Abstract
The COVID-19 pandemic caused major disruptions to healthcare services in 2020, delaying cancer diagnosis and treatment. While early-stage thyroid cancer often progresses slowly, it is crucial to determine whether treatment delays associated with the pandemic have impacted the clinical presentation and management of advanced-stage thyroid cancer. The purpose of our study was to determine the impact of the early COVID-19 pandemic on thyroid cancer presentation and treatment times. Utilizing the National Cancer Database, chi-squared tests and regression analyses were performed to compare patient demographic and clinical characteristics over time for 56,011 patients diagnosed with primary thyroid cancer who were treated at the Commission on Cancer-accredited sites in 2019 and 2020. We found that thyroid cancer diagnoses decreased between 2019 and 2020, with the biggest drop among patients with cT1 disease relative to other T stages. We also found that patients diagnosed with thyroid cancer in 2020 had similar treatment times to patients diagnosed in 2019, as measured by both the time between diagnosis and start of treatment and the time between surgery and start of radioactive iodine therapy. Overall, our study suggests that resources during the pandemic were allocated to patients with advanced thyroid disease, despite a decrease in diagnoses.
Keywords:
thyroid cancer; COVID-19; pandemic; treatment delays; disparities; otolaryngology; oncology 1. Introduction
The COVID-19 pandemic led to unprecedented disruptions in healthcare services including screening, diagnosis, and treatment [1,2,3]. Cancer patients were especially vulnerable to the effects of the COVID-19 pandemic, given the importance of prompt diagnosis and treatment for many types of cancer [4], as well as the increased risk of COVID-19-related complications and mortality for patients with cancer [5]. The delays in cancer treatment and diagnosis caused by the COVID-19 pandemic have led some to predict an increase in patients presenting with late-stage disease and a subsequent increase in cancer mortality in the coming years [6,7].
Studies on thyroid cancer in particular found a decrease in fine needle aspiration biopsies and thyroid cancer diagnoses in 2020 relative to 2019 [8,9]. Additionally, small single-center studies from around the world found that thyroid cancer patients during the pandemic presented with more aggressive cancers when compared to thyroid cancer patients pre-pandemic. Specifically, these patients were more likely to have extrathyroidal extension, lymph node metastases, and multiple lesions [10,11,12]. Medas et al. conducted a multi-institutional study that included 87,467 thyroid cancer patients from 157 centers, only three of which were in the United States, and found an increased occurrence of lymph node metastases and tumor size during the pandemic. Research suggests that while the number of endocrine surgeries overall decreased in 2020 relative to 2019, surgeries for thyroid cancer patients were prioritized and mostly maintained [13,14]. The reduction in surgeries for thyroid nodules was more drastic during the early stages of the pandemic as opposed to the later stages of the pandemic in mid to late 2021 [15]. One study of a university hospital in South Korea found an increase in the number of days between initial visits and surgery during the COVID-19 pandemic relative to pre-pandemic, but this delay did not meet the threshold of statistical significance [16].
Thyroid cancer is relatively unique in that there has been a recent rise in incidence largely attributed to overdiagnosis from screening, as well as from biopsies of nodules incidentally discovered on routine imaging, though some studies suggest that this trend has flattened or even reversed within the past decade [17,18,19,20]. Even prior to the pandemic, there was a shift in American Thyroid Association (ATA) guidelines aimed at minimizing the overdiagnosis of small, incidental thyroid cancers that may not be clinically significant [18,21]. Additionally, the evidence is mixed on whether a modest delay of radioactive iodine (RAI) treatment for thyroid cancer negatively affects outcomes. While many papers suggest that modestly delayed RAI made no difference in overall survival or disease-free survival [16,22,23,24], others found that modestly delayed RAI was associated with worse overall survival and incomplete response to therapy [25,26].
Although early-stage thyroid cancer typically follows an indolent course, it is crucial to understand if delays have affected the presentation and treatment of thyroid malignancies. Given that both patients and healthcare facilities postponed less urgent healthcare services during the early COVID-19 pandemic, patients with thyroid cancer may have been at higher risk of delaying essential treatment compared to those with other types of cancer [27]. The goal of our study was to determine how the early stages of the COVID-19 pandemic impacted treatment times and the stage at presentation of thyroid cancer patients in the United States.
2. Materials and Methods
2.1. Data Source
The data source used for this study was the National Cancer Database, a hospital-based cancer registry managed by the American Cancer Society and the American College of Surgeons Commission on Cancer. This database is collected from over 1500 facilities across the United States and captures over 70% of all patients newly diagnosed with cancer in the United States [28]. This study was approved by the Stanford University School of Medicine. This is a deidentified database and was determined to be exempt from review by the Stanford University institutional review board.
