirumab on Advanced Gastric Cancer in East Asia: The Phase 2 RAINSTORM Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e198243. [Google Scholar] [CrossRef]
- Páez, D.; Tobeña, M.; Fernández-Plana, J.; Sebio, A.; Virgili, A.C.; Cirera, L.; Barnadas, A.; Riera, P.; Sullivan, I.; Salazar, J. Pharmacogenetic Clinical Randomised Phase II Trial to Evaluate the Efficacy and Safety of FOLFIRI with High-Dose Irinotecan (HD-FOLFIRI) in Metastatic Colorectal Cancer Patients According to Their UGT1A 1 Genotype. Br. J. Cancer 2019, 120, 190–195. [Google Scholar] [CrossRef]
- McGregor, L.M.; Skrobanski, H.; Ritchie, M.; Berkman, L.; Miller, H.; Freeman, M.; Patel, N.; Morris, S.; Rees, C.; von Wagner, C. Using Specialist Screening Practitioners (SSPs) to Increase Uptake of Bowel Scope (Flexible Sigmoidoscopy) Screening: Results of a Feasibility Single-Stage Phase II Randomised Trial. BMJ Open 2019, 9, e023801. [Google Scholar] [CrossRef]
- Winther, S.B.; Liposits, G.; Skuladottir, H.; Hofsli, E.; Shah, C.-H.; Poulsen, L.Ø.; Ryg, J.; Osterlund, P.; Berglund, Å.; Qvortrup, C.; et al. Reduced-Dose Combination Chemotherapy (S-1 plus Oxaliplatin) versus Full-Dose Monotherapy (S-1) in Older Vulnerable Patients with Metastatic Colorectal Cancer (NORDIC9): A Randomised, Open-Label Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2019, 4, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Uedo, N.; Tonai, Y.; Arao, M.; Suzuki, S.; Iwatsubo, T.; Kato, M.; Shichijo, S.; Yamasaki, Y.; Matsuura, N.; et al. Efficacy of Vonoprazan in Prevention of Bleeding from Endoscopic Submucosal Dissection-Induced Gastric Ulcers: A Prospective Randomized Phase II Study. J. Gastroenterol. 2019, 54, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kienle, D.L.; Dietrich, D.; Ribi, K.; Wicki, A.; Quagliata, L.; Winterhalder, R.C.; Koeberle, D.; Horber, D.; Bastian, S.; Kueng, M.; et al. Cetuximab Monotherapy and Cetuximab plus Capecitabine as First-Line Treatment in Older Patients with RAS- and BRAF Wild-Type Metastatic Colorectal Cancer. Results of the Multicenter Phase II Trial SAKK 41/10. J. Geriatr. Oncol. 2019, 10, 304–310. [Google Scholar] [CrossRef]
- Parikh, A.R.; Lee, F.-C.; Yau, L.; Koh, H.; Knost, J.; Mitchell, E.P.; Bosanac, I.; Choong, N.; Scappaticci, F.; Mancao, C.; et al. MAVERICC, a Randomized, Biomarker-Stratified, Phase II Study of MFOLFOX6-Bevacizumab versus FOLFIRI-Bevacizumab as First-Line Chemotherapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 2988–2995. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ariyoshi, N.; Miyauchi, H.; Ohira, G.; Kaneya, N.; Yamamoto, K.; Arai, K.; Yamazaki, S.; Matsubara, H.; Suzuki, T.; et al. A Randomized Controlled, Open-Label Early Phase II Trial Comparing Incidence of FOLFIRI.3-Induced Diarrhoea between Hangeshashinto and Oral Alkalization in Japanese Patients with Colorectal Cancer. J. Clin. Pharm. Ther. 2019, 44, 946–951. [Google Scholar] [CrossRef]
- Suwa, Y.; Watanabe, J.; Ota, M.; Suzuki, S.; Suwa, H.; Watanabe, K.; Saito, S.; Nagamine, K.; Momiyama, M.; Ishibe, A.; et al. Randomized Phase II Trial of the Prophylactic Use of Celecoxib for the Prevention of Oxaliplatin-Related Peripheral Vascular Pain in Capeox (YCOG1205). Cancer Chemother. Pharmacol. 2019, 83, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Kang, Y.