Metabolic Imaging as Future Technology and Innovation in Brain-Tumour Surgery: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- Focused on the application of hyperpolarized MRI in patients undergoing neurosurgical procedures or in the management of primary brain tumours.
- Reported quantitative or qualitative outcomes related to imaging.
- Published as peer-reviewed original research articles.
- Reviews, case reports, editorials, or non-peer-reviewed studies.
- Studies involving animal models or in vitro experiments without patient data.
- Articles published in languages other than English.
2.2. Study Selection Process
2.3. Data Extraction
2.4. Quality Assessment
High Bias: if one or more signalling questions are answered ‘no’;
Unclear Bias: if insufficient information is provided.
2.5. Assessment of Heterogeneity and Statistical Analysis
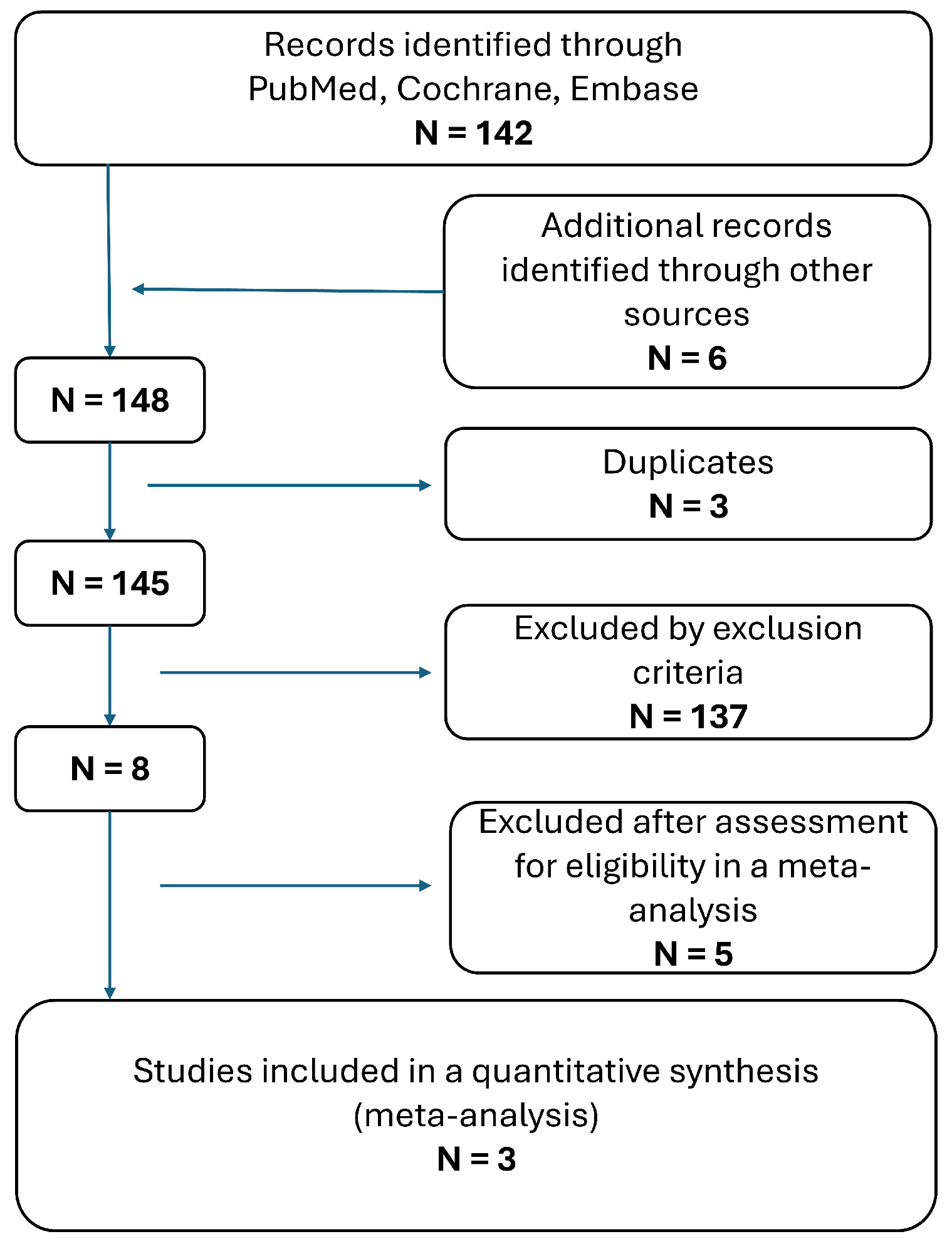
3. Results
3.1. Application of Hyperpolarized MRI in Neurosurgery
3.2. Quality Assessment
3.3. Heterogeneity of the Studies
3.4. Secondary Findings—Hyperpolarized MRI and Contrast Enhanced MRI
4. Discussion
4.1. Current Surgical and Technical Concepts for Primary Brain Tumours
4.2. Imaging Diagnostics in Neurosurgery and Tumour Biology
4.3. Hyperpolarized MRI in Neurosurgery
4.4. Future Neurosurgical Hypotheses and Implications
4.5. Limitations of This Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ALA, ALA | 5-aminolevulinic acid |
| BBB | blood–brain barrier |
| C | Carbon |
| CT | computed tomography |
| CE-MRI | contrast-enhanced MRI |
| kPL | conversion rates of pyruvate to lactate |
| kPB | conversion rates of pyruvate to bicarbonate |
| DESI-MS | desorption electrospray ionisation mass spectrometry |
| df | degrees of freedom |
| DTI | diffusion tensor imaging |
| DCS | direct cortical stimulation |
| EOR | extent of resection |
| fMRI | functional MRI |
| GTR | gross total resection |
| hMRI | hyperpolarized magnetic resonance imaging |
| iUS | intraoperative ultrasound |
| iMRI | intraoperative MRI |
| MR | magnet resonance |
| MRI | magnetic resonance imaging |
| MRSI | magnetic resonance spectroscopic imaging |
| MEP | motor evoked potential |
| nTMS | navigated transcranial magnetic stimulation |
| NABP | normal appearing brain parenchyma |
| NAWM | normal-appearing white matter |
| PET | positron emission tomography imaging |
| PFS | progression-free survival |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies |
| SSEPs | somatosensory evoked potentials |
| SMD | standardized mean differences |
| TCA | tricarboxylic acid |
| WHO | World Health Organization |
References
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current Clinical Brain Tumor Imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Smits, M. Update on neuroimaging in brain tumours. Curr. Opin. Neurol. 2021, 34, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Gillard, J.H. Imaging biomarkers of brain tumour margin and tumour invasion. Br. J. Radiol. 2011, 84, S159–S167. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.; Mangukiya, H.B.; Elfineh, L.; Stockgard, R.; Krona, C.; Gerlee, P.; Nelander, S. Inference of glioblastoma migration and proliferation rates using single time-point images. Commun. Biol. 2023, 6, 402. [Google Scholar] [CrossRef]
- Pichardo-Rojas, P.S.; Zarate, C.; Arguelles-Hernandez, J.; Barron-Lomeli, A.; Sanchez-Velez, R.; Hjeala-Varas, A.; Gutierrez-Herrera, E.; Tandon, N.; Esquenazi, Y. Intraoperative ultrasound for surgical resection of high-grade glioma and glioblastoma: A meta-analysis of 732 patients. Neurosurg. Rev. 2024, 47, 120. [Google Scholar] [CrossRef]
- Pichardo-Rojas, P.S.; Angulo-Lozano, J.C.; Alvarez-Castro, J.A.; Vazquez-Alva, D.; Osuna-Lau, R.A.; Choque-Ayala, L.C.; Tandon, N.; Esquenazi, Y. Intraoperative Magnetic Resonance Imaging (MRI)-Guided Resection of Glioblastoma: A Meta-Analysis of 1,847 Patients. World Neurosurg. 2024, 182, e807–e822. [Google Scholar] [CrossRef]
- Koukoulithras, I.; Gkampenis, A.; Markopoulos, G.S.; Vartholomatos, G.; Siempis, T.; Voulgaris, S.; Alexiou, G.A. Intraoperative methods to maximize gliomas resection: A review of both established and novel techniques. Discov. Med. 2024, 1, 79. [Google Scholar] [CrossRef]
- Han, Q.; Liang, H.; Cheng, P.; Yang, H.; Zhao, P. Gross Total vs. Subtotal Resection on Survival Outcomes in Elderly Patients With High-Grade Glioma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Apra, C.; Bemora, J.S.; Palfi, S. Achieving Gross Total Resection in Neurosurgery: A Review of Intraoperative Techniques and Their Influence on Surgical Goals. World Neurosurg. 2024, 185, 246–253. [Google Scholar] [CrossRef]
- Baik, S.H.; Kim, S.Y.; Na, Y.C.; Cho, J.M. Supratotal Resection of Glioblastoma: Better Survival Outcome than Gross Total Resection. J. Pers. Med. 2023, 13, 383. [Google Scholar] [CrossRef]
- Coburger, J.; Merkel, A.; Scherer, M.; Schwartz, F.; Gessler, F.; Roder, C.; Pala, A.; Konig, R.; Bullinger, L.; Nagel, G.; et al. Low-grade Glioma Surgery in Intraoperative Magnetic Resonance Imaging: Results of a Multicenter Retrospective Assessment of the German Study Group for Intraoperative Magnetic Resonance Imaging. Neurosurgery 2016, 78, 775–786. [Google Scholar] [CrossRef]
- Suchorska, B.; Weller, M.; Tabatabai, G.; Senft, C.; Hau, P.; Sabel, M.C.; Herrlinger, U.; Ketter, R.; Schlegel, U.; Marosi, C.; et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016, 18, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Vychopen, M.; Kuhnapfel, A.; Seidel, C.; Guresir, E. A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers 2023, 15, 1772. [Google Scholar] [CrossRef] [PubMed]
- Tom, M.C.; Varra, V.; Leyrer, C.M.; Park, D.Y.; Chao, S.T.; Yu, J.S.; Suh, J.H.; Reddy, C.A.; Balagamwala, E.H.; Broughman, J.R.; et al. Risk Factors for Progression Among Low-Grade Gliomas After Gross Total Resection and Initial Observation in the Molecular Era. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1099–1105. [Google Scholar] [CrossRef]
- Kreth, F.W.; Thon, N.; Simon, M.; Westphal, M.; Schackert, G.; Nikkhah, G.; Hentschel, B.; Reifenberger, G.; Pietsch, T.; Weller, M.; et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann. Oncol. 2013, 24, 3117–3123. [Google Scholar] [CrossRef]
- Roder, C.; Bisdas, S.; Ebner, F.H.; Honegger, J.; Naegele, T.; Ernemann, U.; Tatagiba, M. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur. J. Surg. Oncol. 2014, 40, 297–304. [Google Scholar] [CrossRef]
- Winther, R.R.; Hjermstad, M.J.; Skovlund, E.; Aass, N.; Helseth, E.; Kaasa, S.; Yri, O.E.; Vik-Mo, E.O. Surgery for brain metastases-impact of the extent of resection. Acta Neurochir. 2022, 164, 2773–2780. [Google Scholar] [CrossRef]