2.2. Study Population and Covariates
The study population included adult patients with well-differentiated thyroid cancer from 2019 to 2020 who were treated at CoC-accredited cancer sites. We included only patients with papillary or follicular histologies. Sociodemographic variables that were included as covariables included age, sex, race, education, household income, distance from treatment facility, insurance status, facility region, and rural–urban classification. Both education and household income were based on the patient’s zip code and classified into quartiles based on US Census data. Insurance status was divided into the following groups: private insurance or managed care, Medicaid, Medicare, and other/government. Facility regions were divided into Northeast, Midwest, West, and South based on the regions used by the US Census Bureau [29]. The rural–urban classification was categorized as metro, urban, rural, and unknown. Distance from the treating facility was grouped into 0–10 miles, 11–20 miles, 21–50 miles, 51–100 miles, and greater than 100 miles.
The clinical variables we analyzed included clinical and pathologic TNM staging, Charlson–Deyo Score, and readmission status. Clinical and pathologic T, N, and M staging were classified according to the Eighth Edition of the American Joint Committee on Cancer (AJCC) Staging Manual [30]. The Charlson–Deyo Score was divided into two categories: the first group included patients with a Charlson–Deyo score of 0 or 1 and the second group included patients with a Charlson–Deyo score of 2 or 3. Readmission status was categorized as planned readmission, unplanned readmission, or unknown.
