Low CD8+ Density Variation and R1 Surgical Margin as Independent Predictors of Early Post-Resection Recurrence in HCC Patients Meeting Milan Criteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemistry
2.3. Digital Image Analysis and Indicator Extraction
2.4. Statistical Analysis and Modeling
3. Results
3.1. Summary of Patient Cohort Characteristics
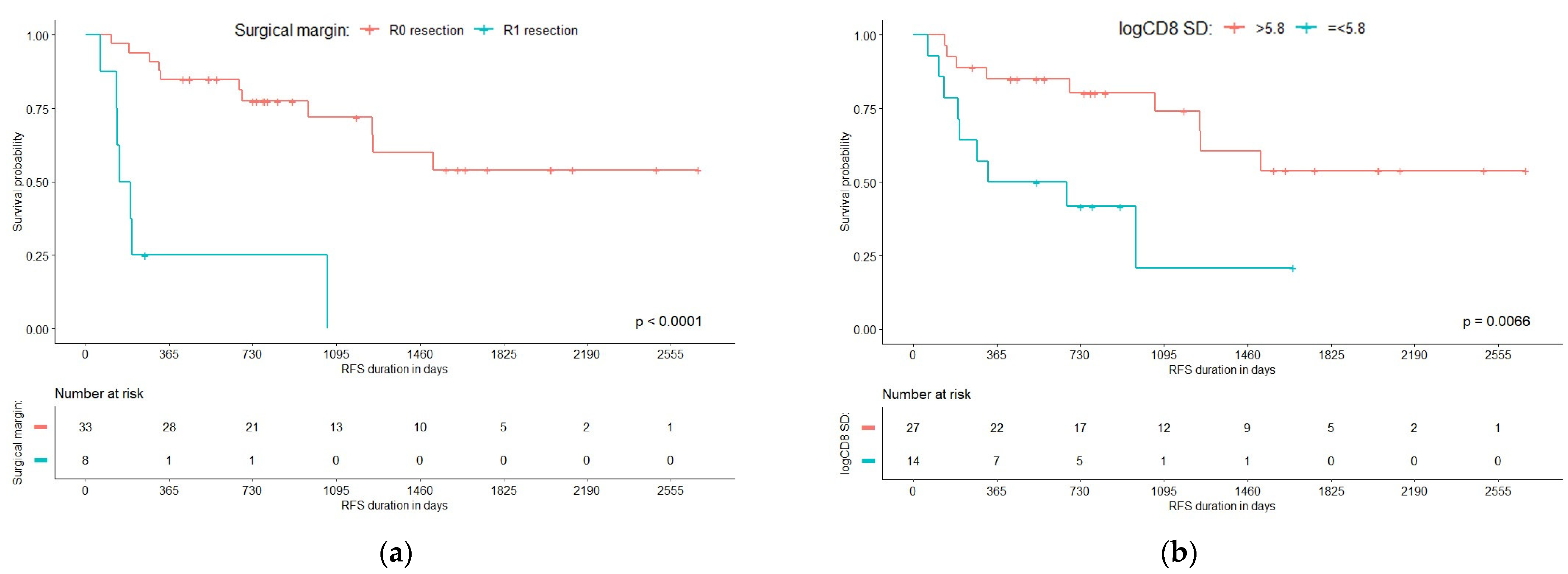
3.2. Predictors of Recurrence-Free Survival
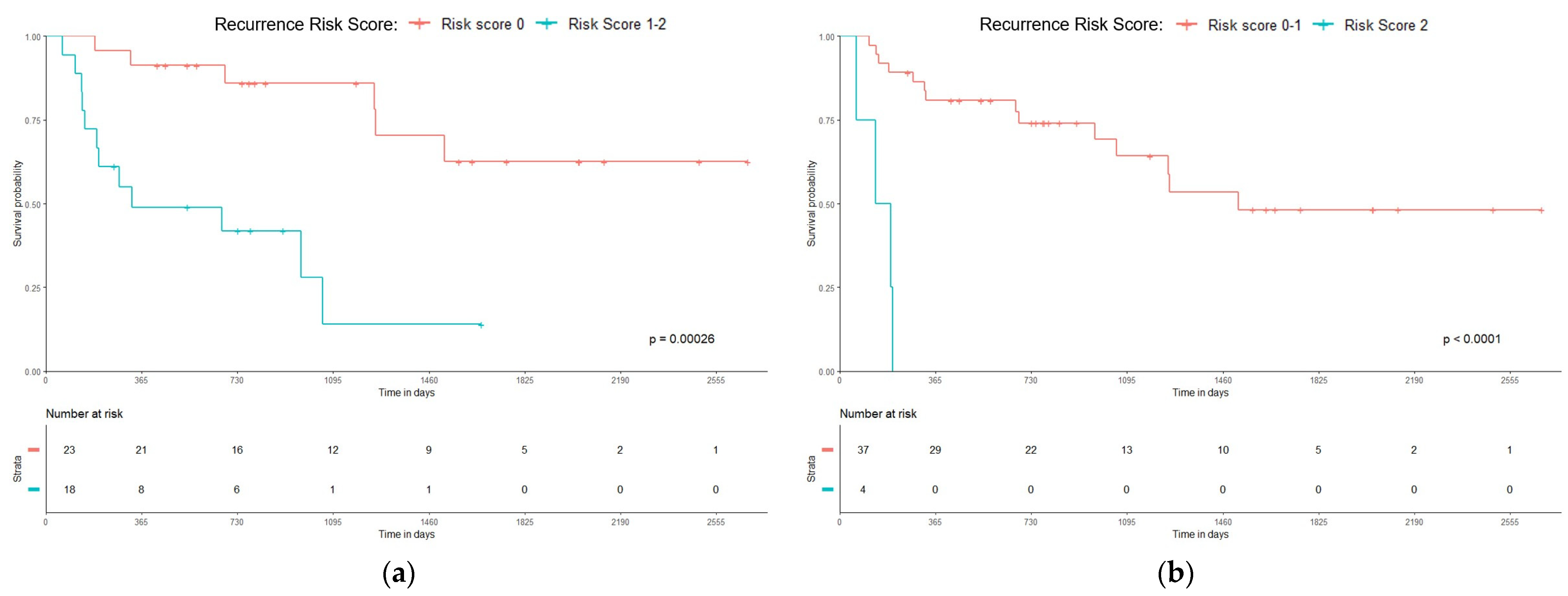
3.3. Recurrence Risk Score
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Chan, A.W.H.; Zhong, J.H.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.Q.; Tada, T.; Chong, C.C.N.; Xiang, B.D.; Li, L.Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef]
- Nevola, R.; Ruocco, R.; Criscuolo, L.; Villani, A.; Alfano, M.; Beccia, D.; Imbriani, S.; Claar, E.; Cozzolino, D.; Sasso, F.C.; et al. Predictors of early and late hepatocellular carcinoma recurrence. World J. Gastroenterol. 2023, 29, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Kotsari, M.; Dimopoulou, V.; Koskinas, J.; Armakolas, A. Immune System and Hepatocellular Carcinoma (HCC): New Insights into HCC Progression. Int. J. Mol. Sci. 2023, 24, 11471. [Google Scholar] [CrossRef]
- Schoenberg, M.B.; Li, X.K.; Li, X.Y.; Han, Y.S.; Hao, J.C.; Miksch, R.C.; Koch, D.; Börner, N.; Beger, N.T.; Bucher, J.N.; et al. The predictive value of tumor infiltrating leukocytes in Hepatocellular Carcinoma: A systematic review and meta-analysis. EJSO-Eur. J. Surg. Oncol. 2021, 47, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Z.; Tan, Y.L.; Qian, Y.; Xue, W.B.; Wang, Y.B.; Du, J.G.; Jin, L.; Ding, W. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+T cells in patients with hepatocellular carcinoma A meta-analysis. Medicine 2019, 98, e13923. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Oi, K.; Hirooka, Y.; Kaibara, N. CD8+lymphocyte infiltration and apoptosis in hepatocellular carcinoma. Eur. J. Surg. Oncol. 2004, 30, 53–57. [Google Scholar] [CrossRef]
- Ramzan, M.; Sturm, N.; Decaens, T.; Bioulac-Sage, P.; Bancel, B.; Merle, P.; Nhieu, J.T.V.; Slama, R.; Letoublon, C.; Zarski, J.P.; et al. Liver-infiltrating CD8+ lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int. 2016, 36, 434–444. [Google Scholar] [CrossRef]