- Karschnia, P.; Vogelbaum, M.A.; van den Bent, M.; Cahill, D.P.; Bello, L.; Narita, Y.; Berger, M.S.; Weller, M.; Tonn, J.C. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur. J. Cancer 2021, 149, 23–33. [Google Scholar] [CrossRef]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016, 18, 96–104. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Attenello, F.J.; Weingart, J.D.; Than, K.; Burger, P.C.; Olivi, A.; Brem, H.; Quinones-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008, 63, 700–707, author reply 707–708. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764, discussion 264–756. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576, discussion 564–576. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Gousias, K.; Schramm, J.; Simon, M. Extent of resection and survival in supratentorial infiltrative low-grade gliomas: Analysis of and adjustment for treatment bias. Acta Neurochir. 2014, 156, 327–337. [Google Scholar] [CrossRef]
- Kubben, P.L.; ter Meulen, K.J.; Schijns, O.E.; ter Laak-Poort, M.P.; van Overbeeke, J.J.; van Santbrink, H. Intraoperative MRI-guided resection of glioblastoma multiforme: A systematic review. Lancet Oncol. 2011, 12, 1062–1070. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Karschnia, P.; Gerritsen, J.K.W.; Teske, N.; Cahill, D.P.; Jakola, A.S.; van den Bent, M.; Weller, M.; Schnell, O.; Vik-Mo, E.O.; Thon, N.; et al. The oncological role of resection in newly diagnosed diffuse adult-type glioma defined by the WHO 2021 classification: A Review by the RANO resect group. Lancet Oncol. 2024, 25, e404–e419. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rivera, V.; Dono, A.; Lewis, C.T.; Chandra, A.; Abdelkhaleq, R.; Sheth, S.A.; Ballester, L.Y.; Esquenazi, Y. Extent of resection and survival outcomes of geriatric patients with glioblastoma: Is there benefit from aggressive surgery? Clin. Neurol. Neurosurg. 2021, 202, 106474. [Google Scholar] [CrossRef] [PubMed]
- Fountain, D.M.; Bryant, A.; Barone, D.G.; Waqar, M.; Hart, M.G.; Bulbeck, H.; Kernohan, A.; Watts, C.; Jenkinson, M.D. Intraoperative imaging technology to maximise extent of resection for glioma: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 1, CD013630. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Ahmeti, H.; Roder, C.; Gessler, F.; Jungk, C.; Pala, A.; Mayer, B.; Senft, C.; Tatagiba, M.; Synowitz, M.; et al. Surgery for Diffuse WHO Grade II Gliomas: Volumetric Analysis of a Multicenter Retrospective Cohort From the German Study Group for Intraoperative Magnetic Resonance Imaging. Neurosurgery 2020, 86, E64–E74. [Google Scholar] [CrossRef] [PubMed]
- Felix, R.; Schorner, W.; Laniado, M.; Niendorf, H.P.; Claussen, C.; Fiegler, W.; Speck, U. Brain tumors: MR imaging with gadolinium-DTPA. Radiology 1985, 156, 681–688. [Google Scholar] [CrossRef]
- Kiviniemi, A.; Gardberg, M.; Ek, P.; Frantzen, J.; Bobacka, J.; Minn, H. Gadolinium retention in gliomas and adjacent normal brain tissue: Association with tumor contrast enhancement and linear/macrocyclic agents. Neuroradiology 2019, 61, 535–544. [Google Scholar] [CrossRef]
- Montagne, A.; Toga, A.W.; Zlokovic, B.V. Blood-Brain Barrier Permeability and Gadolinium: Benefits and Potential Pitfalls in Research. JAMA Neurol. 2016, 73, 13–14. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Autry, A.; Phillips, J.J.; Maleschlijski, S.; Roy, R.; Molinaro, A.M.; Chang, S.M.; Cha, S.; Lupo, J.M.; Nelson, S.J. Characterization of Metabolic, Diffusion, and Perfusion Properties in GBM: Contrast-Enhancing versus Non-Enhancing Tumor. Transl. Oncol. 2017, 10, 895–903. [Google Scholar] [CrossRef]
- Wamelink, I.; Azizova, A.; Booth, T.C.; Mutsaerts, H.; Ogunleye, A.; Mankad, K.; Petr, J.; Barkhof, F.; Keil, V.C. Brain Tumor Imaging without Gadolinium-based Contrast Agents: Feasible or Fantasy? Radiology 2024, 310, e230793. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Z.R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Essig, M.; Weber, M.A.; von Tengg-Kobligk, H.; Knopp, M.V.; Yuh, W.T.; Giesel, F.L. Contrast-enhanced magnetic resonance imaging of central nervous system tumors: Agents, mechanisms, and applications. Top. Magn. Reson. Imaging 2006, 17, 89–106. [Google Scholar] [CrossRef]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The influence of surgery on recurrence pattern of glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Esemen, Y.; Awan, M.; Parwez, R.; Baig, A.; Rahman, S.; Masala, I.; Franchini, S.; Giakoumettis, D. Molecular Pathogenesis of Glioblastoma in Adults and Future Perspectives: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2607. [Google Scholar] [CrossRef] [PubMed]
- Stensjoen, A.L.; Berntsen, E.M.; Jakola, A.S.; Solheim, O. When did the glioblastoma start growing, and how much time can be gained from surgical resection? A model based on the pattern of glioblastoma growth in vivo. Clin. Neurol. Neurosurg. 2018, 170, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.; Fine, H.A.; Magge, R.S. Scientific and Clinical Challenges within Neuro-Oncology. World Neurosurg. 2021, 151, 402–410. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Broekman, M.L.D.; De Vleeschouwer, S.; Schucht, P.; Nahed, B.V.; Berger, M.S.; Vincent, A. Safe surgery for glioblastoma: Recent advances and modern challenges. Neurooncol. Pract. 2022, 9, 364–379. [Google Scholar] [CrossRef]
- Chiariello, M.; Inzalaco, G.; Barone, V.; Gherardini, L. Overcoming challenges in glioblastoma treatment: Targeting infiltrating cancer cells and harnessing the tumor microenvironment. Front. Cell Neurosci. 2023, 17, 1327621. [Google Scholar] [CrossRef]
- Patel, V.; Chavda, V. Intraoperative glioblastoma surgery-current challenges and clinical trials: An update. Cancer Pathog. Ther. 2024, 2, 256–267. [Google Scholar] [CrossRef]
- He, L.; Zhang, H.; Li, T.; Yang, J.; Zhou, Y.; Wang, J.; Saidaer, T.; Liu, X.; Wang, L.; Wang, Y. Distinguishing Tumor Cell Infiltration and Vasogenic Edema in the Peritumoral Region of Glioblastoma at the Voxel Level via Conventional MRI Sequences. Acad. Radiol. 2024, 31, 1082–1090. [Google Scholar] [CrossRef]
- Galijasevic, M.; Steiger, R.; Mangesius, S.; Mangesius, J.; Kerschbaumer, J.; Freyschlag, C.F.; Gruber, N.; Janjic, T.; Gizewski, E.R.; Grams, A.E. Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers 2022, 14, 3197. [Google Scholar] [CrossRef]
- Bulik, M.; Jancalek, R.; Vanicek, J.; Skoch, A.; Mechl, M. Potential of MR spectroscopy for assessment of glioma grading. Clin. Neurol. Neurosurg. 2013, 115, 146–153. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, Q.; Zheng, X.; Wu, C.; Xu, B.N. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: A systematic review and meta-analysis. Eur. J. Radiol. 2014, 83, 2181–2189. [Google Scholar] [CrossRef]