Descriptive statistics showing general trends in thyroid cancer diagnoses and clinical T staging over time were calculated and reported for adult patients with well-differentiated papillary or follicular thyroid cancer treated at CoC-accredited cancer sites between 2004 and 2020. However, the univariable and multivariable analyses were restricted to patients treated between 2019 and 2020 in order to assess the impact of the COVID-19 pandemic.
2.3. Statistical Analysis
The average annual percentage change (AAPC) and 95% confidence intervals (CIs) were used for the calculation of descriptive calculated for thyroid cancer diagnoses and clinical characteristics from 2004 to 2019. Chi-square tests were used to compare differences in patient sociodemographic and clinical characteristics between 2019 and 2020. Multivariable linear regression was performed using sociodemographic and clinical characteristics as covariates. For regression analyses, only patients diagnosed with thyroid cancer between 2019 and 2020 were included. Our treatment time variables were the number of days between diagnosis and the start of any treatment and the number of days between surgery and the start of RAI treatment. Chi-square p-values < 0.05 were considered statistically significant. All analysis was performed using STATA (Version 15.1; StataCorp LLC, College Station, TX, USA). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used to guide the formatting of the manuscript and reporting of the results [31].
3. Results
3.1. Changes in Thyroid Cancer Demographics and Treatment Times from 2004 to 2020
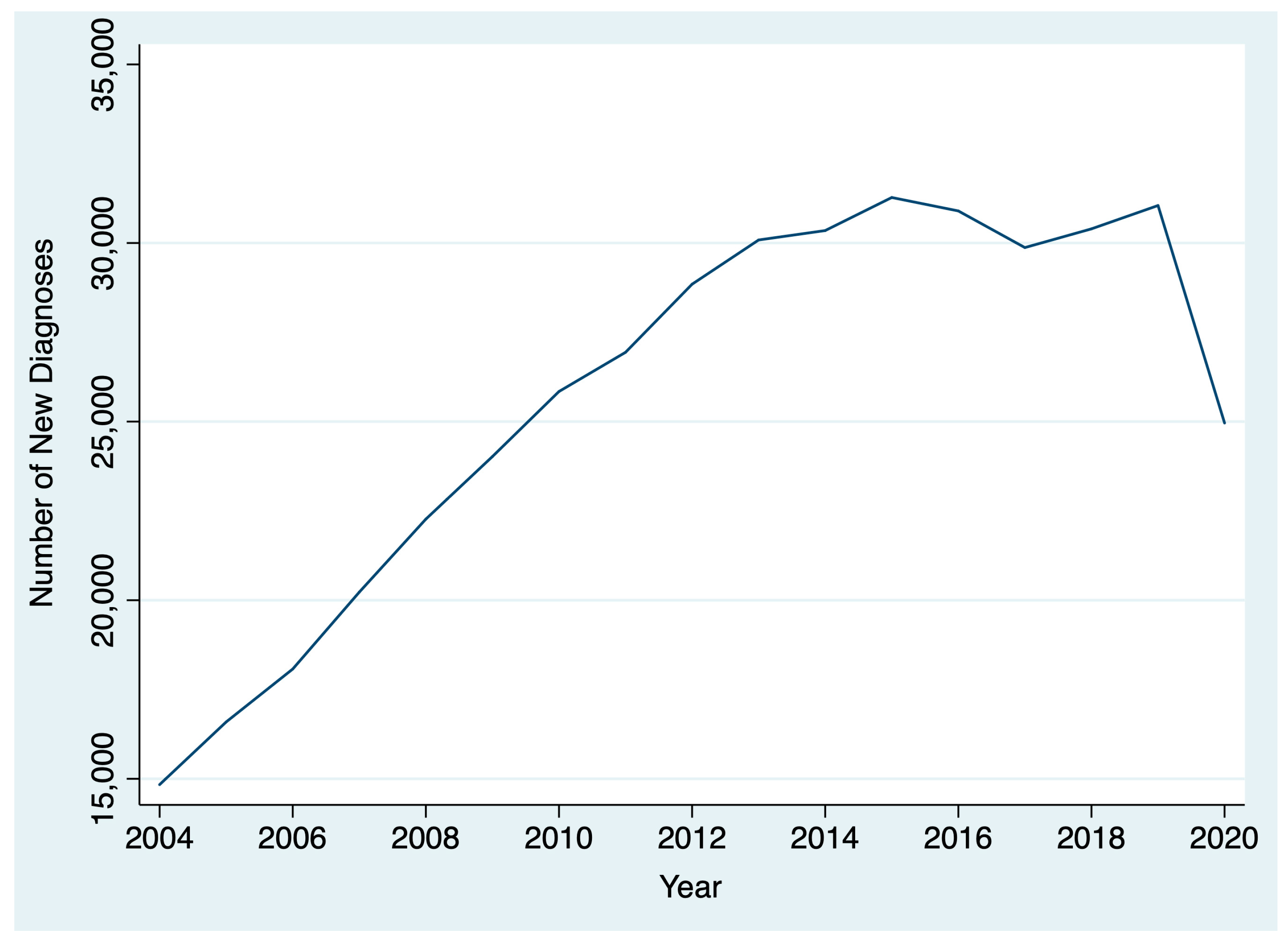
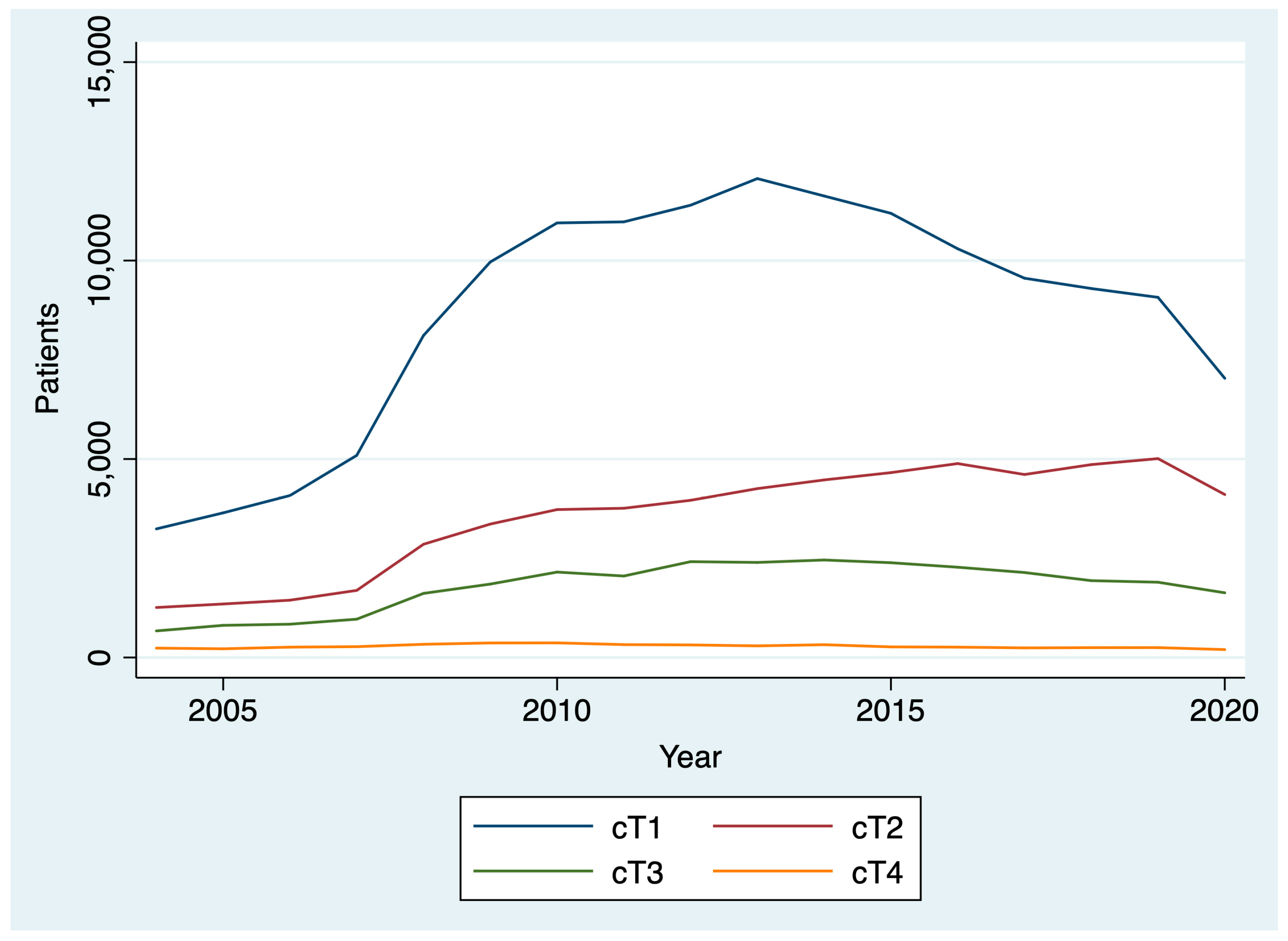