- Ding, W.; Xu, X.Z.; Qian, Y.; Xue, W.B.; Wang, Y.B.; Du, J.G.; Jin, L.; Tan, Y.L. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma A meta-analysis. Medicine 2018, 97, e13301. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.L.; Fu, H.Y.; Li, J.X.; Xu, L.; Wang, G.S.; Wu, H. Peritumoral Tertiary Lymphoid Structures Correlate With Protective Immunity and Improved Prognosis in Patients With Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 648812. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzaferro, V.; Toso, C.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Karakas, S.; Yilmaz, S.; Ince, V.; Akbulut, S.; Dalda, Y.; Akatli, A.N.; Kahraman, A.S.; Kutlu, R.; Carr, B.I. Comparison of liver resection and living donor liver transplantation in patients with hepatocellular carcinoma within Milan criteria and well-preserved liver function. Hepatol. Forum 2023, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Tan, D.J.H.; Ong, Y.; Lim, W.H.; Ng, C.H.; Tay, P.W.L.; Yong, J.N.; Muthiah, M.D.; Tan, E.X.; Pang, N.Q.; et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: A meta-analysis of 18,421 patients. Hepatobil. Surg. Nutr. 2022, 11, 78. [Google Scholar] [CrossRef]
- Anselmo, A.; Siragusa, L.; Brigato, P.; Riccetti, C.; Collini, A.; Sensi, B.; Tisone, G. Primary versus Salvage Liver Transplantation after Curative-Intent Resection or Radiofrequency Ablation for Hepatocellular Carcinoma: Long-Term Oncological Outcomes. Cancers 2023, 15, 5030. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Filgueira, N.A. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef]
- Tabrizian, P.; Holzner, M.L.; Mehta, N.; Halazun, K.; Agopian, V.G.; Yao, F.; Busuttil, R.W.; Roberts, J.; Emond, J.C.; Samstein, B.; et al. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022, 157, 779–788. [Google Scholar] [CrossRef]
- Stulpinas, R.; Zilenaite-Petrulaitiene, D.; Rasmusson, A.; Gulla, A.; Grigonyte, A.; Strupas, K.; Laurinavicius, A. Prognostic Value of CD8+Lymphocytes in Hepatocellular Carcinoma and Perineoplastic Parenchyma Assessed by Interface Density Profiles in Liver Resection Samples. Cancers 2023, 15, 366. [Google Scholar] [CrossRef]
