Tumor-Treating Fields and Related Treatments in the Management of Pediatric Brain Tumors
Abstract
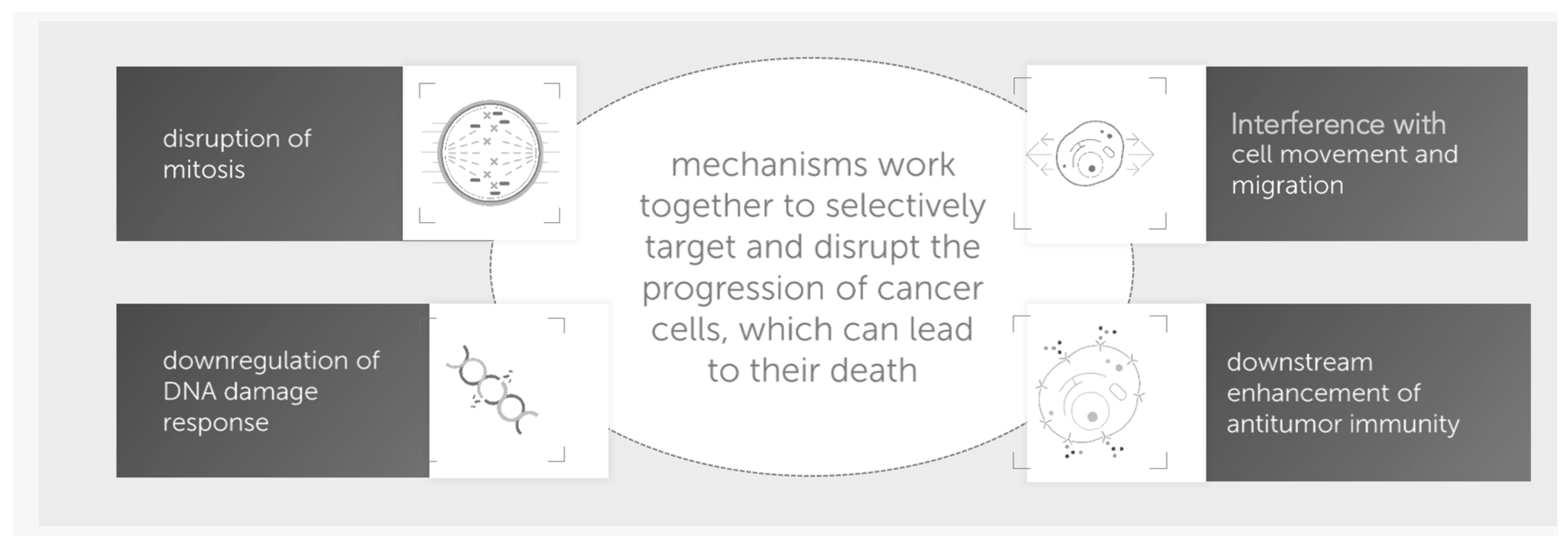
:1. Background
2. Tumor-Treating Fields in Pediatric Brain Tumors
3. Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DBS | Deep brain stimulation |
| EGFR | Epidermal growth factor receptor |
| NCCN | National Comprehensive Cancer Network |
| NSCLC | Non-small cell lung cancer |
| TMZ | Temozolomide |
| TTFIELDS | Tumor-treating fields |
References
- Arvind, R.; Chandana, S.R.; Borad, M.J.; Pennington, D.; Mody, K.; Babiker, H. Tumor-Treating Fields: A fourth modality in cancer treatment, new practice updates. Crit. Rev. Oncol. Hematol. 2021, 168, 103535. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. Available online: http://aacrjournals.org/cancerres/article-pdf/64/9/3288/2529860/zch00904003288.pdf (accessed on 20 January 2025). [CrossRef] [PubMed]
- Kaune, W.T. Introduction to power-frequency electric and magnetic fields. Environ. Health Perspect. 1993, 101 (Suppl. S4), 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gera, N.; Yang, A.; Holtzman, T.S.; Lee, S.X.; Wong, E.T.; Swanson, K.D. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS ONE 2015, 10, e0125269. [Google Scholar] [CrossRef]
- Guo, X.; Yang, X.; Wu, J.; Yang, H.; Li, Y.; Li, J.; Liu, Q.; Wu, C.; Xing, H.; Liu, P.; et al. Tumor-Treating Fields in Glioblastomas: Past, Present, and Future. Cancers 2022, 14, 3669. [Google Scholar] [CrossRef]
- Mun, E.J.; Babiker, H.M.; Weinberg, U.; Kirson, E.D.; Von Hoff, D.D. Tumor-treating fields: A fourth modality in cancer treatment. Clin. Cancer Res. 2018, 24, 266–275. [Google Scholar] [CrossRef]
- Miranda, P.C.; Mekonnen, A.; Salvador, R.; Basser, P.J. Predicting the electric field distribution in the brain for the treatment of glioblastoma. Phys. Med. Biol. 2014, 59, 4137–4147. [Google Scholar] [CrossRef]
- Wenger, C.; Salvador, R.; Basser, P.J.; Miranda, P.C. The electric field distribution in the brain during TTF therapy and its dependence on tissue dielectric properties and anatomy: A computational study. Phys. Med. Biol. 2015, 60, 7339–7357. [Google Scholar] [CrossRef]