Between 2004 and 2020, 436,546 patients were diagnosed with thyroid cancer. The AAPC in thyroid cancer diagnoses between 2004 and 2019 was 5.1% (95% CI: 2.8% to 7.5%), reflecting a general upward trend. However, between 2019 and 2020, there was a 19.6% decrease in thyroid cancer diagnoses (Figure 1). After stratifying by T stage, we found that there was a larger drop between 2019 and 2020 for patients diagnosed with cT1 disease (22.5%; chi-square p < 0.001) compared to patients diagnosed with cT2, cT3, and cT4 disease (18.1%, and 14.1%, 19.7%, respectively; chi-square p < 0.001) (Figure 2). By N stage, the drop in the number of patients diagnosed with cN0 from 2019 to 2020 was only slightly higher than the drop in the number of patients diagnosed with cN+ disease (21.4% and 20.8%, respectively; chi-square p = 0.005). Similarly, the drop in the number of patients diagnosed with cM0 disease from 2019 to 2020 was slightly higher than the number of patients diagnosed with cM0+ disease (21.1% and 19.1%, respectively; chi-square p = 0.004).
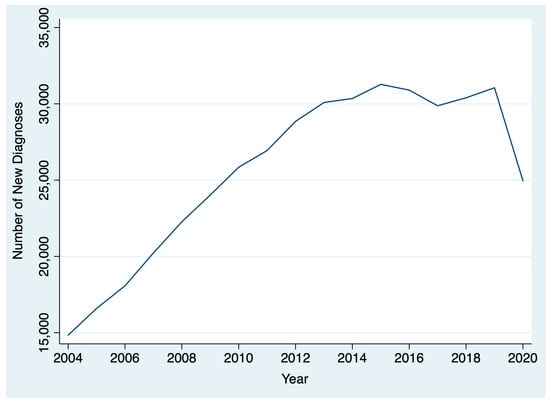
Figure 1.
Change in thyroid cancer diagnoses over time.
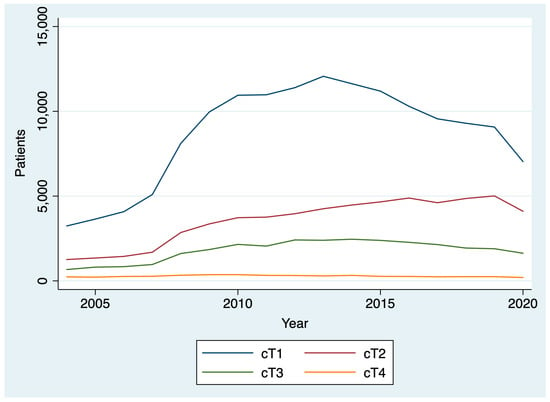
Figure 2.
Change in clinical T stage over time.
When we evaluated treatment time metrics without adjusting for clinical and demographic variables, we found that there was a gradual increase in the number of days between diagnosis and the start of any treatment for thyroid cancer between 2004 and 2019 (AAPC 3.5%, 95% CI: 2.2% to 4.8%) and a relatively flat trend in the number of days between surgery and RAI treatment for thyroid cancer (AAPC 2.4%, 95% CI: −0.8% to 4.1%). Between 2019 and 2020, there was a 6.4% drop (1.72 days) in the average number of days between diagnosis and treatment start from and a 2.4% drop (2.29 days) in the average number of days between surgery and RAI treatment.