- Rasmusson, A.; Zilenaite, D.; Nestarenkaite, A.; Augulis, R.; Laurinaviciene, A.; Ostapenko, V.; Poskus, T.; Laurinavicius, A. Immunogradient Indicators for Antitumor Response Assessment by Automated Tumor-Stroma Interface Zone Detection. Am. J. Pathol. 2020, 190, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Orhan, A.; Khesrawi, F.; Madsen, M.T.; Vogelsang, R.P.; Dohrn, N.; Fiehn, A.M.K.; Gogenur, I. Tumor-Infiltrating Lymphocytes as Biomarkers of Treatment Response and Long-Term Survival in Patients with Rectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 636. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.P.; Sánchez-Canteli, M.; López, F.; Wolf, G.T.; Hernández-Prera, J.C.; Williams, M.D.; Willems, S.M.; Franchi, A.; Coca-Pelaz, A.; Ferlito, A. Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Biomedicines 2021, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Jin, W.J.; Wang, S.S.; Ding, H.G. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 729705. [Google Scholar] [CrossRef]
- Krijgsman, D.; van Leeuwen, M.B.; van der Ven, J.; Almeida, V.; Vlutters, R.; Halter, D.; Kuppen, P.J.K.; van de Velde, C.J.H.; Wimberger-Friedl, R. Quantitative Whole Slide Assessment of Tumor-Infiltrating CD8-Positive Lymphocytes in ER-Positive Breast Cancer in Relation to Clinical Outcome. IEEE J. Biomed. Health 2021, 25, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.L.; Yao, Q.Y.; Wang, Y.F.; Mao, Z.Z.; Zhang, T.C.; Li, J.H.; Nie, Y.; Lei, X.J.; Shi, W.; Song, W.J. Protective effect of tertiary lymphoid structures against hepatocellular carcinoma: New findings from a genetic perspective. Front. Immunol. 2022, 13, 1007426. [Google Scholar] [CrossRef]
- Jia, W.L.; Zhang, T.C.; Yao, Q.Y.; Li, J.H.; Nie, Y.; Lei, X.J.; Mao, Z.Z.; Wang, Y.F.; Shi, W.; Song, W.J. Tertiary Lymphatic Structures in Primary Hepatic Carcinoma: Controversy Cannot Overshadow Hope. Front. Immunol. 2022, 13, 870458. [Google Scholar] [CrossRef]
- Li, J.H.; Nie, Y.; Jia, W.L.; Wu, W.L.; Song, W.J.; Li, Y.X. Effect of Tertiary Lymphoid Structures on Prognosis of Patients with Hepatocellular Carcinoma and Preliminary Exploration of Its Formation Mechanism. Cancers 2022, 14, 5157. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Yang, Y.M.; Deng, X.Q.; Chao, N.N.; Chen, Z.H.; Ye, Y.X.; Zhang, W.Y.; Liu, W.P.; Zhao, S. High CD8+ tumor-infiltrating lymphocytes indicate severe exhaustion and poor prognosis in angioimmunoblastic T-cell lymphoma. Front. Immunol. 2023, 14, 1228004. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331. [Google Scholar] [CrossRef]
