Abstract
A solitary bone plasmacytoma is a rare tumor. Intrahepatic cholangiocarcinoma is the second most common primary liver cancer after hepatocellular carcinoma. We present the case of a 48-year-old female patient who consulted for recent back pain, with a final diagnosis of T10 solitary plasmacytoma and synchronous intrahepatic cholangiocarcinoma. Imaging suggested cholangiocarcinoma with bone metastasis. The patient underwent neurosurgical management with laminectomy, arthrodesis, and arthrectomy, with biopsies revealing monotypic kappa plasmacytic proliferation. Liver biopsies revealed an adenocarcinoma with expression of cytokeratin 19, cytokeratin 7, N-cadherin, and high expression of carbonic anydrase IX. The plasmacytoma was treated with external radiotherapy. The cholangiocarcinoma was treated with selective internal radiation therapy and concomitant systemic treatment with combinations of cisplatin and durvalumab, with capecitabine during radiotherapy, switched for gemcitabine after completion of irradiation. One year after initial management, imaging revealed a partial metabolic response of the intrahepatic cholangiocarcinoma, and a complete metabolic response of the plasmacytoma. This case illustrates the importance of not ignoring two primary tumors and the management of two concomitant treatments exploiting potential therapeutic synergies and limiting expected toxicities.
1. Introduction
This case report outlines the presentation and management of a 48-year-old female patient who initially sought medical attention for dorsal pain. Despite a lack of significant medical history, the diagnostic journey revealed a challenging combination of pathologies—solitary bone plasmacytoma and synchronous unresectable intrahepatic cholangiocarcinoma. The report provides an account of the clinical presentation, radiological characteristics, and the management of therapies aiming at limiting toxicities and exploiting synergistic effects.