- Kirson, E.D.; Schneiderman, R.S.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTF). BMC Med. Phys. 2009, 9, 1. [Google Scholar] [CrossRef]
- Giladi, M.; Weinberg, U.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Voloshin, T.; Blatt, R.; Cahal, S.; Itzhaki, A.; Onn, A.; et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol. 2014, 41, S35–S41. [Google Scholar] [CrossRef]
- Branter, J.; Estevez-Cebrero, M.; Diksin, M.; Griffin, M.; Castellanos-Uribe, M.; May, S.; Rahman, R.; Grundy, R.; Basu, S.; Smith, S. Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors. Int. J. Mol. Sci. 2022, 23, 1982. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Weinberg, U.; Palti, Y. Tumor treating fields: A new frontier in cancer therapy. Ann. N. Y. Acad. Sci. 2013, 1291, 86–95. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Premarket Approval (PMA). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm (accessed on 20 January 2025).
- Novocure. Novocure Announces Positive Topline Results from Phase 3 PANOVA-3 Clinical Trial of Tumor Treating Fields (TTF) Therapy for Pancreatic Cancer. 2024. Available online: https://www.novocure.com/novocure-announces-positive-topline-results-from-phase-3-panova-3-clinical-trial-of-tumor-treating-fields-TTF-therapy-for-pancreatic-cancer/ (accessed on 1 January 2025).
- Mehta, M.P.; Gondi, V.; Ahluwalia, M.S.; Roberge, D.; Ilic, R.; Sio, T.T.W.; Trifiletti, D.M.; Muanza, T.; Krpan, A.M.; Naren, R.; et al. Ramakrishna Results from METIS (EF-25), an International, Multicenter Phase III Randomized Study Evaluating the Efficacy and Safety of Tumor Treating Fields (TTF) Therapy in NSCLC Patients with Brain Metastases. J. Clin. Oncol. 2024, 42, 2008. [Google Scholar]
- Vergote, I.; Copeland, L.J.; Van Gorp, T.; Laenen, A.; Scambia, G.; Thaker, P.H.; Cibula, D.; Colombo, N.; Lea, J.; Gonzalez-Martin, A.; et al. Tumor Treating Fields therapy in platinum-resistant ovarian cancer: Results of the ENGOT-ov50/GOG-3029/INNOVATE-3 pivotal phase 3 randomized study. Eur. J. Cancer 2025, 219, 115306. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)—Central Nervous System Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 20 January 2025).