-K.; Ng, M.; Chung, H.C.; Wainberg, Z.A.; Gendreau, S.; Chan, W.Y.; Xu, N.; Maslyar, D.; Meng, R.; et al. A Phase II, Randomised Study of MFOLFOX6 with or without the Akt Inhibitor Ipatasertib in Patients with Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Cancer. Eur. J. Cancer 2019, 108, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.M.; Horick, N.K.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Azzoli, C.G.; Rubinson, D.A.; Brooks, G.A.; Chan, J.A.; Blaszkowsky, L.S.; et al. FOLFOX plus Ziv-Aflibercept or Placebo in First-Line Metastatic Esophagogastric Adenocarcinoma: A Double-Blind, Randomized, Multicenter Phase 2 Trial. Cancer 2019, 125, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Beck, J.T.; Hofheinz, R.-D.; Garcia-Alfonso, P.; Nechaeva, M.; Cubillo Gracian, A.; Mangel, L.; Elez Fernandez, E.; Deming, D.A.; Ramanathan, R.K.; et al. A Phase 2 Randomised Study of Veliparib plus FOLFIRI±bevacizumab versus Placebo plus FOLFIRI±bevacizumab in Metastatic Colorectal Cancer. Br. J. Cancer 2019, 120, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Sauri, T.; Gracián, A.C.; Alvarez, R.; López-López, C.; García-Alfonso, P.; Hussein, M.; Miron, M.-L.L.; Cervantes, A.; Montagut, C.; et al. The McCAVE Trial: Vanucizumab plus MFOLFOX-6 Versus Bevacizumab plus MFOLFOX-6 in Patients with Previously Untreated Metastatic Colorectal Carcinoma (MCRC). Oncologist 2020, 25, e451–e459. [Google Scholar] [CrossRef]
- Lauzon, S.D.; Zhao, W.; Nietert, P.J.; Ciolino, J.D.; Hill, M.D.; Ramakrishnan, V. Impact of Minimal Sufficient Balance, Minimization, and Stratified Permuted Blocks on Bias and Power in the Estimation of Treatment Effect in Sequential Clinical Trials with a Binary Endpoint. Stat. Methods Med. Res. 2022, 31, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Coart, E.; Bamps, P.; Quinaux, E.; Sturbois, G.; Saad, E.D.; Burzykowski, T.; Buyse, M. Minimization in Randomized Clinical Trials. Stat. Med. 2023, 42, 5285–5311. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, A.; Harsha Shree, B.S.; Karkada, N. Inference under Covariate-Adaptive Randomization: A Simulation Study. Stat. Methods Med. Res. 2021, 30, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
 ) depending on the 2 procedures: Stratified Permuted Block Randomization (left side) and minimization (right side). The characteristics of the 7 patients who were already randomized in the study are shown at the top left of the diagram. The study’s stratification variables are represented using colors and pictograms (see top right of diagram).
) depending on the 2 procedures: Stratified Permuted Block Randomization (left side) and minimization (right side). The characteristics of the 7 patients who were already randomized in the study are shown at the top left of the diagram. The study’s stratification variables are represented using colors and pictograms (see top right of diagram).
 ) depending on the 2 procedures: Stratified Permuted Block Randomization (left side) and minimization (right side). The characteristics of the 7 patients who were already randomized in the study are shown at the top left of the diagram. The study’s stratification variables are represented using colors and pictograms (see top right of diagram).
) depending on the 2 procedures: Stratified Permuted Block Randomization (left side) and minimization (right side). The characteristics of the 7 patients who were already randomized in the study are shown at the top left of the diagram. The study’s stratification variables are represented using colors and pictograms (see top right of diagram).