2. Case Presentation
A 48-year-old female patient presented with back pain. She had no previous history apart from a personal history of colonic polyps (last colonoscopy 5 years ago) and a second-degree family history of colorectal cancer. She was not taking any medication, and had no chronic alcohol abuse. The medical history began with a fall and persistent back pain. The WHO score was 0, and appetite was preserved without any weight loss. There was no neurological deficit, no pain on spinal percussion, and no abdominal pain. Physical examination was unremarkable. Routine laboratory tests were normal, except Gamma glutamyl transferase being three times the upper limit value.
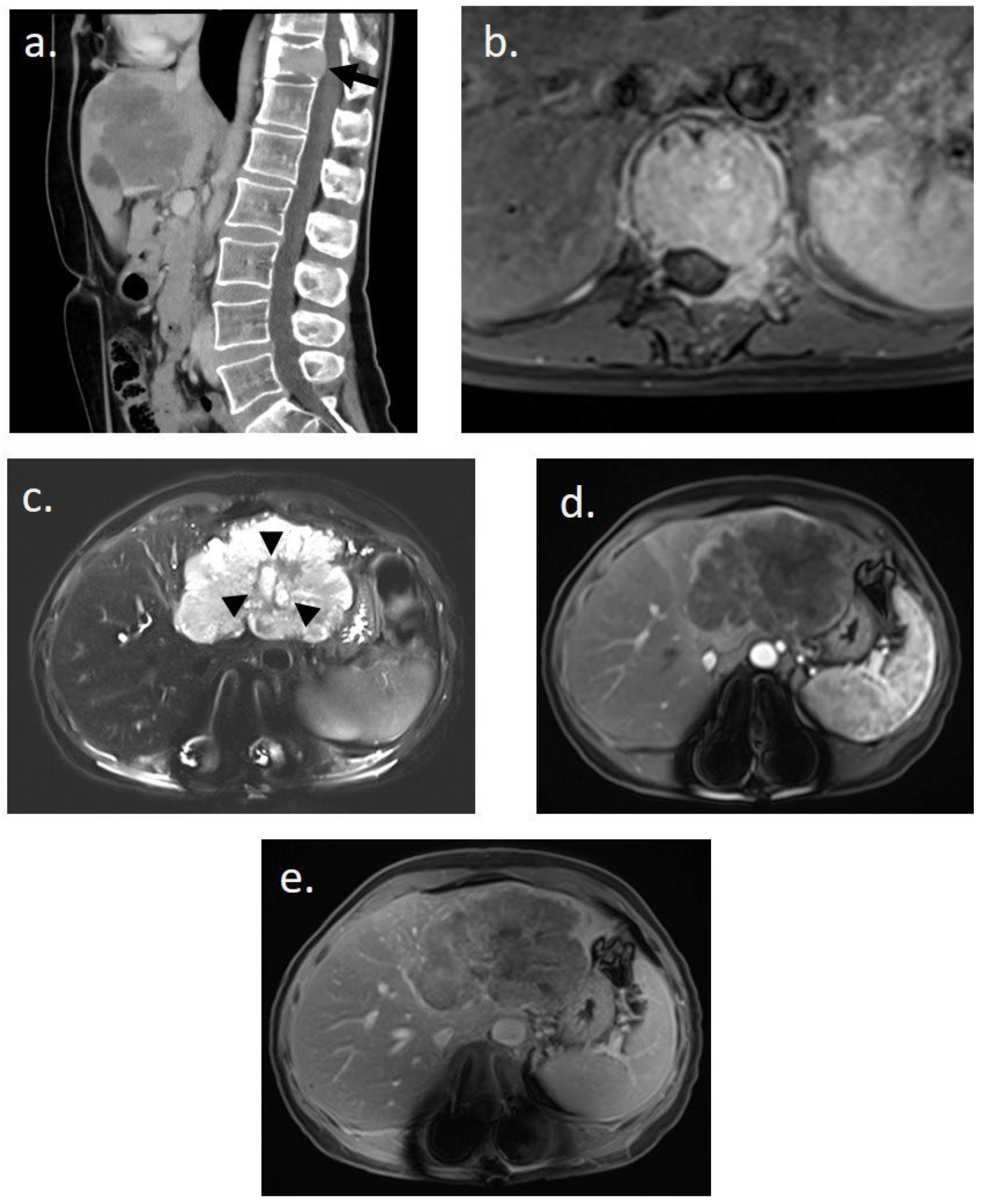
She underwent thoracic–abdominal–pelvic CT scan and spinal MRI on 20 March 2023 (Figure 1). Imaging revealed a 9 cm mass in the left liver, hypodense at different stages of contrast enhancement, with a central artery, dilatation of the intrahepatic bile ducts of segment II, and thrombosis of the left portal branch. There was no hepatic dysmorphism. Imaging also revealed an osteolytic lesion of the vertebral body of T11, with bulging of the posterior wall, canal narrowing, partial extension to the left pedicle, fracture with wedge-shaped compression, and invasion of the T11–D12 foramen on the left. The results suggested in the first instance a primary liver tumor associated with a vertebral metastasis.
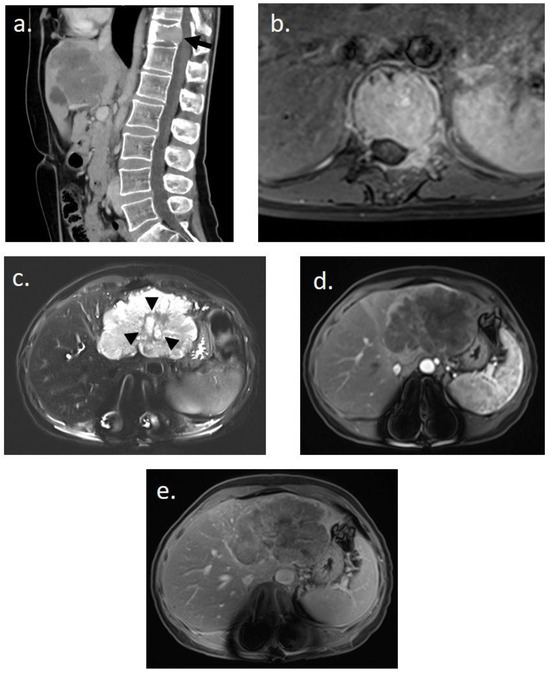
Figure 1.