3.2. Clinical and Demographic Characteristics of Thyroid Cancer Patients in 2020 versus 2019
We identified 56,011 patients who were diagnosed with thyroid cancer between 2019 and 2020. Patient demographic characteristics are shown in Table 1 and were compared to patients diagnosed in 2019 and patients diagnosed in 2020. Age, sex, race, education, and income levels were similar between the two groups. Patients diagnosed in 2020 were more likely to live further away from the treatment facility than patients diagnosed in 2019, with a higher proportion of patients living more than 100 miles away from the treatment facility (20.0% vs. 18.7%; chi-square p = 0.002). Patients diagnosed in 2020, compared to patients diagnosed in 2019, were less likely to be uninsured (3.5% vs. 3.9%) and more likely to be insured by Medicaid (20.1% vs. 9.6%) or Medicare (23.5% vs. 22.9%; chi-square p = 0.002 for all insurance status comparisons).

Table 1.
Patient demographic and clinical characteristics, 2019 versus 2020.
In terms of clinical staging at presentation, patients diagnosed in 2020 were less likely to present with cT1 disease than those diagnosed in 2019 (40.9% vs. 41.3%, chi-square p < 0.001). Patients diagnosed in 2020 were also less likely to present with cN0 cancer (53.4% vs. 54.6%; chi-square p = 0.005) and cM0 cancer (67.9% vs. 69.2%; chi-square p = 0.004) (Table 2).

Table 2.
Patient pathological and clinical staging statistics, 2019 versus 2020.
3.3. Linear Regression Analysis
After adjusting for clinical and demographic characteristics, patients diagnosed with thyroid cancer in 2020 had a shorter interval between diagnosis and the start of treatment than those diagnosed in 2019 (mean difference = −1.46 days; 95% CI, −2.52 to −0.40 (Table 3). Other covariates that had a statistically significant relationship included age, sex, distance from treatment facility, clinical T staging, and clinical M staging. Patients who were 71 years or older had longer treatment times than younger patients (mean difference = 4.35 days; 95% CI, 1.69 to 7.01). Patients who lived between 51–100 miles (mean difference = 5.05 days; 95% CI, 2.77 to 7.33) and 100 miles or greater (mean difference = 10.02 days; 95% CI, 6.89 to 13.16) from the treating facility where much more likely to have delays than those who lived closer. Compared to cT1 cancer, cT2 (mean difference = −6.34 days; 95% CI, −7.79 to −4.90), cT3 (mean difference = −16.93 days; 95% CI, −18.74 to −15.13), and cT4 (mean difference = −10.64 days; 95% CI, −15.85 to −5.43), cancers had a shorter time from diagnosis to treatment.

Table 3.
Linear regression results, time from diagnosis to start of any treatment.
We performed a similar analysis to determine whether the COVID-19 pandemic resulted in any change in the number of days between surgery and the start of RAI. After adjusting for covariates, there was no difference in time between surgery and the start of RAI for patients diagnosed with thyroid cancer in 2019 compared with 2020 (mean difference = −0.01 days; 95% CI, −0.02 to 0.01) (Table 4).

Table 4.
Linear regression results, time from surgery to start of radioactive iodine.
4. Discussion
This analysis found a gradual rise over time in thyroid cancer diagnoses and treatment times from 2004 to 2019 that reversed during the COVID-19 pandemic among patients treated at CoC-accredited cancer sites. During the COVID-19 pandemic, there was a 20% drop in the number of thyroid cancer diagnoses and a 4% decrease in the number of days between diagnosis and surgery. Among all T stages, the decrease was greatest among cT1 patients. Patients diagnosed in 2020 had a shorter interval between diagnosis and the start of treatment than those diagnosed in 2019. However, the difference was small and not clinically significant. There was no difference in the interval between surgery and the start of radioactive iodine in 2020 versus 2019.
The largest decrease in thyroid cancer diagnoses was among those with cT1 thyroid disease, though substantial decreases in those with more advanced disease were also noted. This may be driven by a decrease in the availability of fine needle aspiration biopsies (FNABs) for thyroid nodules, as well as decreased imaging intensity, leading to fewer incidental findings and delayed diagnoses. A study of hospital emergency departments in the United States noted a 43% decrease in visits and a 12% reduction in imaging throughout the pandemic [32]. Small-scale studies of thyroid cancer patients in other countries have reported a 64% decrease in weekly FNABs in May through July 2020 compared to 2019 and early 2020, resulting in a 12% decrease in benign diagnoses and a 6% increase in malignant diagnoses relative to pre-pandemic [9]. The ramifications of the pandemic-driven decline in thyroid cancer diagnoses will have to be monitored closely in the coming years to determine if there is a true increase in advanced thyroid cancer cases due to diagnostic delays.