| Stratification Variable 1 | Stratification Variable 2 | ARM A | ARM B | Difference |
|---|---|---|---|---|
| Total | 50 | 50 | 0 (Total imbalance) | |
| Total Center 1 | 15 | 15 | 0 | |
| Total Center 2 | 11 | 12 | 1 | |
| Total Center 3 | 24 | 23 | 1 | |
| Total Non-smoker | 19 | 19 | 0 | |
| Total Smoker | 31 | 31 | 0 | |
| 2 (Marginal imbalance) | ||||
| Center 1 | Non-smoker | 7 | 3 | 4 |
| Smoker | 8 | 12 | 4 | |
| Center 2 | Non-smoker | 3 | 6 | 3 |
| Smoker | 8 | 6 | 2 | |
| Center 3 | Non-smoker | 9 | 10 | 1 |
| Smoker | 15 | 13 | 2 | |
| Subtotal | 16 (Within-stratum imbalance) | |||
| All Studies | According to Treatment Allocation Procedure | Studies with at Least One Stratification Variable n = 27 | Studies Using Center as Stratification Variable n = 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Randomization | Minimization | Unspecified | Randomization | Minimization | Unspecified | Randomization | Minimization | Unspecified | |
| n = 40 | n = 16 | n = 10 | n = 14 | n = 9 | n = 10 | n = 8 | n = 3 | n = 6 | n = 1 | |
| Number of patients randomized | ||||||||||
| Median (min–max) | 109 (24–376) | 122 (29–311) | 117.5 (67–280) | 72.5 (24–376) | 152 (82.0–311.0) | 117.5 (67–280) | 105.5 (24–376) | 160 (82–311) | 128 (101–280) | 81 |
| Number of arms | ||||||||||
| Median (min–max) | 2 (2–4) | 2 (2–4) | 2 (2–4) | 2 (2–2) | 2 (2–4) | 2 (2–4) | 2 (2–2) | 2 (2–2) | 2.5 (2–4) | 2 |
| Number of stratification variables | ||||||||||
| Median (min–max) | 2 (0–7) ¥ | 1 (0–3) ¥ | 3 (1–7) | 2 (0–4) | 2 (1–3) ¥ | 3 (1–7) | 2.5 (2–4) | 2 (1–2) | 3 (1–4) | 3 |
| Number of centers | ||||||||||
| Median (min–max) | 11 (1–63) | 3 (1–36) | 29 (1–63) § | 10 (1–60) | 3 (1–36) | 29 (1–63) § | 22.5 (1–60) | 18 (3–23) | 39 (25–53) ¥ | 9 |
| >1 center | 31 (77.5%) | 11 (68.8%) | 9 (90%) | 11 (78.6%) | 6 (75%) | 9 (90%) | 7 (87.5%) | |||
| Center as a stratification variable | ||||||||||
| Yes | 10 (25.6%) | 3 (20%) ¥ | 6 (60%) | 1 (7.1%) | 3 (37.5%) ¥ | 6 (60%) | 1 (12.5%) | |||
| Other geographical unit as stratification variable | ||||||||||
| Yes | 5 (12.8%) | 1 (6.7%) ¥ | 0 (0%) | 4 (28.6%) | 1 (12.5%) ¥ | 0 (0%) | 4 (50%) | |||
| Statistical comparison between arms | ||||||||||
| Yes | 33 (82.5%) | 14 (87.5%) | 8 (80%) | 11 (78.6%) | 6 (75%) 7 (77.8%) | 8 (80%) | 7 (87.5%) | 2 (66.7%) | 5 (83.3%) | 1 |
| Adjustments, stratified or subgroup analyses using stratification variable | ||||||||||
| Yes | 8 (20.5%) ¥ | 3 (20%) ¥ | 2 (20%) | 3 (21.4%) | 2 (25%) ¥ | 2 (20%) | 3 (37.5%) | 1 (33.3%) | 2 (33.3%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, E.; Le Malicot, K.; Guérin-Charbonnel, C.; Bocquet, F.; Bouché, O.; Turpin, A.; Aparicio, T.; Legoux, J.-L.; Dahan, L.; Taieb, J.; et al. How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology. Curr. Oncol. 2024, 31, 3513-3528. https://doi.org/10.3390/curroncol31060259
Martin E, Le Malicot K, Guérin-Charbonnel C, Bocquet F, Bouché O, Turpin A, Aparicio T, Legoux J-L, Dahan L, Taieb J, et al. How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology. Current Oncology. 2024; 31(6):3513-3528. https://doi.org/10.3390/curroncol31060259
Chicago/Turabian StyleMartin, Elodie, Karine Le Malicot, Catherine Guérin-Charbonnel, François Bocquet, Olivier Bouché, Anthony Turpin, Thomas Aparicio, Jean-Louis Legoux, Laetitia Dahan, Julien Taieb, and et al. 2024. "How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology" Current Oncology 31, no. 6: 3513-3528. https://doi.org/10.3390/curroncol31060259
APA StyleMartin, E., Le Malicot, K., Guérin-Charbonnel, C., Bocquet, F., Bouché, O., Turpin, A., Aparicio, T., Legoux, J.-L., Dahan, L., Taieb, J., Lepage, C., Dourthe, L.-M., Pétorin, C., Bourgeois, V., Raoul, J.-L., & Seegers, V. (2024). How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology. Current Oncology, 31(6), 3513-3528. https://doi.org/10.3390/curroncol31060259