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma a randomized clinical trial. JAMA-J. Am. Med. Assoc. 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Fallah, J.; Chaudhary, R.T.; Rogers, L.R.; Wei, W.; Brewer, C.J.; Peereboom, D.M.; Ahluwalia, M.S. Clinical outcomes of the combination of bevacizumab and TTF in patients with recurrent glioblastoma: Results of a phase II clinical trial. J. Clin. Oncol. 2020, 38 (Suppl. S15), 2537. [Google Scholar] [CrossRef]
- Lok, E.; San, P.; Liang, O.; White, V.; Wong, E.T. Finite element analysis of Tumor Treating Fields in a patient with posterior fossa glioblastoma. J. Neurooncol. 2020, 147, 125–133. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Demireva, P.; Kanner, A.A.; Pannullo, S.; Mehdorn, M.; Avgeropoulos, N.; Salmaggi, A.; Silvani, A.; Goldlust, S.; David, C.; et al. Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. J. Neurooncol. 2017, 135, 545–552. [Google Scholar] [CrossRef]
- Toms, S.A.; Kim, C.Y.; Nicholas, G.; Ram, Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the EF-14 phase III trial. J. Neurooncol. 2019, 141, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kinzel, A.; Ambrogi, M.; Varshaver, M.; Kirson, E.D. Tumor Treating Fields for Glioblastoma Treatment: Patient Satisfaction and Compliance with the Second-Generation Optune® System. Clin. Med. Insights Oncol. 2019, 13, 1179554918825449. [Google Scholar] [CrossRef] [PubMed]
- Novocure. FDA Approves Novocure’s Innovative HFE Transducer Arrays for Use with Optune Gio® for Glioblastoma. 21 November 2024. Available online: https://www.novocure.com/fda-approves-novocures-innovative-hfe-transducer-arrays-for-use-with-optune-gio-for-glioblastoma/ (accessed on 1 January 2025).
- Mrugala, M.M.; Engelhard, H.H.; Tran, D.D.; Kew, Y.; Cavaliere, R.; Villano, J.L.; Bota, D.A.; Rudnick, J.; Sumrall, A.L.; Zhu, J.-J.; et al. Clinical practice experience with NovoTTF-100ATM system for glioblastoma: The patient registry dataset (PRiDe). Semin. Oncol. 2014, 41, S4–S13. [Google Scholar] [CrossRef]
- Shi, W.; Blumenthal, D.T.; Bush, N.A.O.; Kebir, S.; Lukas, R.V.; Muragaki, Y.; Zhu, J.-J.; Glas, M. Global post-marketing safety surveillance of Tumor Treating Fields (TTF) in patients with high-grade glioma in clinical practice. J. Neurooncol. 2020, 148, 489–500. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Davis, M.E.; Elzinga, G.; Butowski, N.; Tran, D.; Villano, J.L.; DiMeglio, L.; Davies, A.M.; Wong, E.T. Characterization and management of dermatologic adverse events with the NovoTTF-100A system, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin. Oncol. 2014, 41 (Suppl. S4), S1–S14. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Ruzevick, J.; Zlomanczuk, P.; Lukas, R.V. Tumor Treating Fields in Neuro-Oncological Practice. Curr. Oncol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Gentilal, N.; Salvador, R.; Miranda, P.C. Temperature control in TTF therapy of GBM: Impact on the duty cycle and tissue temperature. Phys. Med. Biol. 2019, 64, 225008. [Google Scholar] [CrossRef]
- Gentilal, N.; Miranda, P.C. Heat transfer during TTF treatment: Influence of the uncertainty of the electric and thermal parameters on the predicted temperature distribution. Comput. Methods Programs Biomed. 2020, 196, 105706. [Google Scholar] [CrossRef]
- Novocure. Optune Gio—Instructions for Use. December 2023. Available online: https://assets.novocure.biz/patient/QSD-QR-703%20US%28EN%29%20Commercial%20Optune%20Gio%20INE_IFU%20Rev04.pdf?utm_source=chatgpt.com (accessed on 1 January 2025).
