Self-Screening for Cervical Cancer Offered through a Digital Platform in a Region of British Columbia with Lower Screening Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Recruitment
2.3. Eligibility and Intervention
2.4. Outcome Measures
2.5. HPV Testing and Results Dissemination
2.6. Post-Participation Survey
3. Results
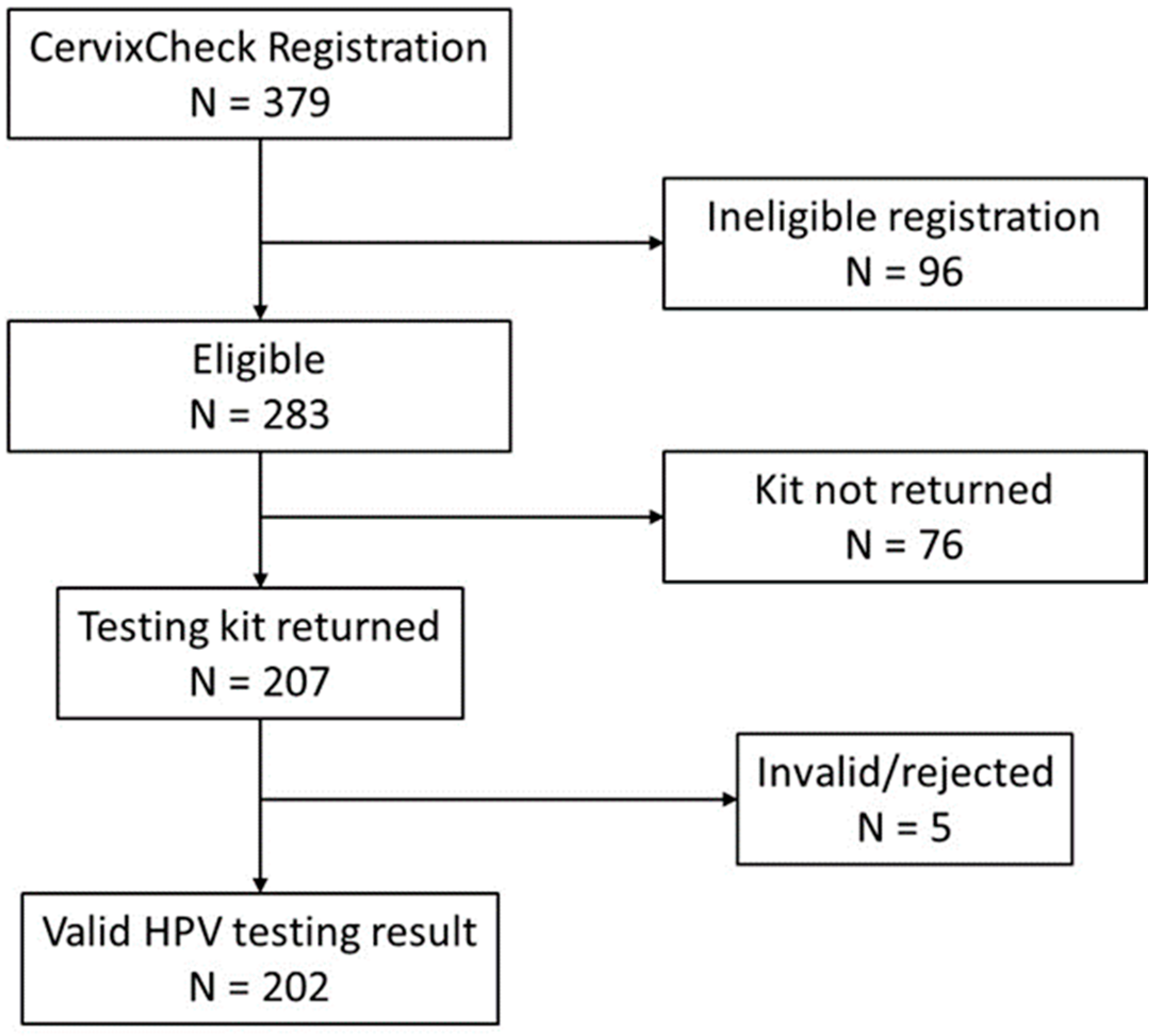
3.1. Self-Screening Completion
3.2. Survey Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 1 August 2024).
- Dickinson, J.A.; Stankiewicz, A.; Popadiuk, C.; Pogany, L.; Onysko, J.; Miller, A.B. Reduced Cervical Cancer Incidence and Mortality in Canada: National Data from 1932 to 2006. BMC Public Health 2012, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society; Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2023. Available online: http://cancer.ca/Canadian-Cancer-Statistics-2023-EN (accessed on 1 August 2024).