- Dadgar, H.; Jokar, N.; Nemati, R.; Larvie, M.; Assadi, M. PET tracers in glioblastoma: Toward neurotheranostics as an individualized medicine approach. Front. Nucl. Med. 2023, 3, 1103262. [Google Scholar] [CrossRef]
- Drake, L.R.; Hillmer, A.T.; Cai, Z. Approaches to PET Imaging of Glioblastoma. Molecules 2020, 25, 568. [Google Scholar] [CrossRef]
- Mansoor, N.M.; Thust, S.; Militano, V.; Fraioli, F. PET imaging in glioma: Techniques and current evidence. Nucl. Med. Commun. 2018, 39, 1064–1080. [Google Scholar] [CrossRef] [PubMed]
- la Fougere, C.; Suchorska, B.; Bartenstein, P.; Kreth, F.W.; Tonn, J.C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro Oncol. 2011, 13, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Alexander, G.S.; Bakas, S.; Nikam, R.; Talekar, K.; Palmer, J.D.; Shi, W. Advanced magnetic resonance imaging in glioblastoma: A review. Chin. Clin. Oncol. 2017, 6, 40. [Google Scholar] [CrossRef]
- De Simone, M.; Iaconetta, G.; Palermo, G.; Fiorindi, A.; Schaller, K.; De Maria, L. Clustering Functional Magnetic Resonance Imaging Time Series in Glioblastoma Characterization: A Review of the Evolution, Applications, and Potentials. Brain Sci. 2024, 14, 296. [Google Scholar] [CrossRef]
- Sollmann, N.; Zhang, H.; Kloth, C.; Zimmer, C.; Wiestler, B.; Rosskopf, J.; Kreiser, K.; Schmitz, B.; Beer, M.; Krieg, S.M. Modern preoperative imaging and functional mapping in patients with intracranial glioma. Rofo 2023, 195, 989–1000. [Google Scholar] [CrossRef]
- Gupta, A.; Shah, A.; Young, R.J.; Holodny, A.I. Imaging of brain tumors: Functional magnetic resonance imaging and diffusion tensor imaging. Neuroimaging Clin. N. Am. 2010, 20, 379–400. [Google Scholar] [CrossRef]
- Pala, A.; Reske, S.N.; Eberhardt, N.; Scheuerle, A.; Konig, R.; Schmitz, B.; Beer, A.J.; Wirtz, C.R.; Coburger, J. Diagnostic accuracy of intraoperative perfusion-weighted MRI and 5-aminolevulinic acid in relation to contrast-enhanced intraoperative MRI and (11)C-methionine positron emission tomography in resection of glioblastoma: A prospective study. Neurosurg. Rev. 2019, 42, 471–479. [Google Scholar] [CrossRef]
- Noh, T.; Mustroph, M.; Golby, A.J. Intraoperative Imaging for High-Grade Glioma Surgery. Neurosurg. Clin. N. Am. 2021, 32, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.D.; Barone, D.G.; Bryant, A.; Vale, L.; Bulbeck, H.; Lawrie, T.A.; Hart, M.G.; Watts, C. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst. Rev. 2018, 1, CD012788. [Google Scholar] [CrossRef] [PubMed]
- Pala, A.; Durner, G.; Braun, M.; Schmitz, B.; Wirtz, C.R.; Coburger, J. The Impact of an Ultra-Early Postoperative MRI on Treatment of Lower Grade Glioma. Cancers 2021, 13, 2914. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.; Sciortino, T.; Secoli, R.; D’Amico, E.; Moccia, S.; Fernandes, B.; Conti Nibali, M.; Gay, L.; Rossi, M.; De Momi, E.; et al. Glioma biopsies Classification Using Raman Spectroscopy and Machine Learning Models on Fresh Tissue Samples. Cancers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Zhang, H.; Guo, Y.; Tang, M.; Wang, J.; Wu, N. Current research status of Raman spectroscopy in glioma detection. Photodiagn. Photodyn. Ther. 2024, 50, 104388. [Google Scholar] [CrossRef]
- Livermore, L.J.; Isabelle, M.; Bell, I.M.; Scott, C.; Walsby-Tickle, J.; Gannon, J.; Plaha, P.; Vallance, C.; Ansorge, O. Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neurooncol. Adv. 2019, 1, vdz008. [Google Scholar] [CrossRef]
- Shahi, M.; Pringle, S.; Morris, M.; Garcia, D.M.; Quinones-Hinojosa, A.; Cooks, R.G. Detection of IDH mutation in glioma by desorption electrospray ionization (DESI) tandem mass spectrometry. Sci. Rep. 2024, 14, 26865. [Google Scholar] [CrossRef]
- Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 6700–6705. [Google Scholar] [CrossRef]
- Zaccagna, F.; McLean, M.A.; Grist, J.T.; Kaggie, J.; Mair, R.; Riemer, F.; Woitek, R.; Gill, A.B.; Deen, S.; Daniels, C.J.; et al. Imaging Glioblastoma Metabolism by Using Hyperpolarized [1-(13)C]Pyruvate Demonstrates Heterogeneity in Lactate Labeling: A Proof of Principle Study. Radiol. Imaging Cancer 2022, 4, e210076. [Google Scholar] [CrossRef]
- Mishkovsky, M.; Gusyatiner, O.; Lanz, B.; Cudalbu, C.; Vassallo, I.; Hamou, M.F.; Bloch, J.; Comment, A.; Gruetter, R.; Hegi, M.E. Hyperpolarized (13)C-glucose magnetic resonance highlights reduced aerobic glycolysis in vivo in infiltrative glioblastoma. Sci. Rep. 2021, 11, 5771. [Google Scholar] [CrossRef]
- Hsieh, K.L.; Chen, Q.; Salzillo, T.C.; Zhang, J.; Jiang, X.; Bhattacharya, P.K.; Shams, S. Hyperpolarized Magnetic Resonance Imaging, Nuclear Magnetic Resonance Metabolomics, and Artificial Intelligence to Interrogate the Metabolic Evolution of Glioblastoma. Metabolites 2024, 14, 448. [Google Scholar] [CrossRef]
- Deen, S.S.; Rooney, C.; Shinozaki, A.; McGing, J.; Grist, J.T.; Tyler, D.J.; Serrao, E.; Gallagher, F.A. Hyperpolarized Carbon 13 MRI: Clinical Applications and Future Directions in Oncology. Radiol. Imaging Cancer 2023, 5, e230005. [Google Scholar] [CrossRef]
- Ross, B.D.; Bhattacharya, P.; Wagner, S.; Tran, T.; Sailasuta, N. Hyperpolarized MR imaging: Neurologic applications of hyperpolarized metabolism. AJNR Am. J. Neuroradiol. 2010, 31, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Larson, P.E.; Zierhut, M.L.; Hu, S.; Bok, R.; Ozawa, T.; Kurhanewicz, J.; Vigneron, D.B.; Vandenberg, S.R.; James, C.D.; et al. Hyperpolarized 13C magnetic resonance metabolic imaging: Application to brain tumors. Neuro Oncol. 2010, 12, 133–144. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, P.C.M.; Brindle, K.; Lu, H.; Barker, P.B.; Edden, R.; Yadav, N.; Knutsson, L. Hyperpolarized MRI, functional MRI, MR spectroscopy and CEST to provide metabolic information in vivo. Curr. Opin. Chem. Biol. 2021, 63, 209–218. [Google Scholar] [CrossRef]
- von Morze, C.; Engelbach, J.A.; Blazey, T.; Quirk, J.D.; Reed, G.D.; Ippolito, J.E.; Garbow, J.R. Comparison of hyperpolarized (13) C and non-hyperpolarized deuterium MRI approaches for imaging cerebral glucose metabolism at 4.7 T. Magn. Reson. Med. 2021, 85, 1795–1804. [Google Scholar] [CrossRef]
- Miloushev, V.Z.; Keshari, K.R.; Holodny, A.I. Hyperpolarization MRI: Preclinical Models and Potential Applications in Neuroradiology. Top. Magn. Reson. Imaging 2016, 25, 31–37. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Mishkovsky, M.; Comment, A. Hyperpolarized MRS: New tool to study real-time brain function and metabolism. Anal. Biochem. 2017, 529, 270–277. [Google Scholar] [CrossRef]
- Bogh, N.; Gordon, J.W.; Hansen, E.S.S.; Bok, R.A.; Blicher, J.U.; Hu, J.Y.; Larson, P.E.Z.; Vigneron, D.B.; Laustsen, C. Initial Experience on Hyperpolarized [1-(13)C]Pyruvate MRI Multicenter Reproducibility-Are Multicenter Trials Feasible? Tomography 2022, 8, 585–595. [Google Scholar] [CrossRef]