Initial imaging assessment for a 48-year-old woman presenting with lower back pain. (a) Abdomino-pelvic CT scan in sagittal slice post-contrast injection revealing a pathological fracture of T11 on a tissue-like lytic lesion (black arrow) and an infiltrating tissue lesion in the left hepatic lobe. (b) Spinal MRI in axial T1-weighted post-contrast injection showing complete tissue infiltration of the T11 vertebral body and the left posterior arch with epidural invasion. (c–e) Hepatic MRI performed immediately after spinal stabilization surgery. (c) Axial T2-weighted image with fat suppression showing a hyperintense polylobed lesion in the left lobe with a central possibly necrotic liquid zone (black arrowhead) on non-dysmorphic liver. (d,e) Enhanced axial T1-weighted images with fat suppression at (d) arterial and (e) 5 min delayed phases reveal a unique infiltrative hypovascular lesion with early peripheral rim enhancement. This imaging assessment was suggestive of a primary malignant hepatic lesion.
She was first hospitalized to undergo urgent neurosurgical management with laminectomy, arthrodesis, and arthrectomy. Preoperative biopsy revealed a well-differentiated, monotypic kappa plasmacytic proliferation. Serum protein electrophoresis revealed monoclonal IgG Kappa (0.7 g/dL). Bone marrow aspirate confirmed the absence of clonal plasma cells. PET-CT performed on 18 April 2023 revealed a unique hypermetabolic bone lesion of T11 (SUVmax 41), in addition to a large hypermetabolic mass of the liver (SUVmax 9) related to cholangiocarcinoma, allowing the diagnosis of solitary bone plasmacytoma.