Among patients who were diagnosed with thyroid cancer in 2020, treatment following diagnosis was timelier than in 2019, although the difference was small and not clinically significant. Our study suggests that the COVID-19 pandemic did not lead to delays in the treatment of thyroid cancer. In other countries, most studies have demonstrated a stable number of thyroid cancer surgeries throughout the pandemic, despite a decline in endocrine surgeries overall, suggesting priority for oncologic care. One study from Turkey found that the annual rate of parathyroidectomy and thyroidectomy for benign goiter decreased in 2020 relative to 2019, but it did not find significant differences in thyroid cancer surgery rates between the two years [13]. Similarly, a nationwide study in Italy found a decrease in the overall number of endocrine surgeries, but with surgeries for oncological patients being maintained [14]. However, a national study in Brazil found that FNABs, oncologic thyroidectomies, and RAI treatments decreased in 2020 relative to 2019, with most procedures, excluding RAI, rebounding closer to pre-pandemic volumes in 2021 [33]. One potential explanation for these conflicting findings is variation in institution-specific pandemic response, as well as differing degrees of the burden of the COVID-19 outbreak on hospital resources and staffing. Research on breast cancer surgery found that, on average, mandated operating room shutdowns during the COVID-19 pandemic in 2020 did not lead to significant treatment delays in a subset of New York City public hospitals. However, there was wide variation between centers, with some being well-equipped to prioritize cancer surgeries and maintain treatment efficiency, while others were less successful and experienced long treatment delays [34].
Patients who benefited from timely thyroid cancer treatment during the pandemic were disproportionately younger, female, and lived closer to the treating facility. A qualitative study of older adults diagnosed with cancer demonstrated that some of the disruptions to cancer care for these patients during the pandemic included physical and social isolation, fear of contracting the virus, and difficulty with telehealth use [35]. Research on thyroid cancer patients in the pre-pandemic era found no significant delay in days to surgery by patient sex [36]. Studies suggest that because thyroid cancer is more commonly diagnosed among women, it is likely that male patients are at a higher risk of being diagnosed later compared to female patients, making timely treatment important [37]. Additionally, even prior to the pandemic, longer distances to the treating facility have been associated with delays in thyroid surgery, as well as presentation with more advanced disease [36]. No studies to date have examined whether the pandemic has exacerbated distance-related disparities in the context of thyroid cancer treatment, making it an interesting point for future research. Further work should focus on how to provide technological support, outreach, and care to our most vulnerable patients during similar situations in the future.
Limitations to our study include the lack of clinical data beyond 2020, preventing a comprehensive assessment of longer-term clinical outcomes post-pandemic. We also did not have data on thyroid ultrasounds or FNABs during the pandemic. Additionally, since the National Cancer Database (NCDB) is a hospital-based registry, our study does not include data from facilities that are not Commission on Cancer (CoC) accredited, limiting the generalizability of our findings and our ability to calculate incidence rates. The absence of data on disease-specific survival also constrains our ability to fully understand the impact of the pandemic on patient outcomes.
The COVID-19 pandemic led to a systemic decrease in the number of thyroid cancer cases diagnosed in the United States among both patients with early and advanced-stage disease, though the greatest decrease was found in early-stage disease. Older patients and patients who lived further away from the treating facility were more likely to experience treatment delays. However, our study suggests that, overall, hospitals were able to provide timely thyroid cancer treatment and effectively prioritize advanced-stage disease. Further work is necessary to determine the long-term impact of the decline in thyroid cancer diagnoses and surgeries performed.
Author Contributions
Conceptualization, M.L.L. and M.M.C.; methodology, M.L.L., M.M.C. and U.C.M.; data analysis, M.L.L. and M.M.C.; data interpretation, M.L.L., U.C.M., A.F., J.E.N. and M.M.C.; writing—original draft preparation, M.L.L. and M.M.C.; writing—review and editing, M.L.L., U.C.M., A.F., J.E.N. and M.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived by the Stanford University Institutional Review Board since this study utilized a deidentified database.
Informed Consent Statement
Patient consent was waived since this study utilized a deidentified database.
Data Availability Statement
The data presented in this study are available from NCDB and provided by the American College of Surgeons, by application.
Acknowledgments
The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigator.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
NCDB: National Cancer Database, CoC: Commission on Cancer, APC: Annual Percentage Change, CI: Confidence Interval, AJCC: American Joint Committee on Cancer, RAI: Radioactive Iodine, ATA: American Thyroid Association.