- Hu, J.Y.; Kim, Y.; Autry, A.W.; Frost, M.M.; Bok, R.A.; Villanueva-Meyer, J.E.; Xu, D.; Li, Y.; Larson, P.E.Z.; Vigneron, D.B.; et al. Kinetic analysis of multi-resolution hyperpolarized (13) C human brain MRI to study cerebral metabolism. Magn. Reson. Med. 2022, 88, 2190–2197. [Google Scholar] [CrossRef]
- Uthayakumar, B.; Soliman, H.; Chen, A.P.; Bragagnolo, N.; Cappelletto, N.I.C.; Endre, R.; Perks, W.J.; Ma, N.; Heyn, C.; Keshari, K.R.; et al. Evidence of (13) C-lactate oxidation in the human brain from hyperpolarized (13) C-MRI. Magn. Reson. Med. 2024, 91, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.T.; Kim, Y.; Gordon, J.W.; Chen, H.Y.; Autry, A.W.; Lee, P.M.; Hu, J.Y.; Tan, C.T.; Suszczynski, C.; Chang, S.M.; et al. Hyperpolarized [2-(13)C]pyruvate MR molecular imaging with whole brain coverage. Neuroimage 2023, 280, 120350. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pinho, M.C.; Harrison, C.E.; Chen, J.; Sun, C.; Hackett, E.P.; Liticker, J.; Ratnakar, J.; Reed, G.D.; Chen, A.P.; et al. Dynamic (13) C MR spectroscopy as an alternative to imaging for assessing cerebral metabolism using hyperpolarized pyruvate in humans. Magn. Reson. Med. 2022, 87, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Le Page, L.M.; Guglielmetti, C.; Taglang, C.; Chaumeil, M.M. Imaging Brain Metabolism Using Hyperpolarized (13)C Magnetic Resonance Spectroscopy. Trends Neurosci. 2020, 43, 343–354. [Google Scholar] [CrossRef]
- Crane, J.C.; Gordon, J.W.; Chen, H.Y.; Autry, A.W.; Li, Y.; Olson, M.P.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Xu, D. Hyperpolarized (13) C MRI data acquisition and analysis in prostate and brain at University of California, San Francisco. NMR Biomed. 2021, 34, e4280. [Google Scholar] [CrossRef]
- Chung, B.T.; Chen, H.Y.; Gordon, J.; Mammoli, D.; Sriram, R.; Autry, A.W.; Le Page, L.M.; Chaumeil, M.M.; Shin, P.; Slater, J.; et al. First hyperpolarized [2-(13)C]pyruvate MR studies of human brain metabolism. J. Magn. Reson. 2019, 309, 106617. [Google Scholar] [CrossRef]
- Miloushev, V.Z.; Granlund, K.L.; Boltyanskiy, R.; Lyashchenko, S.K.; DeAngelis, L.M.; Mellinghoff, I.K.; Brennan, C.W.; Tabar, V.; Yang, T.J.; Holodny, A.I.; et al. Metabolic Imaging of the Human Brain with Hyperpolarized (13)C Pyruvate Demonstrates (13)C Lactate Production in Brain Tumor Patients. Cancer Res. 2018, 78, 3755–3760. [Google Scholar] [CrossRef]
- Grist, J.T.; McLean, M.A.; Riemer, F.; Schulte, R.F.; Deen, S.S.; Zaccagna, F.; Woitek, R.; Daniels, C.J.; Kaggie, J.D.; Matys, T.; et al. Quantifying normal human brain metabolism using hyperpolarized [1-(13)C]pyruvate and magnetic resonance imaging. Neuroimage 2019, 189, 171–179. [Google Scholar] [CrossRef]
- Lai, Y.C.; Hsieh, C.Y.; Juan, Y.H.; Lu, K.Y.; Lee, H.J.; Ng, S.H.; Wan, Y.L.; Lin, G. Hyperpolarized Carbon-13 Magnetic Resonance Imaging: Technical Considerations and Clinical Applications. Korean J. Radiol. 2024, 25, 459–472. [Google Scholar] [CrossRef]
- Lee, C.Y.; Soliman, H.; Bragagnolo, N.D.; Sahgal, A.; Geraghty, B.J.; Chen, A.P.; Endre, R.; Perks, W.J.; Detsky, J.S.; Leung, E.; et al. Predicting response to radiotherapy of intracranial metastases with hyperpolarized [Formula: See text]C MRI. J. Neurooncol. 2021, 152, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Soliman, H.; Geraghty, B.J.; Chen, A.P.; Connelly, K.A.; Endre, R.; Perks, W.J.; Heyn, C.; Black, S.E.; Cunningham, C.H. Lactate topography of the human brain using hyperpolarized (13)C-MRI. Neuroimage 2020, 204, 116202. [Google Scholar] [CrossRef] [PubMed]
- Uthayakumar, B.; Soliman, H.; Bragagnolo, N.D.; Cappelletto, N.I.C.; Lee, C.Y.; Geraghty, B.; Chen, A.P.; Perks, W.J.; Ma, N.; Heyn, C.; et al. Age-associated change in pyruvate metabolism investigated with hyperpolarized (13) C-MRI of the human brain. Hum. Brain Mapp. 2023, 44, 4052–4063. [Google Scholar] [CrossRef]
- Bogh, N.; Grist, J.T.; Rasmussen, C.W.; Bertelsen, L.B.; Hansen, E.S.S.; Blicher, J.U.; Tyler, D.J.; Laustsen, C. Lactate saturation limits bicarbonate detection in hyperpolarized (13) C-pyruvate MRI of the brain. Magn. Reson. Med. 2022, 88, 1170–1179. [Google Scholar] [CrossRef]
- Autry, A.W.; Vaziri, S.; LaFontaine, M.; Gordon, J.W.; Chen, H.Y.; Kim, Y.; Villanueva-Meyer, J.E.; Molinaro, A.; Clarke, J.L.; Oberheim Bush, N.A.; et al. Multi-parametric hyperpolarized (13)C/(1)H imaging reveals Warburg-related metabolic dysfunction and associated regional heterogeneity in high-grade human gliomas. Neuroimage Clin. 2023, 39, 103501. [Google Scholar] [CrossRef]
- Dong, Y.; Eskandari, R.; Ray, C.; Granlund, K.L.; Santos-Cunha, L.D.; Miloushev, V.Z.; Tee, S.S.; Jeong, S.; Aras, O.; Chen, Y.B.; et al. Hyperpolarized MRI Visualizes Warburg Effects and Predicts Treatment Response to mTOR Inhibitors in Patient-Derived ccRCC Xenograft Models. Cancer Res. 2019, 79, 242–250. [Google Scholar] [CrossRef]
- Jorgensen, S.H.; Bogh, N.; Hansen, E.; Vaeggemose, M.; Wiggers, H.; Laustsen, C. Hyperpolarized MRI—An Update and Future Perspectives. Semin. Nucl. Med. 2022, 52, 374–381. [Google Scholar] [CrossRef]
- Chen, J.; Patel, T.R.; Pinho, M.C.; Choi, C.; Harrison, C.E.; Baxter, J.D.; Derner, K.; Pena, S.; Liticker, J.; Raza, J.; et al. Preoperative imaging of glioblastoma patients using hyperpolarized (13)C pyruvate: Potential role in clinical decision making. Neurooncol. Adv. 2021, 3, vdab092. [Google Scholar] [CrossRef]
- Mishkovsky, M.; Comment, A.; Gruetter, R. In vivo detection of brain Krebs cycle intermediate by hyperpolarized magnetic resonance. J. Cereb. Blood Flow Metab. 2012, 32, 2108–2113. [Google Scholar] [CrossRef]
- Lipso, K.W.; Magnusson, P.; Ardenkjaer-Larsen, J.H. Hyperpolarized (13)C MR Angiography. Curr. Pharm. Des. 2016, 22, 90–95. [Google Scholar] [CrossRef]
- Svensson, J.; Mansson, S.; Johansson, E.; Petersson, J.S.; Olsson, L.E. Hyperpolarized 13C MR angiography using trueFISP. Magn. Reson. Med. 2003, 50, 256–262. [Google Scholar] [CrossRef]
- Shepelytskyi, Y.; Hane, F.T.; Grynko, V.; Li, T.; Hassan, A.; Albert, M.S. Hyperpolarized (129)Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics 2020, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Mansson, S.; Wirestam, R.; Svensson, J.; Petersson, J.S.; Golman, K.; Stahlberg, F. Cerebral perfusion assessment by bolus tracking using hyperpolarized 13C. Magn. Reson. Med. 2004, 51, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Vaziri, S.; Bogh, N.; Kim, Y.; Autry, A.W.; Bok, R.A.; Li, Y.; Laustsen, C.; Xu, D.; Larson, P.E.Z.; et al. Investigating cerebral perfusion with high resolution hyperpolarized [1-(13) C]pyruvate MRI. Magn. Reson. Med. 2023, 90, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Stewart, N.J.; Griffiths, P.D.; Norquay, G.; Wild, J.M. Imaging Human Brain Perfusion with Inhaled Hyperpolarized (129)Xe MR Imaging. Radiology 2018, 286, 659–665. [Google Scholar] [CrossRef]