She had a liver biopsy of the hepatic lesion, which revealed an adenocarcinoma with expression of cytokeratin 19, cytokeratin 7, N-cadherin, and high expression of carbonic anydrase IX. In immunohistochemistry analysis, there was no HER2 expression, nor mismatch repair deficiency. No targetable mutation or fusion abnormality was detected by DNA next-generation panel sequencing (BRAF wild-type and microsatellite stable status, no IDH1 mutation) and RNA panel sequencing (notably, no FGFR or NTRK fusion).
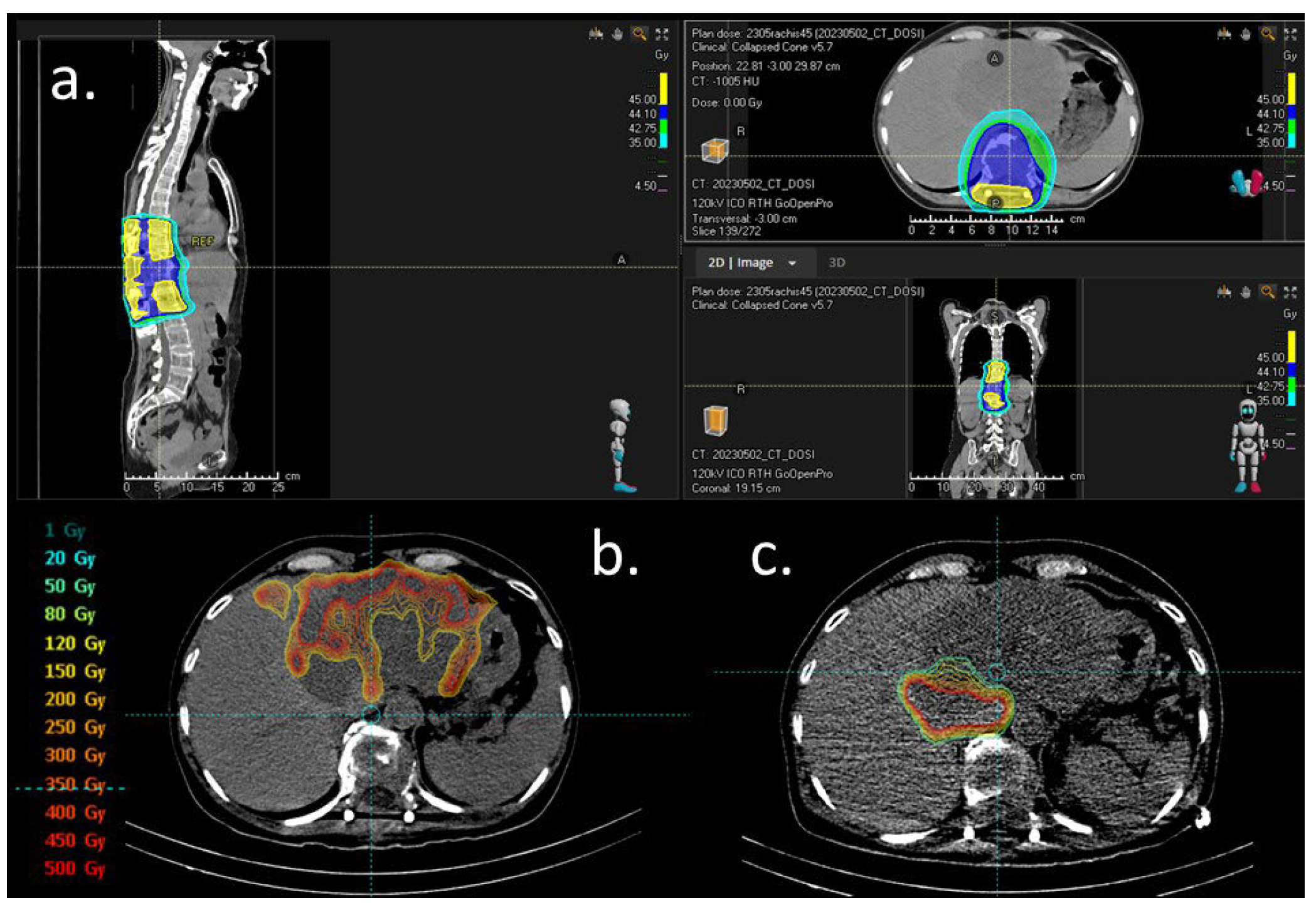
Systemic therapy was initiated on 10 May 2023 with two cycles of capecitabine/cisplatin/durvalumab chemotherapy. The plasmacytoma was treated with IMRT to 45 Gy in 25 fractions from 17 May 2023 to 28 June 2023 (Figure 2a). In October 2023, imaging and biochemistry assessment of SP confirmed a complete response.
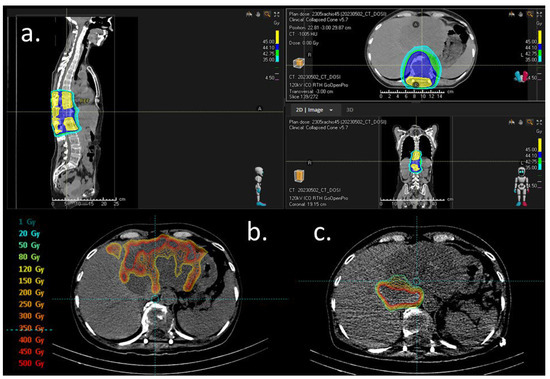
Figure 2.
Dosimetry figures. (a) External beam radiation for solitary plasmocytoma. Isodose distribution in color wash, scale right. (b) Dosimetry simulation after 1st work-up with 99 mTc-macroaggregated albumin (MAA) injection in the left branch of the hepatic artery (23 May 2023). Distribution of the radiotracer on the left hepatic lesion with less uptake in two areas: segment II (initially attributed to a necrosis plage) and the second right side of the tumor (depending on another feeding artery). (c) Dosimetry simulation after second work-up with 99 mTc-MAA injection on a right artery branch (segment I and partially segment VIII on the CBCT) (8 June 2023). The distribution of the radiotracer showed less uptake than that in the first work-up.