- Nitta, R.T.; Luo, E.J.; Lim, M.; Li, G. Can tumor treating fields induce DNA damage and reduce cell motility in medulloblastoma cell lines? J. Neurosurg. Pediatr. 2022, 30, 555–566. [Google Scholar] [CrossRef]
- Di Sebastiano, A.R.; Deweyert, A.; Benoit, S.; Iredale, E.; Xu, H.; De Oliveira, C.; Wong, E.; Schmid, S.; Hebb, M.O. Preclinical outcomes of Intratumoral Modulation Therapy for glioblastoma. Sci Rep. 2018, 8, 7301. [Google Scholar] [CrossRef]
- Crawford, J.; Saria, M.G.; Dhall, G.; Margol, A.; Kesari, S. Feasibility of Treating High Grade Gliomas in Children with Tumor-Treating Fields: A Case Series. Cureus 2020, 5, e10804. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Margol, A.; Hwang, E.I.; Tanaka, K.; Suchorska, B.; Crawford, J.R.; Kesari, S. Safety of Tumor Treating Fields (TTF) therapy in pediatric patients with malignant brain tumors: Post-marketing surveillance data. Front. Oncol. 2022, 12, 958637. [Google Scholar] [CrossRef]
- O’Connell, D.; Shen, V.; Loudon, W.; Bota, D.A. First report of tumor treating fields use in combination with bevacizumab in a pediatric patient: A case report. CNS Oncol. 2017, 6, 11–18. [Google Scholar] [CrossRef]
- Green, A.L.; Levy, J.M.M.; Vibhakar, R.; Hemenway, M.; Madden, J.; Foreman, N.; Dorris, K. Tumor treating fields in pediatric high-grade glioma. Child’s Nerv. Syst. 2017, 33, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Toledano, H.; Campino, G.A.; Dvir, R.; Postovsky, S.; Yalon, M. INNV-29. Experience with TTFields (OPTUNE®) in Pediatric High Grade Glioma Patients in Israel. Neuro Oncol. 2018, 20 (Suppl. S6), vi144. [Google Scholar] [CrossRef]
- Wölfl, M.; Miller, E.; Eyrich, M.; Krauss, J.; Schlegel, P.G. PDCT-07. Tumor Treating Fields for Pediatric Patients with High Grade Glioma: First Case Series in Germany. Neuro Oncol. 2017, 19 (Suppl. S6), vi185. [Google Scholar] [CrossRef]
- Gött, H.; Kiez, S.; Dohmen, H.; Kolodziej, M.; Stein, M. Tumor treating fields therapy is feasible and safe in a 3-year-old patient with diffuse midline glioma H3K27M—A case report. Child’s Nerv. Syst. 2022, 38, 1791–1796. [Google Scholar] [CrossRef]
- Lei, K.F.; Hsieh, S.C.; Goh, A.; Kuo, R.L.; Tsang, N.M. Proliferation arrest, selectivity, and chemosensitivity enhancement of cancer cells treated by a low-intensity alternating electric field. Biomed. Microdevices 2018, 20, 90. [Google Scholar] [CrossRef]
- Ye, E.; Lee, J.E.; Lim, Y.S.; Yang, S.H.; Park, S.M. Effect of duty cycles of tumor-treating fields on glioblastoma cells and normal brain organoids. Int. J. Oncol. 2022, 60, 8. [Google Scholar] [CrossRef]
- National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 19 January 2025).