References
- Mayo, M.; Potugari, B.; Bzeih, R.; Scheidel, C.; Carrera, C.; Shellenberger, R.A. Cancer Screening during the COVID-19 Pandemic: A Systematic Review and Meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 1109–1117. [Google Scholar] [CrossRef]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sullivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Lei, X.; Zhao, H.; Scheet, P.; Giordano, S.H. Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients With or Without Cancer. JAMA Oncol. 2022, 8, 69–78. [Google Scholar] [CrossRef]
- Blay, J.Y.; Boucher, S.; Le Vu, B.; Cropet, C.; Chabaud, S.; Perol, D.; Barranger, E.; Campone, M.; Conroy, T.; Coutant, C.; et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open 2021, 6, 100134. [Google Scholar] [CrossRef]
- Malagón, T.; Yong, J.H.E.; Tope, P.; Miller, W.H.; Franco, E.L.; McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int. J. Cancer 2022, 150, 1244–1254. [Google Scholar] [CrossRef]
- Tsang, V.H.M.; Gild, M.; Glover, A.; Clifton-Bligh, R.; Robinson, B.G. Thyroid cancer in the age of COVID-19. Endocr.-Relat. Cancer 2020, 27, R407–R416. [Google Scholar] [CrossRef]
- Palladino, R.; Migliatico, I.; Sgariglia, R.; Nacchio, M.; Iaccarino, A.; Malapelle, U.; Vigliar, E.; Salvatore, D.; Troncone, G.; Bellevicine, C. Thyroid fine-needle aspiration trends before, during, and after the lockdown: What we have learned so far from the COVID-19 pandemic. Endocrine 2021, 71, 20–25. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, L.; Guo, L.; Yu, X.; Li, L.; Feng, H.; Yang, D.; Xu, Z.; Tu, Y.; Chen, C.; et al. More Aggressive Cancer Behaviour in Thyroid Cancer Patients in the Post-COVID-19 Pandemic Era: A Retrospective Study. Int. J. Gen. Med. 2021, 14, 7197–7206. [Google Scholar] [CrossRef]
- Kim, S.H.; Min, E.; Hwang, Y.M.; Choi, Y.S.; Yi, J.W. Impact of COVID-19 Pandemic on Thyroid Surgery in a University Hospital in South Korea. Cancers 2022, 14, 4338. [Google Scholar] [CrossRef]
- Vigliar, E.; Cepurnaite, R.; Iaccarino, A.; Pisapia, P.; De Luca, C.; Malapelle, U.; Bellevicine, C.; Troncone, G. Cytopathology practice during the COVID-19 postlockdown: An Italian experience. Cancer Cytopathol. 2021, 129, 548–554. [Google Scholar] [CrossRef]
- Tunca, F. Impact of the COVID-19 Pandemic on the Annual Thyroid, Parathyroid, and Adrenal Surgery Volume in a Tertiary Refferal Endocrine Surgery Center in 2020. Sisli Etfal [Internet]. 2021. Available online: https://sislietfaltip.org/jvi.aspx?un=SETB-64920&volume= (accessed on 17 August 2023).
- Medas, F.; Ansaldo, G.L.; Avenia, N.; Basili, G.; Boniardi, M.; Bononi, M.; Bove, A.; Carcoforo, P.; Casaril, A.; Cavallaro, G.; et al. The THYCOVIT (Thyroid Surgery during COVID-19 pandemic in Italy) study: Results from a nationwide, multicentric, case-controlled study. Updates Surg. 2021, 73, 1467–1475. [Google Scholar] [CrossRef]
- Medas, F.; Dobrinja, C.; Al-Suhaimi, E.A.; Altmeier, J.; Anajar, S.; Arikan, A.E.; Azaryan, I.; Bains, L.; Basili, G.; Bolukbasi, H.; et al. Effect of the COVID-19 pandemic on surgery for indeterminate thyroid nodules (THYCOVID): A retrospective, international, multicentre, cross-sectional study. Lancet Diabetes Endocrinol. 2023, 11, 402–413. [Google Scholar] [CrossRef]
- Cheng, F.; Xiao, J.; Huang, F.; Shao, C.; Ding, S.; Yun, C.; Jia, H. Delay of initial radioactive iodine therapy beyond 3 months has no effect on clinical responses and overall survival in patients with thyroid carcinoma: A cohort study and a meta-analysis. Cancer Med. 2022, 11, 2386–2396. [Google Scholar] [CrossRef]
- Li, M.; Dal Maso, L.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020, 8, 468–470. [Google Scholar] [CrossRef]
- Nickel, B.; Glover, A.; Miller, J.A. Delays to Low-risk Thyroid Cancer Treatment during COVID-19—Refocusing from What Has Been Lost to What May Be Learned and Gained. JAMA Otolaryngol.–Head Neck Surg. 2021, 147, 5–6. [Google Scholar] [CrossRef]