Selective internal radiation therapy (SIRT) with Yttrium-90 microspheres (Theraspheres®) was performed with Theraspheres® injected into the left branch of the hepatic artery (23 May 2023) and during a second procedure in a branch of the right hepatic artery (8 June 2023, enabling targeting almost all of the lesion (a central plage with less uptake was initially attributed to the necrosis part of the lesion)). The total injected dose of Yttriu-m-90 was 7.5 GBq (Figure 2b,c).
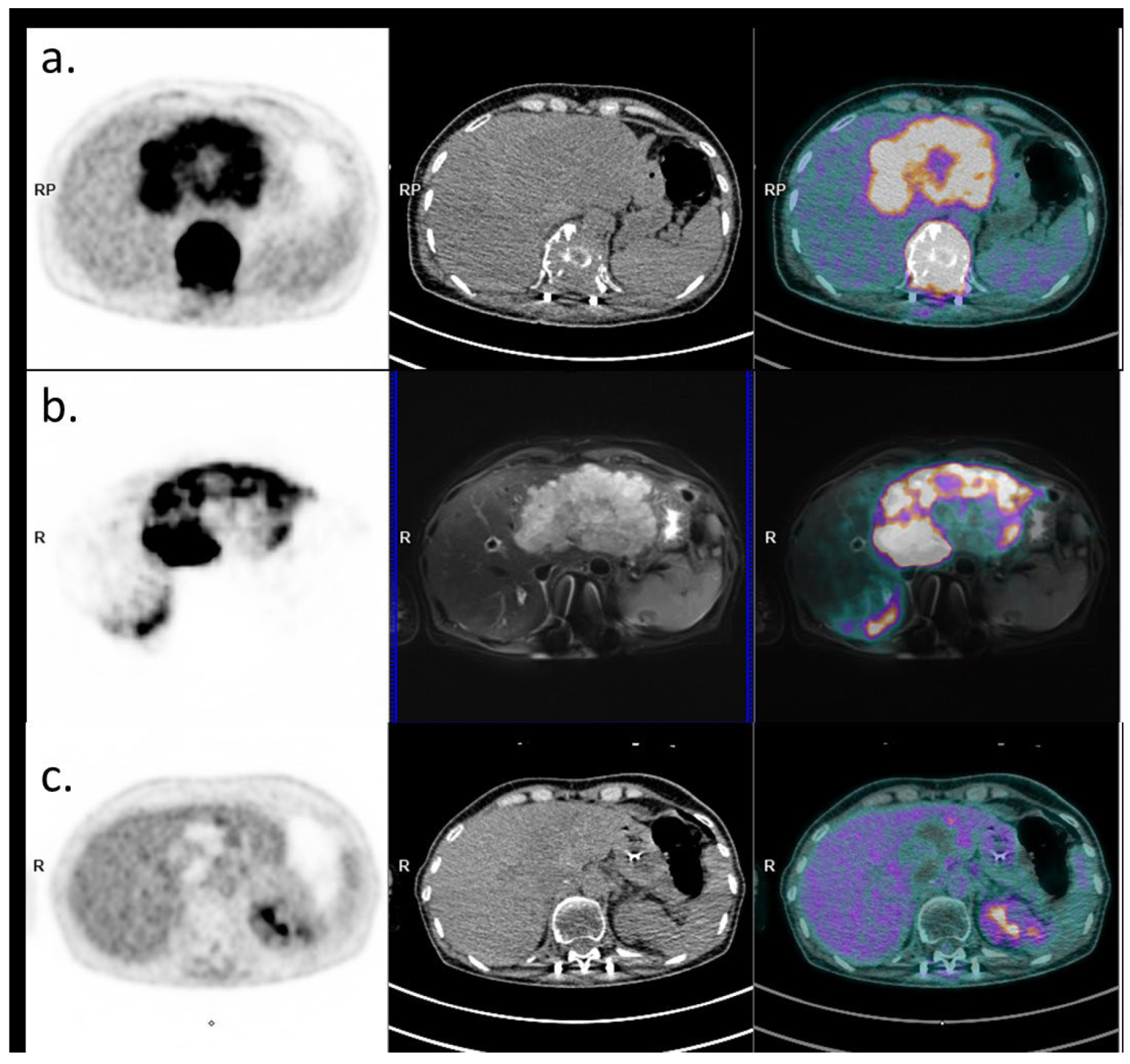
Following both external radiation and SIRT, the chemotherapy regimen was switched to a GEMCIS durvalumab protocol for subsequent cycles. A CT scan in September 2023 showed RECIST stability and a partial mRECIST response in the cholangiocarcinoma. PET-CT performed in December 2023 revealed a partial metabolic response of the intrahepatic cholangiocarcinoma, and a complete metabolic response of the plasmocytoma (Figure 3). Durvalumab was continued as monotherapy and interrupted in May 2024 after one year of treatment. A surveillance program was then initiated.