- Detre, J.A.; Rao, H.; Wang, D.J.; Chen, Y.F.; Wang, Z. Applications of arterial spin labeled MRI in the brain. J. Magn. Reson. Imaging 2012, 35, 1026–1037. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Lai, Y.C.; Lu, K.Y.; Lin, G. Advancements, Challenges, and Future Prospects in Clinical Hyperpolarized Magnetic Resonance Imaging: A Comprehensive Review. Biomed. J. 2024, 48, 100802. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Bankson, J.A.; Brindle, K.M.; Epstein, S.; Gallagher, F.A.; Grashei, M.; Guglielmetti, C.; Kaggie, J.D.; Keshari, K.R.; Knecht, S.; et al. New Horizons in Hyperpolarized (13)C MRI. Mol. Imaging Biol. 2024, 26, 222–232. [Google Scholar] [CrossRef]
- Ruiz-Rodado, V.; Brender, J.R.; Cherukuri, M.K.; Gilbert, M.R.; Larion, M. Magnetic resonance spectroscopy for the study of cns malignancies. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 122, 23–41. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Liu, D.; Li, Y.; Chen, W.; Wang, Y.; Wang, Y.; Xing, H.; Xia, Y.; Li, J.; et al. Clinical updates on gliomas and implications of the 5th edition of the WHO classification of central nervous system tumors. Front. Oncol. 2023, 13, 1131642. [Google Scholar] [CrossRef]
- Agadi, K.; Dominari, A.; Tebha, S.S.; Mohammadi, A.; Zahid, S. Neurosurgical Management of Cerebrospinal Tumors in the Era of Artificial Intelligence: A Scoping Review. J. Korean Neurosurg. Soc. 2023, 66, 632–641. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.T.; Chan, K.W. Developing MR probes for molecular imaging. Adv. Cancer Res. 2014, 124, 297–327. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.W.; Gordon, J.W.; Chen, H.Y.; LaFontaine, M.; Bok, R.; Van Criekinge, M.; Slater, J.B.; Carvajal, L.; Villanueva-Meyer, J.E.; Chang, S.M.; et al. Characterization of serial hyperpolarized (13)C metabolic imaging in patients with glioma. Neuroimage Clin. 2020, 27, 102323. [Google Scholar] [CrossRef]
- Ahumada-Vizcaino, J.C.; Wuo-Silva, R.; Hernandez, M.M.; Chaddad-Neto, F. The art of combining neuroanatomy and microsurgical skills in modern neurosurgery. Front. Neurol. 2022, 13, 1076778. [Google Scholar] [CrossRef]
- Satoer, D.; Visch-Brink, E.; Dirven, C.; Vincent, A. Glioma surgery in eloquent areas: Can we preserve cognition? Acta Neurochir. 2016, 158, 35–50. [Google Scholar] [CrossRef]
- Krieg, S.M.; Bernhard, D.; Ille, S.; Meyer, B.; Combs, S.; Rotenberg, A.; Fruhwald, M.C. Neurosurgery for eloquent lesions in children: State-of-the-art rationale and technical implications of perioperative neurophysiology. Neurosurg. Focus 2022, 53, E4. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Introduction: Surgical Management of Eloquent Area Tumors. Neurosurgery 2020, 87, 1076–1077. [Google Scholar] [CrossRef]
- Staub-Bartelt, F.; Rapp, M.; Sabel, M. Resection of Eloquent Located Brain Tumors by Mapping Only-A Feasibility Study. Brain Sci. 2023, 13, 1366. [Google Scholar] [CrossRef]
- Zuniga, J.R.; Zenn, M.R. Principles of microsurgery. Oral Maxillofac. Surg. Clin. N. Am. 2001, 13, 331–342. [Google Scholar] [CrossRef]
- Margalit, E.; Lee, H.; Finzi, D.; DiCarlo, J.J.; Grill-Spector, K.; Yamins, D.L.K. A Unifying Principle for the Functional Organization of Visual Cortex. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Siddiqui, K.; Baig, A.; Shamim, M.S. Role of diffusion tensor imaging for brain tumour resection. J. Pak. Med. Assoc. 2022, 72, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhylka, A.; Sollmann, N.; Kofler, F.; Radwan, A.; De Luca, A.; Gempt, J.; Wiestler, B.; Menze, B.; Schroeder, A.; Zimmer, C.; et al. Reconstruction of the Corticospinal Tract in Patients with Motor-Eloquent High-Grade Gliomas Using Multilevel Fiber Tractography Combined with Functional Motor Cortex Mapping. AJNR Am. J. Neuroradiol. 2023, 44, 283–290. [Google Scholar] [CrossRef]
- Kang, K.M.; Kim, K.M.; Kim, I.S.; Kim, J.H.; Kang, H.; Ji, S.Y.; Dho, Y.S.; Oh, H.; Park, H.P.; Seo, H.G.; et al. Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging for Language Mapping in Brain Tumor Surgery: Validation With Direct Cortical Stimulation and Cortico-Cortical Evoked Potential. Korean J. Radiol. 2023, 24, 553–563. [Google Scholar] [CrossRef]
- Mansouri, A.; Ibrahim, S.; Bello, L.; Martino, J.; Velasquez, C. The current state of the art of primary motor mapping for tumor resection: A focused survey. Clin. Neurol. Neurosurg. 2023, 229, 107685. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, T.; Chong, S.T.; Hsu, S.P.; Chu, Y.H.; Hsu, Y.C.; Xu, G.; Ko, Y.T.; Kuo, K.T.; Yang, Z.; et al. Automatic bundle-specific white matter fiber tracking tool using diffusion tensor imaging data: A pilot trial in the application of language-related glioma resection. Front. Oncol. 2023, 13, 1089923. [Google Scholar] [CrossRef]
- Huisman, T.; Patel, R.; Kralik, S.; Desai, N.K.; Meoded, A.; Chen, K.; Weiner, H.L.; Curry, D.J.; Lequin, M.; Kranendonk, M.; et al. Advances in Imaging Modalities for Pediatric Brain and Spinal Cord Tumors. Pediatr. Neurosurg. 2023, 58, 240–258. [Google Scholar] [CrossRef]
- Urcuyo, J.C.; Curtin, L.; Langworthy, J.M.; De Leon, G.; Anderies, B.; Singleton, K.W.; Hawkins-Daarud, A.; Jackson, P.R.; Bond, K.M.; Ranjbar, S.; et al. Image-localized biopsy mapping of brain tumor heterogeneity: A single-center study protocol. PLoS ONE 2023, 18, e0287767. [Google Scholar] [CrossRef]
- Napolitano, M.; Vaz, G.; Lawson, T.M.; Docquier, M.A.; van Maanen, A.; Duprez, T.; Raftopoulos, C. Glioblastoma surgery with and without intraoperative MRI at 3.0T. Neurochirurgie 2014, 60, 143–150. [Google Scholar] [CrossRef]
- Altawalbeh, G.; Goldberg, M.; Mondragon-Soto, M.G.; Negwer, C.; Wagner, A.; Gempt, J.; Meyer, B.; Aftahy, A.K. Navigating Brain Metastases: Unveiling the Potential of 3-Tesla Intraoperative Magnetic Resonance Imaging. Cancers 2024, 16, 2774. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Segovia von Riehm, J.; Ganslandt, O.; Wirtz, C.R.; Renovanz, M. Is There an Indication for Intraoperative MRI in Subtotal Resection of Glioblastoma? A Multicenter Retrospective Comparative Analysis. World Neurosurg. 2018, 110, e389–e397. [Google Scholar] [CrossRef]
