Skeletal Muscle Radiation Attenuation at C3 Predicts Survival in Head and Neck Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Deep Learning Pipeline for SMA and SMRA Quantification
2.3. Automatic Detection
2.4. Automatic Segmentation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics (Supplementary Table S1)
3.2. Distribution of SMA and SMRA
3.3. Association Between SMA and SMRA Groups and LRC
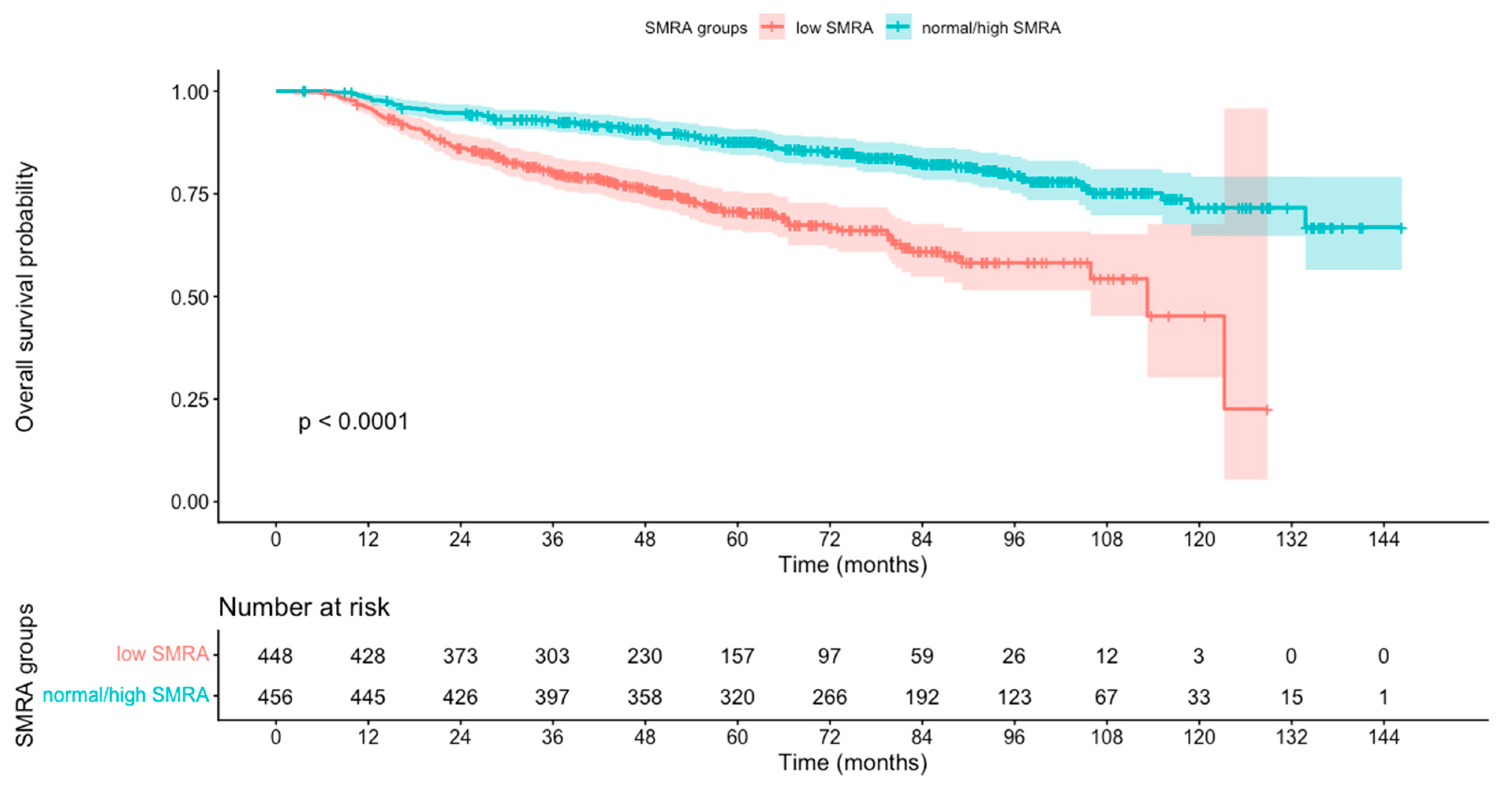
3.4. Association Between SMA and SMRA Groups and OS
4. Discussion
4.1. Limitations
4.2. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C3 | Third Cervical Vertebra |
| CI | Confidence Interval |
| CRT | Chemoradiation |
| CUP | Cancer of Unknown Primary |
| CSA | Cross-Sectional Area |
| DL | Deep Learning |
| DLT | Dose-Limiting Toxicity |
| H&N | Head and Neck |
| HNC | Head and Neck Cancer |
| HPV | Human Papillomavirus |
| HR | Hazard Ratio |
| L3 | Third Lumbar Vertebra |
| LRC | Locoregional Control |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| RT | Radiotherapy |
| SMA | Skeletal Muscle Area |
| SMD | Skeletal Muscle Density |
| SMRA | Skeletal Muscle Radiation Attenuation |
References
- Almada-Correia, I.; Neves, P.M.; Mäkitie, A.; Ravasco, P. Body Composition Evaluation in Head and Neck Cancer Patients: A Review. Front. Oncol. 2019, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Yao, N.; Sun, L.; Hu, W.; Li, X.; Yang, Y.; Wang, Y.; Zhu, W.; Li, B. The Effect of HPV DNA and P16 Status on the Prognosis of Patients with Hypopharyngeal Carcinoma: A Meta-Analysis. BMC Cancer 2022, 22, 658. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Chamchod, S.; Fuller, C.D.; Mohamed, A.S.R.; Heukelom, J.; Eichelberger, H.; Kantor, M.E.; Hutcheson, K.A.; Gunn, G.B.; Garden, A.S.; et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016, 2, 782. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn-Dekker, M.I.; Van Den Bosch, L.; Van Den Hoek, J.G.M.; Bijl, H.P.; Van Aken, E.S.M.; Van Der Hoorn, A.; Oosting, S.F.; Halmos, G.B.; Witjes, M.J.H.; Van Der Laan, H.P.; et al. Impact of Sarcopenia on Survival and Late Toxicity in Head and Neck Cancer Patients Treated with Radiotherapy. Radiother. Oncol. 2020, 147, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Schaeffers, A.W.M.A.; Scholten, H.A.; Van Beers, M.A.; Meussen, B.W.; Smid, E.J.; Van Gils, C.H.; Devriese, L.A.; De Bree, R. The Effect of Skeletal Muscle Mass on Dose-Limiting Toxicities during (Chemo)Radiotherapy in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. Oral Oncol. 2024, 157, 106978. [Google Scholar] [CrossRef] [PubMed]