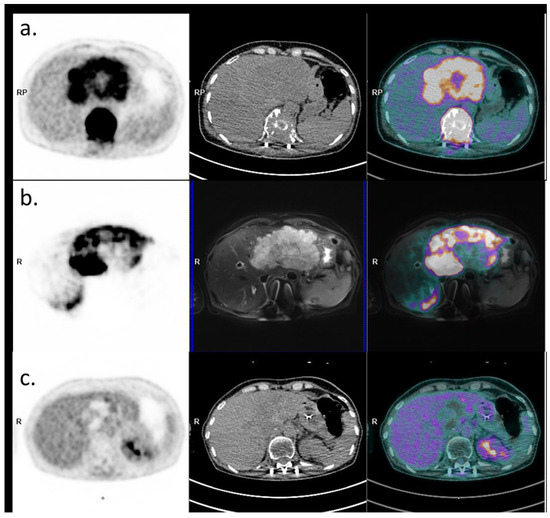
Figure 3.
Baseline and post-therapeutic PET imaging (PET image on the left, CT on the middle, and fusion image on the right). (a) Initial 18FDG PET-CT evaluation (18 April 2024) showing the large hypermetabolic liver lesion and the hypermetabolic plasmacytoma. (b) PET-MRI evaluation post-Yttrium-90 injection with PET image on the left, MRI (axial T2 fatSat sequence) in the middle, and fusion on the right, showing good targeting of the liver lesion (22 June 2024). (c) 18FDG PET-CT evaluation 6 months later showing a complete metabolic response of the plasmacytoma and an excellent response of the liver lesion (persistence of a viable upper segment II residue).
3. Discussion
We report the case of a synchronous presentation of a hepatic lesion and a vertebral lesion, leading to the concomitant diagnosis of intrahepatic cholangiocarcinoma and vertebral plasmacytoma. Such a situation is a diagnostic trap, potentially leading to the conclusion of a metastatic lesion. A precise diagnosis and biopsy of two synchronous lesions should be discussed at a multidisciplinary meeting, to avoid misdiagnosing two different synchronous histological lesions, and to consider the treatment of both primary tumors rather than a metastatic disease. In the case of two primary tumors, one challenge is to identify the best therapeutic strategy, aiming at exploiting synergistic therapeutic effects and limiting cumulative toxicities. In the present case, radiation therapy has been used both for the treatment of plasmacytoma (external beam radiation therapy) and cholangiocarcinoma (intra-arterial radiation therapy). The durvalumab plus gemcitabine and cisplatin protocol was modified at the time of radiotherapy (SIRT and external radiotherapy) by replacing gemcitabine with capecitabine, to avoid the potential toxicity of the combination of gemcitabine and irradiation [1]. Both irradiations were concomitant with durvalumab.