- Kumaria, A. Tumor treating fields in pediatric brain tumors: Overcoming challenges. Child’s Nerv. Syst. 2022, 38, 1847–1848. [Google Scholar] [CrossRef]
- Segar, D.J.; Bernstock, J.D.; Arnaout, O.; Bi, W.L.; Friedman, G.K.; Langer, R.; Traverso, G.; Rampersad, S.M. Modeling of intracranial tumor treating fields for the treatment of complex high-grade gliomas. Sci. Rep. 2023, 13, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Fort, M.K.; Naveh, A.; McClelland, S., III; Gilman, C.K.; Fort, M.; Mendez, M.; Matta, J.; Bomzon, Z.; Lange, C.S. Computational simulations establish a novel transducer array placement arrangement that extends delivery of therapeutic TTF to the infratentorium of patients with brainstem gliomas. Rep. Pract. Oncol. Radiother. 2021, 26, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Le, S.B.; Hutchinson, T.E.; Calinescu, A.-A.; Sebastian, M.; Jin, D.; Liu, T.; Ghiaseddin, A.; Rahman, M.; Tran, D.D. Tumor Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Investig. 2022, 132, e149258. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Kaynan, N.; Davidi, S.; Porat, Y.; Shteingauz, A.; Schneiderman, R.S.; Zeevi, E.; Munster, M.; Blat, R.; Brami, C.T.; et al. Tumor-treating fields (TTF) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol. Immunother. 2020, 69, 1191–1204. [Google Scholar] [CrossRef]
- Tran, D.; Ghiaseddin, A.; Chen, D.; Le, S. 581 Final analysis of 2-THE-TOP: A phase 2 study of TTF (Optune) plus pembrolizumab plus maintenance temozolomide (TMZ) in patients with newly diagnosed glioblastoma. BMJ 2023, 11, A661. [Google Scholar] [CrossRef]
- Korshoej, A.R.; Lukacova, S.; Lassen-Ramshad, Y.; Rahbek, C.; Severinsen, K.E.; Guldberg, T.L.; Mikic, N.; Jensen, M.H.; Cortnum, S.O.S.; von Oettingen, G.; et al. OptimalTTF-1: Enhancing tumor treating fields therapy with skull remodeling surgery. A clinical phase I trial in adult recurrent glioblastoma. Neurooncol. Adv. 2020, 2, vdaa121. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Helekar, S.A. Rotating Magnetic Fields Inhibit Mitochondrial Respiration, Promote Oxidative Stress and Produce Loss of Mitochondrial Integrity in Cancer Cells. Front. Oncol. 2021, 11, 768758. [Google Scholar] [CrossRef]
- Helekar, S.A.; Hambarde, S.; Ijare, O.B.; Pichumani, K.; Baskin, D.S.; Sharpe, M.A. Selective induction of rapid cytotoxic effect in glioblastoma cells by oscillating magnetic fields. J. Cancer Res. Clin. Oncol. 2021, 147, 3577–3589. [Google Scholar] [CrossRef]
- Murphy, M.; Dowling, A.; Thien, C.; Priest, E.; Morgan Murray, D.; Kesari, S. A feasibility study of the Nativis Voyager® device in patients with recurrent glioblastoma in Australia. CNS Oncol. 2019, 8, CNS31. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Foster, H.; Butters, M.; Yoon, J.-G.; Ozawa, T.; Nicolaides, T.; Figueroa, X.; Hothi, P.; Prados, M.; Butters, J.; et al. Precision knockdown of EGFR gene expression using radio frequency electromagnetic energy. J. Neurooncol. 2017, 133, 257–264. [Google Scholar] [CrossRef]
- Iwamoto, K.S.; Sandstrom, R.E.; Bryan, M.; Liu, Y.; Elgart, S.R.; Sheng, K.; Steinberg, M.L.; McBride, W.H.; Low, D.A. Weak Magnetic Fields Enhance the Efficacy of Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100645. [Google Scholar] [CrossRef]
- Kumar, R.; Vijay Kumar, R. Quantum Magnetic Resonance Therapy: Targeting Biophysical Cancer Vulnerabilities to Effectively Treat and Palliate. J. Clin. Exp. Oncol. 2016, 5, 2. [Google Scholar] [CrossRef]