- Van Heusden, H.C.; Van Beers, M.A.; Schaeffers, A.W.M.A.; Swartz, E.; Swartz, J.E.; De Bree, R. The Predictive and Prognostic Role of Radiologically Defined Sarcopenia in Head and Neck Cancer: A Systematic Review and Multi-Level Meta-Analysis. Br. J. Cancer 2025, 133, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Syziu, A.; Schache, A. The Prognostic Value of Pre-Treatment Sarcopenia in Overall Survival in Head and Neck Cancer Patients: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2025, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Erul, E.; Guven, D.C.; Ozbay, Y.; Altunbulak, A.Y.; Kahvecioglu, A.; Ercan, F.; Yesil, M.F.; Ucdal, M.T.; Cengiz, M.; Yazici, G.; et al. Evaluation of Sarcopenia as a Prognostic Biomarker in Locally Advanced Head and Neck Squamous Cell Carcinoma. Biomark. Med. 2023, 17, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.L.; Platek, M.E.; Singh, A.K.; Ma, S.J.; Farrugia, M.; Wilding, G.; Ray, A.D.; Ochs-Balcom, H.M.; Noyes, K. Effect of Musculature on Mortality, a Retrospective Cohort Study. BMC Cancer 2022, 22, 688. [Google Scholar] [CrossRef] [PubMed]
- Bardoscia, L.; Besutti, G.; Pellegrini, M.; Pagano, M.; Bonelli, C.; Bonelli, E.; Braglia, L.; Cozzi, S.; Roncali, M.; Iotti, C.; et al. Impact of Low Skeletal Muscle Mass and Quality on Clinical Outcomes in Patients with Head and Neck Cancer Undergoing (Chemo)Radiation. Front. Nutr. 2022, 9, 994499. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Kay-Rivest, E.; Perrin, L.J.; Liem, X.; Furstoss, C.; Aerts, H.J.W.L.; Khaouam, N.; Nguyen-Tan, P.F.; Wang, C.-S.; Sultanem, K.; et al. Radiomics Strategies for Risk Assessment of Tumour Failure in Head-and-Neck Cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef] [PubMed]
- Zuley, M.L.; Jarosz, R.; Kirk, S.; Lee, Y.; Colen, R.; Garcia, K.; Delbeke, D.; Pham, M.; Nagy, P.; Sevinc, G.; et al. The Cancer Genome Atlas Head-Neck Squamous Cell Carcinoma Collection (TCGA-HNSC) (Version 6) [Data Set]. The Cancer Imaging Archive. Available online: https://www.cancerimagingarchive.net/collection/tcga-hnsc/ (accessed on 5 June 2023).