There is growing evidence that the combination of radiotherapy and immunotherapy could have a synergistic effect. Immunity has been identified as a key factor in the response to radiotherapy. A dead tumor cell releases a cascade of signals and ligands into the microenvironment and expresses surface receptors that activate immunity. This process then results in the release of cytokines, cell death and damage factors, leading to interactions with T cells and dendritic cells [2]. Enhanced immunogenicity can be achieved with anti-CTLA-4, anti-PD-1, and anti-PD-L1 monoclonal antibodies [3,4]. Combining radiotherapy with an immunomodulator can induce a distant effect on non-irradiated sites, which is called the abscopal effect, highlighting the potential synergy [5]. Immunity has been identified as a key factor in the response to radiotherapy. A dead tumor cell releases a cascade of signals and ligands into the microenvironment and expresses surface receptors that activate immunity. This process then results in the release of cytokines, cell death and damage factors, leading to interactions with dendritic cells and T cells [2]. Anti-CTLA-4, anti-PD-1, and anti-PD-L1 monoclonal antibodies can potentiate tumor immunogenicity [3,4]. Radiation can also enhance the immune response by upregulating MHC class I, activating dendritic cells, increasing cross-presentation of tumor antigens, and promoting immune cell infiltration [2,5,6].
Biliary tract cancers may be classified into immune “hot” and “cold” depending on their cytotoxic lymphocyte (CTL) density. The immune “hot” type has a high density of CTLs and is associated with higher response rates to ICB, and vice versa [7]. In a retrospective analysis of surgical specimens of patients treated for HCC, without preoperative treatment (n = 32), after preoperative TACE (n = 16), or after preoperative SIRT (n = 2), SIRT was associated with a significant increase in Tumor-Infiltrating Lymphocytes, CD4+ and CD8+ T cells, and Granzyme-B expression compared to TACE or no preoperative treatment [8]. The combination of SIRT with gemcitabine and cisplatin demonstrated promising results in a prospective phase II study [9,10]. A recently published analysis derived from prospective clinical trials suggests that SIRT combined with chemotherapy might also improve outcomes over chemotherapy alone in patients with advanced liver-only iCCA [11]. The first-line treatment for advanced cholangiocarcinoma is now the combination of chemotherapy (gemcitabine/cisplatin) plus immunotherapy (durvalumab or pembrolizumab), regardless of mutational status [12,13]. Thus, there is a rationale for prospective randomized trials investigating combinations of SIRT and systemic treatments including immunotherapy.
A solitary bone plasmacytoma is defined as a single lytic lesion caused by monoclonal plasma cell infiltration, with or without soft tissue extension. Solitary plasmacytomas are highly sensitive to radiation, with local control ranging between 79 and 91%, giving the possibility for radiation therapy to be a curative treatment [14]. Depending on tumor size and localization, the recommended dose ranges between 40 and 50 Gy [15]. Radiotherapy is the standard of care for the treatment of solitary bone plasmacytoma, and no systemic therapy has been approved or recommended to date for these patients [16,17]. Even if the PD1/PDL1 axis seems to be an interesting target because most multiple myeloma cells express PDL1, the use of immune checkpoint inhibitors (ICIs) in monotherapy did not provide any clinical benefit in these patients in previous reports [18]. To our knowledge, there is no published experience of anti-PD-1/PD-L1 immunotherapy in patients with solitary plasmacytoma and therefore no data to support a potential synergy between this class of drug with radiotherapy in this context.
4. Conclusions
This case highlights the complexity of managing synchronous plasmacytoma and cholangiocarcinoma, with a challenging diagnostic approach, and the need for multidisciplinary management to exploit potential synergistic therapeutic effects, particularly those of combining radiotherapy and immunotherapy.