- Familiari, P.; Frati, A.; Pesce, A.; Miscusi, M.; Cimatti, M.; Raco, A. Real Impact of Intraoperative Magnetic Resonance Imaging in Newly Diagnosed Glioblastoma Multiforme Resection: An Observational Analytic Cohort Study From a Single Surgeon Experience. World Neurosurg. 2018, 116, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Caras, A.; Mugge, L.; Miller, W.K.; Mansour, T.R.; Schroeder, J.; Medhkour, A. Usefulness and Impact of Intraoperative Imaging for Glioma Resection on Patient Outcome and Extent of Resection: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 134, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Trifiletti, D.M.; Alonso, C.; Grover, S.; Fadul, C.E.; Sheehan, J.P.; Showalter, T.N. Prognostic Implications of Extent of Resection in Glioblastoma: Analysis from a Large Database. World Neurosurg. 2017, 103, 330–340. [Google Scholar] [CrossRef]
- Al-Adli, N.N.; Young, J.S.; Scotford, K.; Sibih, Y.E.; Payne, J.; Berger, M.S. Advances in Intraoperative Glioma Tissue Sampling and Infiltration Assessment. Brain Sci. 2023, 13, 1637. [Google Scholar] [CrossRef]
- Bander, E.D.; Magge, R.; Ramakrishna, R. Advances in Glioblastoma Operative Techniques. World Neurosurg. 2018, 116, 529–538. [Google Scholar] [CrossRef]
- Coburger, J.; Wirtz, C.R.; Konig, R.W. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field magnetic resonance imaging. J. Neurosurg. Sci. 2017, 61, 233–244. [Google Scholar] [CrossRef]
- Coburger, J.; Nabavi, A.; Konig, R.; Wirtz, C.R.; Pala, A. Contemporary use of intraoperative imaging in glioma surgery: A survey among EANS members. Clin. Neurol. Neurosurg. 2017, 163, 133–141. [Google Scholar] [CrossRef]
- Guzzi, G.; Ricciuti, R.A.; Della Torre, A.; Lo Turco, E.; Lavano, A.; Longhini, F.; La Torre, D. Intraoperative Neurophysiological Monitoring in Neurosurgery. J. Clin. Med. 2024, 13, 2966. [Google Scholar] [CrossRef] [PubMed]
- Jeltema, H.R.; Ohlerth, A.K.; de Wit, A.; Wagemakers, M.; Rofes, A.; Bastiaanse, R.; Drost, G. Comparing navigated transcranial magnetic stimulation mapping and “gold standard” direct cortical stimulation mapping in neurosurgery: A systematic review. Neurosurg. Rev. 2021, 44, 1903–1920. [Google Scholar] [CrossRef] [PubMed]
- Yamao, Y.; Matsumoto, R.; Kikuchi, T.; Yoshida, K.; Kunieda, T.; Miyamoto, S. Intraoperative Brain Mapping by Cortico-Cortical Evoked Potential. Front. Hum. Neurosci. 2021, 15, 635453. [Google Scholar] [CrossRef]
- Seidel, K.; Szelenyi, A.; Bello, L. Intraoperative mapping and monitoring during brain tumor surgeries. Handb. Clin. Neurol. 2022, 186, 133–149. [Google Scholar] [CrossRef]
- Tuleasca, C.; Leroy, H.A.; Strachowski, O.; Derre, B.; Maurage, C.A.; Peciu-Florianu, I.; Reyns, N. Combined use of intraoperative MRI and awake tailored microsurgical resection to respect functional neural networks: Preliminary experience. Swiss Med. Wkly. 2023, 153, 40072. [Google Scholar] [CrossRef]
- Tuleasca, C.; Peciu-Florianu, I.; Strachowski, O.; Derre, B.; Vannod-Michel, Q.; Reyns, N. How to combine the use of intraoperative magnetic resonance imaging (MRI) and awake craniotomy for microsurgical resection of hemorrhagic cavernous malformation in eloquent area: A case report. J. Med. Case Rep. 2023, 17, 160. [Google Scholar] [CrossRef]
- Coburger, J.; Musahl, C.; Henkes, H.; Horvath-Rizea, D.; Bittl, M.; Weissbach, C.; Hopf, N. Comparison of navigated transcranial magnetic stimulation and functional magnetic resonance imaging for preoperative mapping in rolandic tumor surgery. Neurosurg. Rev. 2013, 36, 65–75, discussion 75–66. [Google Scholar] [CrossRef]
- Schucht, P.; Seidel, K.; Beck, J.; Murek, M.; Jilch, A.; Wiest, R.; Fung, C.; Raabe, A. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: Evaluation of resection rates and neurological outcome. Neurosurg. Focus 2014, 37, E16. [Google Scholar] [CrossRef]
- Hauser, S.B.; Kockro, R.A.; Actor, B.; Sarnthein, J.; Bernays, R.L. Combining 5-Aminolevulinic Acid Fluorescence and Intraoperative Magnetic Resonance Imaging in Glioblastoma Surgery: A Histology-Based Evaluation. Neurosurgery 2016, 78, 475–483. [Google Scholar] [CrossRef]
- Peters, D.R.; Halimi, F.; Ozduman, K.; Levivier, M.; Conti, A.; Reyns, N.; Tuleasca, C. Resection of the contrast-enhancing tumor in diffuse gliomas bordering eloquent areas using electrophysiology and 5-ALA fluorescence: Evaluation of resection rates and neurological outcome-a systematic review and meta-analysis. Neurosurg. Rev. 2023, 46, 185. [Google Scholar] [CrossRef]
- Dixon, L.; Lim, A.; Grech-Sollars, M.; Nandi, D.; Camp, S. Intraoperative ultrasound in brain tumor surgery: A review and implementation guide. Neurosurg. Rev. 2022, 45, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.M.; Maione, M.; Peschillo, S.; Signorelli, F.; Visocchi, M.; Sortino, G.; Fiumano, G.; Certo, F. Intraoperative computed tomography, navigated ultrasound, 5-amino-levulinic acid fluorescence and neuromonitoring in brain tumor surgery: Overtreatment or useful tool combination? J. Neurosurg. Sci. 2024, 68, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Scheuerle, A.; Pala, A.; Thal, D.; Wirtz, C.R.; Konig, R. Histopathological Insights on Imaging Results of Intraoperative Magnetic Resonance Imaging, 5-Aminolevulinic Acid, and Intraoperative Ultrasound in Glioblastoma Surgery. Neurosurgery 2017, 81, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.M.; Ius, T.; La Rocca, G.; Gaudino, S.; Isola, M.; Pignotti, F.; Rapisarda, A.; Mazzucchi, E.; Giordano, C.; Dragonetti, V.; et al. 5-Aminolevulinic Acid and Contrast-Enhanced Ultrasound: The Combination of the Two Techniques to Optimize the Extent of Resection in Glioblastoma Surgery. Neurosurgery 2020, 86, E529–E540. [Google Scholar] [CrossRef]
- Kleihues, P.; Burger, P.C.; Scheithauer, B.W. The new WHO classification of brain tumours. Brain Pathol. 1993, 3, 255–268. [Google Scholar] [CrossRef]
- Kleihues, P.; Louis, D.N.; Scheithauer, B.W.; Rorke, L.B.; Reifenberger, G.; Burger, P.C.; Cavenee, W.K. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 215–225, discussion 226–219. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Donohue, K.E.; Gooch, C.; Katz, A.; Wakelee, J.; Slavotinek, A.; Korf, B.R. Pitfalls and challenges in genetic test interpretation: An exploration of genetic professionals experience with interpretation of results. Clin. Genet. 2021, 99, 638–649. [Google Scholar] [CrossRef]
- Horbinski, C.; Ligon, K.L.; Brastianos, P.; Huse, J.T.; Venere, M.; Chang, S.; Buckner, J.; Cloughesy, T.; Jenkins, R.B.; Giannini, C.; et al. The medical necessity of advanced molecular testing in the diagnosis and treatment of brain tumor patients. Neuro Oncol. 2019, 21, 1498–1508. [Google Scholar] [CrossRef]
- Blobner, J.; Dengler, L.; Blobner, S.; Eberle, C.; Weller, J.; Teske, N.; Karschnia, P.; Ruhlmann, K.; Heinrich, K.; Ziemann, F.; et al. Significance of molecular diagnostics for therapeutic decision-making in recurrent glioma. Neurooncol. Adv. 2023, 5, vdad060. [Google Scholar] [CrossRef]