- Powers, A.E.; Marcadis, A.R.; Lee, M.; Morris, L.G.T.; Marti, J.L. Changes in Trends in Thyroid Cancer Incidence in the United States, 1992 to 2016. JAMA 2019, 322, 2440–2441. [Google Scholar] [CrossRef]
- Megwalu, U.C.; Moon, P.K. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000–2018. Thyroid 2022, 32, 560–570. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Suman, P.; Wang, C.H.; Abadin, S.S.; Block, R.; Raghavan, V.; Moo-Young, T.A.; Prinz, R.A.; Winchester, D.J. Timing of radioactive iodine therapy does not impact overall survival in high-risk papillary thyroid carcinoma. Endocr. Pract. 2016, 22, 822–831. [Google Scholar] [CrossRef]
- Kim, M.; Han, M.; Jeon, M.J.; Kim, W.G.; Kim, I.J.; Ryu, J.S.; Kim, W.B.; Shong, Y.K.; Kim, T.Y.; Kim, B.H. Impact of delayed radioiodine therapy in intermediate-/high-risk papillary thyroid carcinoma. Clin. Endocrinol. 2019, 91, 449–455. [Google Scholar] [CrossRef]
- Matrone, A.; Gambale, C.; Torregrossa, L.; Piaggi, P.; Bianchi, F.; Valerio, L.; Viola, D.; Agate, L.; Molinaro, E.; Materazzi, G.; et al. Delayed 131-I first treatment after surgery has no impact on the median term outcome of patients with intermediate risk differentiated thyroid cancer. Endocr. Pract. 2020, 26, 58–71. [Google Scholar] [CrossRef]
- Higashi, T.; Nishii, R.; Yamada, S.; Nakamoto, Y.; Ishizu, K.; Kawase, S.; Togashi, K.; Itasaka, S.; Hiraoka, M.; Misaki, T.; et al. Delayed initial radioactive iodine therapy resulted in poor survival in patients with metastatic differentiated thyroid carcinoma: A retrospective statistical analysis of 198 cases. J. Nucl. Med. 2011, 52, 683–689. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.Q.; Wang, C.; Zhang, X.; Li, X.; Lin, Y.S. Delayed initial radioiodine therapy related to incomplete response in low- to intermediate-risk differentiated thyroid cancer. Clin. Endocrinol. 2018, 88, 601–606. [Google Scholar] [CrossRef]
- Gertz, A.H.; Pollack, C.C.; Schultheiss, M.D.; Brownstein, J.S. Delayed medical care and underlying health in the United States during the COVID-19 pandemic: A cross-sectional study. Prev. Med. Rep. 2022, 28, 101882. [Google Scholar] [CrossRef]
- Boffa, D.J.; Rosen, J.E.; Mallin, K.; Loomis, A.; Gay, G.; Palis, B.; Thoburn, K.; Gress, D.; McKellar, D.P.; Shulman, L.N.; et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017, 3, 1722–1728. [Google Scholar] [CrossRef]
- us_regdiv.pdf [Internet]. Available online: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf (accessed on 3 April 2023).
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef]
- Sharperson, C.; Hanna, T.N.; Herr, K.D.; Zygmont, M.E.; Gerard, R.L.; Johnson, J.O. The effect of COVID-19 on emergency department imaging: What can we learn? Emerg. Radiol. 2021, 28, 339–347. [Google Scholar] [CrossRef]
- Silveira, V.B.; Schwengber, W.K.; Hetzel, G.M.; Zanella, A.B.; Scheffel, R.S.; Maia, A.L.; Dora, J.M. Effect of COVID-19 pandemic on diagnosis and treatment of thyroid cancer in Brazil. Front. Endocrinol. 2022, 13, 995329. [Google Scholar] [CrossRef]
- Escobar, N.; DiMaggio, C.; Pocock, B.; Pescovitz, A.; McCalla, S.; Joseph, K.A. Effects of COVID-19 on Surgical Delays in Patients with Breast Cancer in NYC Public Hospitals: A Multicenter Study. Ann. Surg. Oncol. 2023, 30, 23–30. [Google Scholar] [CrossRef]
- Pergolotti, M.; Pisegna, J.; Chien, L.C.; BrintzenhofeSzoc, K.; Kaur, A.; Battisti, N.; Canin, B.; Malone, M.V.; Shahrokni, A.; Plotkin, E.; et al. Healthcare providers’ experiences of continuing care for older adults with cancer during the COVID-19 pandemic. J. Cancer Surviv. 2023, 1–8. [Google Scholar]
- Lopez, B.; Fligor, S.C.; Randolph, G.W.; James, B.C. Inequities in Thyroid Cancer Care: Populations Most at Risk for Delays in Diagnosis and Treatment. Thyroid 2023, 33, 724–731. [Google Scholar] [CrossRef]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).