Author Contributions
Y.T., M.B., L.P.-F., B.J., E.S., L.O. and D.B. were involved in the clinical management of the patients and were major contributors in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper and accompanying images.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Yann Touchefeu has received fees for advisory/consultancy roles from Astra Zeneca and MSD. Matthieu Barbaud has received fees for advisory/consultancy roles from Boston Scientific. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Masson, I.; Supiot, S.; Doutriaux-Dumoulin, I.; Thillays, F. Report of a Unique Case of Gemcitabine-Induced Radiation Recall Myelitis Following Spinal Cord Irradiation. BJR Case Rep. 2020, 6, 20190118. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and Checkpoint Blockade Immunotherapy: Radiosensitisation and Potential Mechanisms of Synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Probst, H.C.; Vuong, V.; Landshammer, A.; Muth, S.; Yagita, H.; Schwendener, R.; Pruschy, M.; Knuth, A.; van den Broek, M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 2012, 189, 558–566. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, C.; Lu, S.; Xu, Y.; Li, Z.; Jiang, H.; Ma, Y. Tumor-Associated Macrophages in Cholangiocarcinoma: Complex Interplay and Potential Therapeutic Target. EBioMedicine 2021, 67, 103375. [Google Scholar] [CrossRef] [PubMed]
- Craciun, L.; de Wind, R.; Demetter, P.; Lucidi, V.; Bohlok, A.; Michiels, S.; Bouazza, F.; Vouche, M.; Tancredi, I.; Verset, G.; et al. Retrospective Analysis of the Immunogenic Effects of Intra-Arterial Locoregional Therapies in Hepatocellular Carcinoma: A Rationale for Combining Selective Internal Radiation Therapy (SIRT) and Immunotherapy. BMC Cancer 2020, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-Line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Goislard de Monsabert, C.; Touchefeu, Y.; Guiu, B.; Campillo-Gimenez, B.; Farges, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Beuzit, L.; Pracht, M.; et al. Selective Internal Radiation Combined with Chemotherapy Maintains the Quality of Life in Intrahepatic Cholangiocarcinomas. Curr. Oncol. 2021, 28, 4530–4541. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Bridgewater, J.; Campillo-Gimenez, B.; Neveu, E.; Phelip, J.-M.; Neuzillet, C.; Boudjema, K.; Rolland, Y.; Valle, J.W.; Garin, E.; et al. Chemotherapy with or without Selective Internal Radiation Therapy for Intrahepatic Cholangiocarcinoma: Data from Clinical Trials. Hepatology 2024, 79, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Durvalumab Plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer|NEJM Evidence. Available online: https://evidence.nejm.org/doi/full/10.1056/EVIDoa2200015 (accessed on 17 May 2024).
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. KEYNOTE-966 Investigators. Pembrolizumab in Combination with Gemcitabine and Cisplatin Compared with Gemcitabine and Cisplatin Alone for Patients with Advanced Biliary Tract Cancer (KEYNOTE-966): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, L.; Debbi, K.; To, N.-H.; Cailleteau, A.; Supiot, S.; Mervoyer, A.; Guimas, V.; Belkacémi, Y. Is Oligometastatic Disease an Applicable and Useful Concept in Haematologic Malignancies? A Narrative Review of Radiation Therapy Standards, Modern Techniques, and Innovations. Cancer Radiother. 2024, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.W.; Campbell, B.A.; Goda, J.S.; Kelsey, C.R.; Kirova, Y.M.; Parikh, R.R.; Ng, A.K.; Ricardi, U.; Suh, C.-O.; Mauch, P.M.; et al. Radiation Therapy for Solitary Plasmacytoma and Multiple Myeloma: Guidelines From the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzeau, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, Treatment, and Response Assessment in Solitary Plasmacytoma: Updated Recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Mheidly, K.; Lamy De La Chapelle, T.; Hunault, M.; Benboubker, L.; Benchalal, M.; Moreau, P.; Baugier de Materre, A.; Decaux, O.; Laribi, K. New Insights in the Treatment of Patients with Solitary Bone Plasmacytoma. Leuk. Lymphoma 2019, 60, 2810–2813. [Google Scholar] [CrossRef] [PubMed]
- Le Calvez, B.; Moreau, P.; Touzeau, C. Immune Checkpoint Inhibitors for the Treatment of Myeloma: Novel Investigational Options. Expert. Opin. Investig. Drugs 2021, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).