| Authors | Year of Publication | Type of Study | Number of Pediatric Participants (<18 Years Old) | Age | Tumor Type(s) | Tumor Location | Main Findings |
|---|---|---|---|---|---|---|---|
| Goldman et al. [37] | 2022 | Post-marketing safety surveillance study | 81 | 3–17 | AA (13), AEPN (2), GBM (59), HGG NOS (3), atypical meningioma (1), PNET (1), PXA (1) | Supratentorial (71), infratentorial (4), unknown (6) | Median treatment duration was 81 days; 51 (63%) patients reported at least 1 grade 1 AE; skin reactions were the most common adverse event (36%) and were similar across age groups; 28 SAEs reported, none deemed related to TTfields. |
| Shi et al. [28] | 2020 | Post-marketing safety surveillance study | 52 | 3–17 | ndGBM (19), rGBM (22), AA/AO (8), others (3) | Unknown | Pediatric participants reported overall less AEs (58%) compared to adult and elderly participants (63–66%). Skin disorders were the most commonly reported AEs (37%). |
| Crawford et al. [36] | 2020 | Case series | 4 | 4–16 | rHGG | Supratentorial | Treatment duration up to 4 months. No device-related AE; 53–92% compliance rate. All patients died within 2–6 months after TTfield initiation |
| Green et al. [39] | 2017 | Case series | 3 | 10–15 | GBM (1), HGG (1), DMG (1) | Supratentorial | Patients were treated with TTfields for 5–6 months, until tumor progression. Two patients with partial response, possibly related to RT. Two patients reported no AEs. One patient reported grade 2 skin toxicity with ulceration requiring a 2-day treatment interruption. |
| Toledano et al. [40] * | 2018 | Case series | 5 | 11–17 | DMG (4), DHG (1) | Supratentorial (1), unknown (4) | Three patients maintained > 90% daily use, one patient with 80% daily use, one patient with 60% daily use. Two patients had disease progression after 86 and 142 days on treatment; treatment duration unknown for the remaining patients. Twelve patient reported minor cutaneous symptoms. Four patients maintained normal activities. Three patients had programmable shunts. |
| Wölfl et al. [41] * | 2017 | Case series | 3 | 7–11 | AA (1), GBM (2) | Unknown | Duration of use not reported. Average daily use ranged from 71 to 92%. Treatment was well tolerated without significant impact on quality of life. Treatment outcomes not reported. |
| Gött et al. [42] | 2022 | Case report | 1 | DMG | Infratentorial | Infratentorial layout used, in combination with maintenance TMZ. Treatment duration was almost 9 months. Initial daily use was low at 40% but gradually increased to 76% during months 4 to 8. No treatment-related AEs were observed. Repeat brain MRI 1 year after initial biopsy showed response to treatment. | |
| O’Connell et al. [38] | 2017 | Case report | 1 | 13 | rGBM | Supratentorial | Patient maintained ≥ 75% daily use for the entire treatment duration of 13 months. Treatment was well tolerated with minimal scalp irritation. Patient had stable disease for 7 months while administered TTfields. |
| Study ID | Study Name | Phase | Country | Population | Pathology | Intervention | Primary Objective/Primary Outcome Measure | Status | Estimated Study Completion |
|---|---|---|---|---|---|---|---|---|---|
| ADULTS | |||||||||
| NCT04471844 | TRIDENT | 3 | International | Adults | Glioblastoma, newly diagnosed | TTfields concomitant to TMZ and RT | Efficacy (OS) | Active, not recruiting | 1/2026 |
| NTC06556563 | EF-41 | 3 | International | Adults | Glioblastoma, newly diagnosed | TTfields concomitant to TMZ and pembrolizumab | Efficacy (OS) | Recruiting | 4/2029 |