- Bertero, L.; Mangherini, L.; Ricci, A.A.; Cassoni, P.; Sahm, F. Molecular neuropathology: An essential and evolving toolbox for the diagnosis and clinical management of central nervous system tumors. Virchows Arch. 2024, 484, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Fortin Ensign, S.; Hrachova, M.; Chang, S.; Mrugala, M.M. Assessing the utility and attitudes toward molecular testing in neuro-oncology: A survey of the Society for Neuro-Oncology members. Neurooncol. Pract. 2021, 8, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Allwohn, L.; Wolfgang, J.; Onken, J.; Wasilewski, D.; Roohani, S.; Zips, D.; Ehret, F.; Kaul, D. Treating oligodendroglioma—An analysis of a homogeneous 1p/19q-codeleted and isocitrate dehydrogenase-mutant patient cohort. Clin. Transl. Radiat. Oncol. 2023, 41, 100626. [Google Scholar] [CrossRef]
- Clark, V.E.; Cahill, D.P. Extent of Resection Versus Molecular Classification: What Matters When? Neurosurg. Clin. N. Am. 2019, 30, 95–101. [Google Scholar] [CrossRef]
- Yoo, J.; Yoon, S.J.; Kim, K.H.; Jung, I.H.; Lim, S.H.; Kim, W.; Yoon, H.I.; Kim, S.H.; Sung, K.S.; Roh, T.H.; et al. Patterns of recurrence according to the extent of resection in patients with IDH-wild-type glioblastoma: A retrospective study. J. Neurosurg. 2022, 137, 533–543. [Google Scholar] [CrossRef]
- Nie, S.; Zhu, Y.; Yang, J.; Xin, T.; Xue, S.; Sun, J.; Mu, D.; Chen, Z.; Sun, P.; Yu, J.; et al. Clinicopathologic analysis of microscopic tumor extension in glioma for external beam radiotherapy planning. BMC Med. 2021, 19, 269. [Google Scholar] [CrossRef]
- Demuth, T.; Berens, M.E. Molecular mechanisms of glioma cell migration and invasion. J. Neurooncol. 2004, 70, 217–228. [Google Scholar] [CrossRef]
- Tonn, J.C.; Goldbrunner, R. Mechanisms of glioma cell invasion. In Local Therapies for Glioma; Westphal, M., Tonn, J.C., Ram, Z., Eds.; Springer: Vienna, Austria, 2003; Volume 1. [Google Scholar]
- Nilsson, M.; Englund, E.; Szczepankiewicz, F.; van Westen, D.; Sundgren, P.C. Imaging brain tumour microstructure. Neuroimage 2018, 182, 232–250. [Google Scholar] [CrossRef]
- Geraldes, C.F.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Dorfner, F.J.; Patel, J.B.; Kalpathy-Cramer, J.; Gerstner, E.R.; Bridge, C.P. A review of deep learning for brain tumor analysis in MRI. NPJ Precis. Oncol. 2025, 9, 2. [Google Scholar] [CrossRef]
- Autry, A.W.; Kim, Y.; Dang, D.; Chen, H.Y.; Slater, J.B.; Bok, R.A.; Xu, D.; Lupo, J.M.; Gordon, J.W.; Larson, P.E.Z.; et al. Clinical Translation of Hyperpolarized (13)C Metabolic Probes for Glioma Imaging. AJNR Am. J. Neuroradiol. 2025, 46, 1754–1764. [Google Scholar] [CrossRef]
- Allouche-Arnon, H.; Gamliel, A.; Barzilay, C.M.; Nalbandian, R.; Gomori, J.M.; Karlsson, M.; Lerche, M.H.; Katz-Brull, R. A hyperpolarized choline molecular probe for monitoring acetylcholine synthesis. Contrast Media Mol. Imaging 2011, 6, 139–147. [Google Scholar] [CrossRef]
- Ehrhardt, M.J.; Gallagher, F.A.; McLean, M.A.; Schonlieb, C.B. Enhancing the spatial resolution of hyperpolarized carbon-13 MRI of human brain metabolism using structure guidance. Magn. Reson. Med. 2022, 87, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Shepelytskyi, Y.; Grynko, V.; Li, T.; Hassan, A.; Granberg, K.; Albert, M.S. The effects of an initial depolarization pulse on dissolved phase hyperpolarized (129) Xe brain MRI. Magn. Reson. Med. 2021, 86, 3147–3155. [Google Scholar] [CrossRef]
- Grynko, V.; Shepelytskyi, Y.; Li, T.; Hassan, A.; Granberg, K.; Albert, M.S. Hyperpolarized (129) Xe multi-slice imaging of the human brain using a 3D gradient echo pulse sequence. Magn. Reson. Med. 2021, 86, 3175–3181. [Google Scholar] [CrossRef]
- Shang, H.; Larson, P.E.Z.; Kerr, A.; Reed, G.; Sukumar, S.; Elkhaled, A.; Gordon, J.W.; Ohliger, M.A.; Pauly, J.M.; Lustig, M.; et al. Multiband RF pulses with improved performance via convex optimization. J. Magn. Reson. 2016, 262, 81–90. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, H.Y.; Autry, A.W.; Villanueva-Meyer, J.; Chang, S.M.; Li, Y.; Larson, P.E.Z.; Brender, J.R.; Krishna, M.C.; Xu, D.; et al. Denoising of hyperpolarized (13) C MR images of the human brain using patch-based higher-order singular value decomposition. Magn. Reson. Med. 2021, 86, 2497–2511. [Google Scholar] [CrossRef]
- Keshari, K.R.; Kurhanewicz, J.; Bok, R.; Larson, P.E.; Vigneron, D.B.; Wilson, D.M. Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18606–18611. [Google Scholar] [CrossRef]
- Datta, K.; Spielman, D. MRI of [2-(13) C]Lactate without J-coupling artifacts. Magn. Reson. Med. 2021, 85, 1522–1539. [Google Scholar] [CrossRef]
- Larson, P.E.; Kerr, A.B.; Reed, G.D.; Hurd, R.E.; Kurhanewicz, J.; Pauly, J.M.; Vigneron, D.B. Generating super stimulated-echoes in MRI and their application to hyperpolarized C-13 diffusion metabolic imaging. IEEE Trans. Med. Imaging 2012, 31, 265–275. [Google Scholar] [CrossRef]
- Gordon, J.W.; Autry, A.W.; Tang, S.; Graham, J.Y.; Bok, R.A.; Zhu, X.; Villanueva-Meyer, J.E.; Li, Y.; Ohilger, M.A.; Abraham, M.R.; et al. A variable resolution approach for improved acquisition of hyperpolarized (13) C metabolic MRI. Magn. Reson. Med. 2020, 84, 2943–2952. [Google Scholar] [CrossRef]
- Yeung, K.; Ng, K.L.; McGing, J.J.; Axford, A.; Birkhoelzer, S.; Shinozaki, A.; Ricchi, M.; Sgambelluri, N.; Zaccagna, F.; Mills, R.; et al. Evaluation of an integrated variable flip angle protocol to estimate coil B(1) for hyperpolarized MRI. Magn. Reson. Med. 2025, 93, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Park, J.M. Super-Resolution Hyperpolarized (13)C Imaging of Human Brain Using Patch-Based Algorithm. Tomography 2020, 6, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized (13)C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Chekmenev, E.Y.; Perman, W.H.; Harris, K.C.; Lin, A.P.; Norton, V.A.; Tan, C.T.; Ross, B.D.; Weitekamp, D.P. Towards hyperpolarized (13)C-succinate imaging of brain cancer. J. Magn. Reson. 2007, 186, 150–155. [Google Scholar] [CrossRef]
- Najac, C.; Ronen, S.M. MR Molecular Imaging of Brain Cancer Metabolism Using Hyperpolarized 13C Magnetic Resonance Spectroscopy. Top. Magn. Reson. Imaging 2016, 25, 187–196. [Google Scholar] [CrossRef]
- Park, I.; Larson, P.E.Z.; Gordon, J.W.; Carvajal, L.; Chen, H.Y.; Bok, R.; Van Criekinge, M.; Ferrone, M.; Slater, J.B.; Xu, D.; et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn. Reson. Med. 2018, 80, 864–873. [Google Scholar] [CrossRef]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar] [CrossRef]
- Gierse, M.; Nagel, L.; Keim, M.; Lucas, S.; Speidel, T.; Lobmeyer, T.; Winter, G.; Josten, F.; Karaali, S.; Fellermann, M.; et al. Parahydrogen-Polarized Fumarate for Preclinical in Vivo Metabolic Magnetic Resonance Imaging. J. Am. Chem. Soc. 2023, 145, 5960–5969. [Google Scholar] [CrossRef]