- Li, K.K.; Adams, D.H. Antitumor CD8+ T Cells in Hepatocellular Carcinoma: Present but Exhausted. Hepatology 2014, 59, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Barsch, M.; Salié, H.; Schlaak, A.E.; Zhang, Z.; Hess, M.; Mayer, L.S.; Tauber, C.; Otto-Mora, P.; Ohtani, T.; Nilsson, T.; et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 2022, 77, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Drefs, M.; Schoenberg, M.B.; Börner, N.; Koliogiannis, D.; Koch, D.T.; Schirren, M.J.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Guba, M.O. Changes of long-term survival of resection and liver transplantation in hepatocellular carcinoma throughout the years: A meta-analysis. EJSO-Eur. J. Surg. Oncol. 2024, 50, 107952. [Google Scholar] [CrossRef]
- Donadon, M.; Terrone, A.; Procopio, F.; Cimino, M.; Palmisano, A.; Viganò, L.; Del Fabbro, D.; Di Tommaso, L.; Torzilli, G. Is R1 vascular hepatectomy for hepatocellular carcinoma oncologically adequate? Analysis of 327 consecutive patients. Surgery 2019, 165, 897–904. [Google Scholar] [CrossRef]
- Lim, C.; Salloum, C.; Lahat, E.; Sotirov, D.; Eshkenazy, R.; Shwaartz, C.; Azoulay, D. Impact of narrow margin and R1 resection for hepatocellular carcinoma on the salvage liver transplantation strategy. An intention-to-treat analysis. HPB 2019, 21, 1295–1302. [Google Scholar] [CrossRef]
- Field, W.B.S.; Rostas, J.W.; Philps, P.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G. Wide versus narrow margins after partial hepatectomy for hepatocellular carcinoma: Balancing recurrence risk and liver function. Am. J. Surg. 2017, 214, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Guo, R.P.; Lin, X.J.; Zhang, Y.Q.; Chen, M.S.; Zhang, C.Q.; Lau, W.Y.; Li, J.Q. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma—A prospective randomized trial. Ann. Surg. 2007, 245, 36–43. [Google Scholar] [CrossRef]
- Shimada, K.; Sakamoto, Y.; Esaki, M.; Kosuge, T. Role of the width of the surgical margin in a hepatectomy for small hepatocellular carcinomas eligible for percutaneous local ablative therapy. Am. J. Surg. 2008, 195, 775–781. [Google Scholar] [CrossRef]
- Poon, R.T.P.; Fan, S.T.; Ng, I.O.L.; Wong, J. Significance of resection margin in hepatectomy for hepatocellular carcinoma—A critical reappraisal. Ann. Surg. 2000, 231, 544–551. [Google Scholar] [CrossRef]
- Mohan, B.P.; Iriana, S.; Khan, S.R.; Yarra, P.; Ponnada, S.; Gallegos-Orozco, J.F. Outcomes of liver transplantation in patients 70 years or older: A systematic review and meta-analysis. Ann. Hepatol. 2022, 27, 100741. [Google Scholar] [CrossRef]