- National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (CPTAC). The Clinical Proteomic Tumor Analysis Consortium Head and Neck Squamous Cell Carcinoma Collection (CPTAC-HNSCC) (Version 19) [Dataset]. The Cancer Imaging Archive. 2018. Available online: https://wiki.cancerimagingarchive.net/display/Public/CPTAC-HNSCC (accessed on 5 June 2023).
- Wee, L.; Dekker, A. Data from HEAD-NECK-RADIOMICS-HN1 [Data Set]. The Cancer Imaging Archive. 2019. Available online: https://wiki.cancerimagingarchive.net/display/Public/Head-Neck-Radiomics-HN1 (accessed on 5 June 2023).
- Dickstein, D.R.; Powers, A.E.; Vujovic, D.; Roof, S.; Bakst, R.L. Clinical and Therapeutic Considerations for Older Adults with Head and Neck Cancer. Clin. Interv. Aging 2023, 18, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, D.; Hanai, N.; Suzuki, H.; Koide, Y.; Beppu, S.; Hasegawa, Y. The Impact of Skeletal Muscle Depletion on Head and Neck Squamous Cell Carcinoma. ORL 2018, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.R.; Roh, J.-L.; Kim, J.S.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Prognostic Value of Body Composition on Recurrence and Survival of Advanced-Stage Head and Neck Cancer. Eur. J. Cancer 2019, 116, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Ueno, T.; Hirai, N.; Komori, T.; Nakanishi, Y.; Kondo, S.; Wakisaka, N.; Yoshizaki, T. Low Skeletal Muscle Mass Is a Risk Factor for Aspiration Pneumonia During Chemoradiotherapy. Laryngoscope 2021, 131, E1524–E1529. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and Prognosis in Cancer: Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Pituskin, E.; Mackey, J.R.; Grenier, J.G.; Ian Paterson, D.; Haykowsky, M.J.; Thompson, R.B. Longitudinal Changes in Skeletal Muscle Metabolism, Oxygen Uptake, and Myosteatosis During Cardiotoxic Treatment for Early-Stage Breast Cancer. Oncologist 2022, 27, e748–e754. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K. Assessment of Muscle Quantity, Quality and Function. J. Obes. Metab. Syndr. 2022, 31, 9–16. [Google Scholar] [CrossRef]
| Univariate Analysis | Multivariable Analysis * | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Locoregional control | ||||||
| SMRA | 2.22 | [1.57–3.14] | <0.001 | 1.76 | [1.22–2.54] | <0.001 |
| SMA | 1.89 | [1.34–2.66] | <0.001 | 1.85 | [1.19–2.88] | <0.001 |
| Overall survival | ||||||
| SMRA | 2.58 | [1.92–3.39] | <0.001 | 2.13 | [1.58–2.88] | <0.001 |
| SMA | 1.46 | [1.09–1.96] | 0.01 | 1.53 | [1.06–2.20] | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barajas Ordonez, F.; Xie, K.; Ferreira, A.; Siepmann, R.; Chargi, N.; Nebelung, S.; Truhn, D.; Bergé, S.; Bruners, P.; Egger, J.; et al. Skeletal Muscle Radiation Attenuation at C3 Predicts Survival in Head and Neck Cancer. Curr. Oncol. 2025, 32, 587. https://doi.org/10.3390/curroncol32100587
Barajas Ordonez F, Xie K, Ferreira A, Siepmann R, Chargi N, Nebelung S, Truhn D, Bergé S, Bruners P, Egger J, et al. Skeletal Muscle Radiation Attenuation at C3 Predicts Survival in Head and Neck Cancer. Current Oncology. 2025; 32(10):587. https://doi.org/10.3390/curroncol32100587
Chicago/Turabian StyleBarajas Ordonez, Felix, Kunpeng Xie, André Ferreira, Robert Siepmann, Najiba Chargi, Sven Nebelung, Daniel Truhn, Stefaan Bergé, Philipp Bruners, Jan Egger, and et al. 2025. "Skeletal Muscle Radiation Attenuation at C3 Predicts Survival in Head and Neck Cancer" Current Oncology 32, no. 10: 587. https://doi.org/10.3390/curroncol32100587
APA StyleBarajas Ordonez, F., Xie, K., Ferreira, A., Siepmann, R., Chargi, N., Nebelung, S., Truhn, D., Bergé, S., Bruners, P., Egger, J., Hölzle, F., Wirth, M., Kuhl, C., & Hinrichs-Puladi, B. (2025). Skeletal Muscle Radiation Attenuation at C3 Predicts Survival in Head and Neck Cancer. Current Oncology, 32(10), 587. https://doi.org/10.3390/curroncol32100587