- Nagel, L.; Gierse, M.; Gottwald, W.; Ahmadova, Z.; Grashei, M.; Wolff, P.; Josten, F.; Karaali, S.; Muller, C.A.; Lucas, S.; et al. Parahydrogen-Polarized [1-(13) C]Pyruvate for Reliable and Fast Preclinical Metabolic Magnetic Resonance Imaging. Adv. Sci. 2023, 10, e2303441. [Google Scholar] [CrossRef]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.; Duckett, S.B.; Green, G.G.; Khazal, I.G.; Lopez-Serrano, J.; Williamson, D.C. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Morshed, R.A.; Chunduru, P.; Pandian, B.; Young, J.; Villanueva-Meyer, J.E.; Tihan, T.; Sloan, E.A.; Aghi, M.K.; Molinaro, A.M.; et al. Detection of glioma infiltration at the tumor margin using quantitative stimulated Raman scattering histology. Sci. Rep. 2021, 11, 12162. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Engwer, C.; Wenske, M. Estimating the extent of glioblastoma invasion: Approximate stationalization of anisotropic advection-diffusion-reaction equations in the context of glioblastoma invasion. J. Math. Biol. 2021, 82, 10. [Google Scholar] [CrossRef]
- Duffau, H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: A consecutive series with 11-year follow-up. Acta Neurochir. 2016, 158, 51–58. [Google Scholar] [CrossRef]
- Vamsidhar, D.; Desai, P.; Joshi, S.; Kolhar, S.; Deshpande, N.; Gite, S. Hybrid model integration with explainable AI for brain tumor diagnosis: A unified approach to MRI analysis and prediction. Sci. Rep. 2025, 15, 20542. [Google Scholar] [CrossRef]
- Satushe, V.; Vyas, V.; Metkar, S.; Singh, D.P. AI in MRI brain tumor diagnosis: A systematic review of machine learning and deep learning advances (2010–2025). Chemom. Intell. Lab. Syst. 2025, 263, 105414. [Google Scholar] [CrossRef]
- Holdhoff, M.; Grossman, S.A. Controversies in the adjuvant therapy of high-grade gliomas. Oncologist 2011, 16, 351–358. [Google Scholar] [CrossRef]
- Khan, M.K.; Hunter, G.K.; Vogelbaum, M.; Suh, J.H.; Chao, S.T. Evidence-based adjuvant therapy for gliomas: Current concepts and newer developments. Indian J. Cancer 2009, 46, 96–107. [Google Scholar] [CrossRef]
- Zhu, P.; Du, X.L.; Hwang, L.Y.; Lairson, D.; Li, R.; Esquenazi, Y.; Zhu, J.J. Impact of timing to initiate adjuvant therapy on survival of elderly glioblastoma patients using the SEER-Medicare and national cancer databases. Sci. Rep. 2023, 13, 3266. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Castellano, A.; Bailo, M.; Cicone, F.; Carideo, L.; Quartuccio, N.; Mortini, P.; Falini, A.; Cascini, G.L.; Minniti, G. Advanced Imaging Techniques for Radiotherapy Planning of Gliomas. Cancers 2021, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, T.C.; Mawoneke, V.; Weygand, J.; Shetty, A.; Gumin, J.; Zacharias, N.M.; Gammon, S.T.; Piwnica-Worms, D.; Fuller, G.N.; Logothetis, C.J.; et al. Measuring the Metabolic Evolution of Glioblastoma throughout Tumor Development, Regression, and Recurrence with Hyperpolarized Magnetic Resonance. Cells 2021, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Bok, R.; Ozawa, T.; Phillips, J.J.; James, C.D.; Vigneron, D.B.; Ronen, S.M.; Nelson, S.J. Detection of early response to temozolomide treatment in brain tumors using hyperpolarized 13C MR metabolic imaging. J. Magn. Reson. Imaging 2011, 33, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, S.; Pucciarelli, D.; Song, J.; Choi, J.M.; Lee, K.H.; Kim, Y.H.; Jung, S.; Yoon, W.; Nakamura, J.L. Differentiating Radiation Necrosis from Brain Tumor Using Hyperpolarized Carbon-13 MR Metabolic Imaging. Mol. Imaging Biol. 2021, 23, 417–426. [Google Scholar] [CrossRef]
- Lim, H.; Martinez-Santiesteban, F.; Jensen, M.D.; Chen, A.; Wong, E.; Scholl, T.J. Monitoring Early Changes in Tumor Metabolism in Response to Therapy Using Hyperpolarized (13)C MRSI in a Preclinical Model of Glioma. Tomography 2020, 6, 290–300. [Google Scholar] [CrossRef]
| Study | Mean Age (± SD) | Male/Female | Design | Population | Field Strength | HP Agent | Main Outcomes | Control Region |
|---|---|---|---|---|---|---|---|---|
| Autry et al. (2020) [116] | 45.6 ± 10.0 | 2:3 | Prospective observational | 5 patients with glioma | 3T MRI | 13C pyruvate | kPL and kPB values | NAWM (normal-appearing white matter) |
| Chen et al. (2021) [98] | 67.0 ± 4.2 | 2:1 | Case series | 3 patients with glioblastoma | 3T MRI | 13C pyruvate | Relative lactate and bicarbonate signals | Contralateral NAWM |
| Zaccagna et al. (2022) [69] | 60.0 ± 10 | 6:2 | Prospective observational | 7 patients with glioma | 3T MRI | 13C pyruvate | Lactate/Bicarbonate ratio Lactate/Pyruvate ratio kPL and kPB values | Contralateral NABP |
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Outcome Variable | Group A (Mean ± SD) Non-Tumour Side | Group B (Mean ± SD) Tumour | Group Sizes (A/B) | Test Reported in Study | Statistical Method for SMD Computation | p-Value | SMD | Standard Error | 95% CI |
| Zaccagna et al. [69] | Lactate/Bicarbonate Ratio | 0.1043 ± 0.0412 | 0.0571 ± 0.0281 | n = 7/n = 7 | Wilcoxon test | Cohen’s d (pooled) | 0.002 | 1.34 | 0.600 | −2.51–0.16 |
| kPL (GBM vs. NABP) | 16.51 ± 7.86 | 16.09 ± 6.18 | 0.730 | 0.06 | 0.535 | −0.99–1.11 | ||||
| Autry et al. [116] | kPL (T2L vs. NAWM) | 0.0198 ± 0.0055 | 0.0216 ± 0.0054 | n = 5/n = 5 | Not specified | Cohen’s d (pooled) | Not reported | −0.33 | 0.638 | −1.58–0.92 |
| Chen et al. [98] | Relative Lactate/Bicarbonate Signal | Not reported in analyzable format | Not reported in analyzable format | n = 3/n = 3 | Qualitative only | Not applicable | Not applicable | Not calculable | Not calculable | Not calculable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapapa, T.; König, R.; Coburger, J.; Mayer, B.; Kreiser, K.; Rasche, V. Metabolic Imaging as Future Technology and Innovation in Brain-Tumour Surgery: A Systematic Review. Curr. Oncol. 2025, 32, 597. https://doi.org/10.3390/curroncol32110597
Kapapa T, König R, Coburger J, Mayer B, Kreiser K, Rasche V. Metabolic Imaging as Future Technology and Innovation in Brain-Tumour Surgery: A Systematic Review. Current Oncology. 2025; 32(11):597. https://doi.org/10.3390/curroncol32110597
Chicago/Turabian StyleKapapa, Thomas, Ralph König, Jan Coburger, Benjamin Mayer, Kornelia Kreiser, and Volker Rasche. 2025. "Metabolic Imaging as Future Technology and Innovation in Brain-Tumour Surgery: A Systematic Review" Current Oncology 32, no. 11: 597. https://doi.org/10.3390/curroncol32110597
APA StyleKapapa, T., König, R., Coburger, J., Mayer, B., Kreiser, K., & Rasche, V. (2025). Metabolic Imaging as Future Technology and Innovation in Brain-Tumour Surgery: A Systematic Review. Current Oncology, 32(11), 597. https://doi.org/10.3390/curroncol32110597