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 1 August 2024).
- Caird, H.; Simkin, J.; Smith, L.; Van Niekerk, D.; Ogilvie, G. The Path to Eliminating Cervical Cancer in Canada: Past, Present and Future Directions. Curr. Oncol. 2022, 29, 1117–1122. [Google Scholar] [CrossRef]
- Canadian Partnership against Cancer. Action Plan for the Elimination of Cervical Cancer in Canada 2020–2030. Available online: https://s22457.pcdn.co/wp-content/uploads/2020/11/Elimination-cervical-cancer-action-plan-EN.pdf (accessed on 1 August 2024).
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.H.; et al. Introduction of Molecular HPV Testing as the Primary Technology in Cervical Cancer Screening: Acting on Evidence to Change the Current Paradigm. Prev. Med. 2017, 98, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Vn, N.; Santesso, N.; Bryant, A.; Ppl, M.; Ra, M.; Schünemann, H.; Koliopoulos, G.; Vn, N.; Santesso, N.; et al. Cytology versus HPV Testing for Cervical Cancer Screening in the General Population (Review). Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.S.; Van Niekerk, D.; Krajden, M.; Smith, L.W.; Cook, D.; Gondara, L.; Ceballos, K.; Quinlan, D.; Lee, M.; Martin, R.E.; et al. Effect of Screening with Primary Cervical HPV Testing vs Cytology Testing on High-Grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial. JAMA 2018, 320, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide Use of HPV Self-Sampling for Cervical Cancer Screening. Prev. Med. 2022, 154, 199–203. [Google Scholar] [CrossRef]
- World Health Organization. Implementation of Self-Care Interventions for Health and Well-Being: Guidance for Health Systems. Available online: https://www.who.int/publications/i/item/9789240094888 (accessed on 1 August 2024).
- Madzima, T.R.; Vahabi, M.; Lofters, A. Emerging Role of HPV Self-Sampling in Cervical Cancer Screening for Hard-To-Reach Women. Can. Fam. Physician 2017, 63, 597–601. [Google Scholar]
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.S.; Snijders, P.J.F.; Arbyn, M. Reaching Women Who Do Not Participate in the Regular Cervical Cancer Screening Programme by Offering Self-Sampling Kits: A Systematic Review and Meta-Analysis of Randomised Trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Verberckmoes, B.; Castle, P.E.; Arbyn, M. Offering HPV Self-Sampling Kits: An Updated Meta-Analysis of the Effectiveness of Strategies to Increase Participation in Cervical Cancer Screening. Br. J. Cancer 2023, 128, 805–813. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting Cervical Precancer and Reaching Underscreened Women by Using HPV Testing on Self Samples: Updated Meta-Analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef]
- Pretsch, P.K.; Spees, L.P.; Brewer, N.T.; Hudgens, M.G.; Sanusi, B.; Rohner, E.; Miller, E.; Jackson, S.L.; Barclay, L.; Carter, A.; et al. Effect of HPV Self-Collection Kits on Cervical Cancer Screening Uptake among under-Screened Women from Low-Income US Backgrounds (MBMT-3): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet Public Health 2023, 8, e411–e421. [Google Scholar] [CrossRef] [PubMed]
- Racey, C.S.; Gesink, D.C. Barriers and Facilitators to Cervical Cancer Screening Among Women in Rural Ontario, Canada: The Role of Self-Collected HPV Testing. J. Rural Health 2016, 32, 136–145. [Google Scholar] [CrossRef]
- Wilson, E.; Free, C.; Morris, T.P.; Syred, J.; Ahamed, I.; Menon-Johansson, A.S.; Palmer, M.J.; Barnard, S.; Rezel, E.; Baraitser, P. Internet-Accessed Sexually Transmitted Infection (e-STI) Testing and Results Service: A Randomised, Single-Blind, Controlled Trial. PLoS Med. 2017, 14, e1002479. [Google Scholar] [CrossRef]
- Gilbert, M.; Salway, T.; Haag, D.; Fairley, C.K.; Wong, J.; Grennan, T.; Uddin, Z.; Buchner, C.S.; Wong, T.; Krajden, M.; et al. Use of Get Checked Online, a Comprehensive Web-Based Testing Service for Sexually Transmitted and Blood-Borne Infections. J. Med. Internet Res. 2017, 19, e81. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategy on Digital Health 2020–2025. Available online: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf (accessed on 1 August 2024).