| Characteristic | Value (%) |
|---|---|
| Patients | 41 (100%) |
| Age, years: Mean (SD) Median (IQR) | 64.4 (9.16) 66 (12) |
| Age distribution <50 years 50–59 years 60–69 years 70–79 years ≥80 years | 1 (2.4%) 10 (24.4%) 18 (43.9%) 11 (26.8%) 1 (2.4%) |
| Gender Males Females | 28 (68.3%) 13 (31.7%) |
| Tumor grade G1 G2 G3 | 4 (9.8%) 29 (70.7%) 8 (19.5%) |
| pT stage: pT1 pT2 | 27 (65.9%) 14 (34.1%) |
| Intravascular invasion LVI present LVI absent | 12 (29.3%) 29 (70.7%) |
| Resection margin R0 R1 | 33 (80.5%) 8 (19.5%) |
| Number of tumors One tumor Two tumors Three tumors | 32 (78.0%) 7 (17.1%) 2 (4.9%) |
| Tumor size in the pathology report, mm Mean (SD) Median (IQR) | 28 (11) 25 (20) |
| Recurrences HCC recurrence No recurrence | 18 (43.9%) 23 (56.1%) |
| RFS time, days Mean (SD) Median (IQR) | 904.4 (702.9) 749 (933) |
| Indicator | DF | Parameter Estimates | Standard Error | Chi-Square | p-Value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | |
|---|---|---|---|---|---|---|---|---|
| R1 resection | 1 | 1.96882 | 0.59935 | 10.7909 | 0.0010 | 7.162 | 2.213 | 23.185 |
| SD of CD8 at tumor edge | 1 | −1.40113 | 0.58769 | 5.6842 | 0.0171 | 0.246 | 0.078 | 0.779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stulpinas, R.; Jakiunaite, I.; Sidabraite, A.; Rasmusson, A.; Zilenaite-Petrulaitiene, D.; Strupas, K.; Laurinavicius, A.; Gulla, A. Low CD8+ Density Variation and R1 Surgical Margin as Independent Predictors of Early Post-Resection Recurrence in HCC Patients Meeting Milan Criteria. Curr. Oncol. 2024, 31, 5344-5353. https://doi.org/10.3390/curroncol31090394
Stulpinas R, Jakiunaite I, Sidabraite A, Rasmusson A, Zilenaite-Petrulaitiene D, Strupas K, Laurinavicius A, Gulla A. Low CD8+ Density Variation and R1 Surgical Margin as Independent Predictors of Early Post-Resection Recurrence in HCC Patients Meeting Milan Criteria. Current Oncology. 2024; 31(9):5344-5353. https://doi.org/10.3390/curroncol31090394
Chicago/Turabian StyleStulpinas, Rokas, Ieva Jakiunaite, Agne Sidabraite, Allan Rasmusson, Dovile Zilenaite-Petrulaitiene, Kestutis Strupas, Arvydas Laurinavicius, and Aiste Gulla. 2024. "Low CD8+ Density Variation and R1 Surgical Margin as Independent Predictors of Early Post-Resection Recurrence in HCC Patients Meeting Milan Criteria" Current Oncology 31, no. 9: 5344-5353. https://doi.org/10.3390/curroncol31090394