- Erku, D.; Khatri, R.; Endalamaw, A.; Wolka, E.; Nigatu, F.; Zewdie, A.; Assefa, Y. Digital Health Interventions to Improve Access to and Quality of Primary Health Care Services: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 6854. [Google Scholar] [CrossRef] [PubMed]
- The BC Cancer Agency. BC Cancer Cervix Screening 2018 Program Results . Available online: http://www.bccancer.bc.ca/screening/Documents/Cervix-Program-Results-2018.pdf (accessed on 1 August 2024).
- Leinonen, M.K.; Schee, K.; Jonassen, C.M.; Lie, A.K.; Nystrand, C.F.; Rangberg, A.; Furre, I.E.; Johansson, M.J.; Tropé, A.; Sjøborg, K.D.; et al. Safety and Acceptability of Human Papillomavirus Testing of Self-Collected Specimens: A Methodologic Study of the Impact of Collection Devices and HPV Assays on Sensitivity for Cervical Cancer and High-Grade Lesions. J. Clin. Virol. 2018, 99–100, 22–30. [Google Scholar] [CrossRef]
- Mclarty, J.W.; Williams, D.L.; Loyd, S.; Hagensee, M.E. Cervical Human Papillomavirus Testing With Two Home Self-Collection Methods Compared With a Standard Clinically Collected Sampling Method. Sex. Transm. Dis. 2019, 46, 670–675. [Google Scholar] [CrossRef]
- El-Zein, M.; Bouten, S.; Louvanto, K.; Gilbert, L.; Gotlieb, W.; Hemmings, R.; Behr, M.A.; Franco, E.L. Validation of a New HPV Self-Sampling Device for Cervical Cancer Screening: The Cervical and Self-Sample In Screening (CASSIS) Study. Gynecol. Oncol. 2018, 149, 491–497. [Google Scholar] [CrossRef]
- Sechi, I.; Muresu, N.; Puci, M.V.; Saderi, L.; Del Rio, A.; Cossu, A.; Muroni, M.R.; Castriciano, S.; Martinelli, M.; Cocuzza, C.E.; et al. Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women. Pathogens 2023, 12, 1169. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Di Gennaro, G.; Licata, F.; Trovato, A.; Bianco, A. Does Self-Sampling for Human Papilloma Virus Testing Have the Potential to Increase Cervical Cancer Screening? An Updated Meta-Analysis of Observational Studies and Randomized Clinical Trials. Front. Public Health 2022, 10, 1003461. [Google Scholar] [CrossRef] [PubMed]
- Taro, I.; Onuma, T.; Kurokawa, T.; Chino, Y.; Shinagawa, A.; Yoshida, Y. Evaluating Opt-In Vaginal Human Papillomavirus Self-Sampling: Participation Rates and Detection of High-Grade Lesions (CIN2+) among Unscreened Japanese Women Aged 30–39. Healthcare 2024, 12, 599. [Google Scholar] [CrossRef]
- Lam, J.U.H.; Rebolj, M.; Møller Ejegod, D.; Pedersen, H.; Rygaard, C.; Lynge, E.; Thirstrup Thomsen, L.; Krüger Kjaer, S.; Bonde, J. Human Papillomavirus Self-Sampling for Screening Nonattenders: Opt-in Pilot Implementation with Electronic Communication Platforms. Int. J. Cancer 2017, 140, 2212–2219. [Google Scholar] [CrossRef]
- Feltri, G.; Valenti, G.; Isidoro, E.; Kaur, J.; Treleani, M.; Bartelloni, A.; Mauro, C.; Spiga, F.; Ticich, G.; Di Napoli, M.; et al. Evaluation of Self-Sampling-Based Cervical Cancer Screening Strategy Using HPV Selfy CE-IVD Test Coupled with Home-Collection Kit: A Clinical Study in Italy. Eur. J. Med. Res. 2023, 28, 582. [Google Scholar] [CrossRef]
- Decker, K.; Demers, A.; Chateau, D.; Musto, G.; Nugent, Z.; Lotocki, R.; Harrison, M. Papanicolaou Test Utilization and Frequency of Screening Opportunities among Women Diagnosed with Cervical Cancer. Open Med. 2009, 3, 140–147. [Google Scholar]
- Nieminen, P.; Kallio, M.; Anttila, A.; Hakama, M. Organised vs. Spontaneous Pap-Smear Screening for Cervical Cancer: A Case-Control Study. Int. J. Cancer 1999, 83, 55–58. [Google Scholar] [CrossRef]
- Sancho-Garnier, H.; Tamalet, C.; Halfon, P.; Leandri, F.X.; Le Retraite, L.; Djoufelkit, K.; Heid, P.; Davies, P.; Piana, L. HPV Self-Sampling or the Pap-Smear: A Randomized Study among Cervical Screening Nonattenders from Lower Socioeconomic Groups in France. Int. J. Cancer 2013, 133, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Enerly, E.; Bonde, J.; Schee, K.; Pedersen, H.; Lönnberg, S.; Nygård, M. Self-Sampling for Human Papillomavirus Testing among Non-Attenders Increases Attendance to the Norwegian Cervical Cancer Screening Programme. PLoS ONE 2016, 11, e0151978. [Google Scholar] [CrossRef]
| Total Eligible (N = 283) | Kit Returned (N = 207) | Kit Not Returned (N = 76) | p-Value * | |
|---|---|---|---|---|
| Age when the kit was sent (median, IQR) | 45 (37–53) | 45 (37–53) | 44 (38–51) | 0.55 |
| Screening history | 0.90 | |||
| Due (3–4 years) | 193 (68.2) | 140 (67.6) | 53 (69.7) | |
| Overdue (≥5 years) | 61 (21.6) | 46 (22.2) | 15 (19.7) | |
| Never screened | 29 (10.2) | 21 (10.1) | 8 (10.5) | |
| Years since last screened (median, IQR) | n = 254 | n = 186 | n = 68 | 0.82 |
| 3.9 (3.4–4.9) | 3.9 (4.4–5.0) | 4.0 (3.4–4.7) | ||
| Time from when the kit was sent to when the sample was collected in days (median, IQR) | - | 22 (10–48) | - | - |
| Total N = 202 | Due (3–4 Years) N = 140 | Overdue (≥5 Years) N = 42 | Never Screened N = 20 | |
|---|---|---|---|---|
| HPV Positive | 15 | 11 | 3 | 1 |
| (7.4%, CI: 4.5–12.0) | (7.8%, CI: 4.3–13.6) | (7.1%, CI: 1.8–19.7) | (5.0%, CI: 0.9–25.4) |
| Returned Kit and Fully Completed Survey N = 42 * | |
|---|---|
| Age at kit sent (median, IQR) | 48 (40–54) |
| Ethnicity (n = 39) | |
| Asian | 25 (64.1%) |
| Indigenous | 1 (2.6%) |
| White | 12 (30.8%) |
| Other/Multiethnic | 1 (2.6%) |
| Languages can comfortably communicate in (check all that apply) | |
| English | 35 (83.3%) |
| Punjabi | 11 (26.2%) |
| Hindi | 10 (23.8%) |
| French | 1 (2.4%) |
| Mandarin | 1 (2.4%) |
| Tagalog | 1 (2.4%) |
| Other | 2 (4.8%) |
| Prefer not to answer | 1 (2.4%) |
| Household income in 2018 (n = 29) | |
| Under CAD 30,000 | 2 (6.9%) |
| CAD 30,000–59,999 | 6 (20.7%) |
| CAD 60,000–89,999 | 7 (24.1%) |
| Over CAD 90,000 | 14 (48.3%) |
| Reasons for participating in CervixCheck (check all that apply) | |
| Convenience | 27/51 (52.9%) |
| Time | 20/51 (39.2%) |
| Stress | 16/51 (31.4%) |
| Comfort | 22/51 (43.1%) |
| Other | 5/51 (9.8%) |
| Experience of using the CervixCheck website | |
| Did the CervixCheck website provide enough information about cervical cancer and HPV to answer any questions? | Yes |
| 39/45 (86.7%) | |
| Did the CervixCheck website provide enough information about participating in the project? | Yes |
| 42/46 (91.3%) | |
| Signing up online for a CervixCheck account was… | Easy/Very Easy * |
| 43/46 (93.5%) | |
| It was easy to use the website | Agree/Strongly Agree |
| 37/44 (84.1%) | |
| The language used on the website was easy to understand | Agree/Strongly Agree |
| 38/43 (88.4%) | |
| Felt confident about the confidentiality and privacy | Agree/Strongly Agree |
| 36/43 (83.7%) | |
| Were satisfied with the content and features of the website | Agree/Strongly Agree |
| 35/43 (81.4%) | |
| Experience of HPV self-screening Among participants who completed self-screening | |
| The instructions in the kit were easy to follow | Agree/Strongly Agree |
| 35/38 (92.1%) | |
| Self-collecting † a cervical sample was easy to perform | Agree/Strongly Agree |
| 33/38 (86.8%) | |
| I felt that I was collecting the sample correctly | Agree/Strongly Agree |
| 33/38 (86.8%) | |
| The self-collection device was comfortable to use | Agree/Strongly Agree |
| 35/38 (92.1%) | |
| Did you experience any pain or discomfort that discouraged you from self-screening? | No |
| 35/38 (92.1%) | |
| Receiving results online Among participants who completed self-screening | |
| How did you feel about receiving your screening results online through the CervixCheck website? | Satisfied/Very Satisfied |
| 33/38 (86.8%) | |
| Did you understand your online results? | Yes |
| 35/38 (92.1%) | |
| Future screening intentions and recommendations Among participants who completed self-screening | |
| Assuming that both HPV self-collection and having a healthcare provider collect a cervical sample are equally safe and effective for testing, what would you prefer in a future screening program? | |
| Self-screening | 32/38 (84.2%) |
| Sample taken by doctor | 2/38 (5.3%) |
| No preference | 4/38 (10.5%) |
| If you had the opportunity, how likely are you to use self-collection again in the future for cervical cancer screening? | Likely/Very Likely |
| 35/38 (92.1%) | |
| How likely are you to recommend self-collection to other women you know? | Likely/Very Likely |
| 35/38 (92.1%) | |
| Preferred methods for receiving self-collection kits in the future | |
| By signing up online through a website like CervixCheck | 12/51 (23.5%) |
| By visiting my family doctor and getting the kit during my visit | 7/51 (13.7%) |
| Automatically receiving one in the mail from the screening program when I am due for screening | 28/51 (54.9%) |
| Having the option to request a kit be sent from the screening program when I am due for screening | 14/51 (27.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, L.W.; Booth, A.; Racey, C.S.; Smith, B.; Prabhakaran, A.; Dabee, S.; Hong, Q.; Niazi, N.; Ogilvie, G.S. Self-Screening for Cervical Cancer Offered through a Digital Platform in a Region of British Columbia with Lower Screening Rates. Curr. Oncol. 2024, 31, 5399-5411. https://doi.org/10.3390/curroncol31090399
Smith LW, Booth A, Racey CS, Smith B, Prabhakaran A, Dabee S, Hong Q, Niazi N, Ogilvie GS. Self-Screening for Cervical Cancer Offered through a Digital Platform in a Region of British Columbia with Lower Screening Rates. Current Oncology. 2024; 31(9):5399-5411. https://doi.org/10.3390/curroncol31090399
Chicago/Turabian StyleSmith, Laurie W., Amy Booth, C. Sarai Racey, Brenda Smith, Ashwini Prabhakaran, Smritee Dabee, Quan Hong, Nazia Niazi, and Gina S. Ogilvie. 2024. "Self-Screening for Cervical Cancer Offered through a Digital Platform in a Region of British Columbia with Lower Screening Rates" Current Oncology 31, no. 9: 5399-5411. https://doi.org/10.3390/curroncol31090399
APA StyleSmith, L. W., Booth, A., Racey, C. S., Smith, B., Prabhakaran, A., Dabee, S., Hong, Q., Niazi, N., & Ogilvie, G. S. (2024). Self-Screening for Cervical Cancer Offered through a Digital Platform in a Region of British Columbia with Lower Screening Rates. Current Oncology, 31(9), 5399-5411. https://doi.org/10.3390/curroncol31090399