| NCT03405792 | 2-THE-TOP | 2 | United States | Adults | Glioblastoma, newly diagnosed | TTfields concomitant to TMZ and pembrolizumab | Efficacy (PFS) | Active, not recruiting | 9/2025 |
| NCT06140875 | BRAIN-RF | 1 | Germany | Adults | Glioblastoma, newly diagnosed | Combined chemoradiation and radiofrequency electromagnetic field | Efficacy (PFS) | Recruiting | 5/2029 |
| NCT03223103 | N/A | 1 | USA | Adults | Glioblastoma, newly diagnosed | TTfields with MTA-based vaccine during adjuvant TMZ | Safety | Active, not recruiting | 5/2025 |
| NCT04474353 | N/A | 1 | USA | Adults | Glioblastoma, newly diagnosed | TTfields, combined chemoradiation with SRS, followed by adjuvant TMZ | Safety | Active, not recruiting | 11/2025 |
| NCT05086497 | N/A | N/A | USA | Adults | Glioblastoma, newly diagnosed | TTfields with advanced array placement based on advanced MRI with spectroscopy | Efficacy (PFS) | Recruiting | 6/2026 |
| NCT06558214 | OPTIMUS PRIME | 2 | United States | Adults | Glioblastoma, recurrent/progressive | TTfields, MRI-guided laser ablation, and pembrolizumab | Safety and feasibility | Recruiting | 10/2029 |
| NCT04223999 | OptimalTTF-2 | 2 | Denmark | Adults | Glioblastoma, recurrent/progressive | skull remodeling plus TTfields plus best practice medical management | Efficacy (12-month OS) | Recruiting | 3/2026 |
| NCT04671459 | TaRRGET | 2 | Poland | Adults | Glioblastoma, recurrent/progressive | TTfields and SRS | Efficacy (1-year survival rate) | Active. not recruiting | 12/2024 |
| NCT04221503 | N/A | 2 | USA | Adults | Glioblastoma, recurrent/progressive | TTfields and niraparib | Efficacy (CR, PR, or SD) | Active, not recruiting | 12/2025 |
| NCT04397679 | N/A | 1 | USA | Adults | Grade 4 glioma, newly diagnosed | partial brain radiation therapy, temozolomide, chloroquine, and tumor-treating fields | Safety (high-grade dermatitis) | Active, not recruiting | 4/2026 |
| NCT05310448 | N/A | 1 | USA | Adults | Brainstem glioma | SOC plus TTfields | Safety and tolerability | Recruiting | 11/2025 |
| NCT01892397 | N/A | 2 | USA | Adults | Recurrent atypical and malignant meningioma | TTfields monotherapy | Efficacy (PFS) | Active, not recruiting | 6/2025 |
| NCT02847559 | N/A | 2 | USA | Adults | Recurrent atypical and malignant meningioma | TTfields plus bevacizumab | Efficacy (PFS-6) | Recruiting | 12/2026 |
| NCT02831959 | METIS | 3 | International | Adults | Brain metastases from NSCLC | TTfields following SRS | Time to intracranial progression | Active, not recruiting | 12/2024 |
| NCT05341349 | N/A | 1 | USA | Adults | Brain metastases from melanoma | stereotactic radiosurgery and immune checkpoint inhibitors with NovoTTF-100M | Safety | Recruiting | 3/2025 |
| PEDIATRICS | |||||||||
| NCT03128047 | HUMC 1612 | 1 | USA | Pediatrics | Recurrent high-grade glioma and ependymoma | Optune NovoTTF-200A system combined with temozolomide and bevacizumab | Safety and tolerability | Active, not recruiting | 6/2024 |
| NCT03033992 | PBTC-048 | 1/2 | USA | Pediatrics | Recurrent/progressive/refractory supratentorial high-grade glioma and ependymoma (stratum 1), newly diagnosed DIPG (stratum 2) | Optune NovoTTF-200A system (stratum 1), Optune NovoTTF-200A in combination with RT (stratum 2) | Safety and feasibility (stratum 1), safety, feasibility, and efficacy (stratum 2) | Active, recruiting | 9/2032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, J.; Lapointe, S.; Roberge, D. Tumor-Treating Fields and Related Treatments in the Management of Pediatric Brain Tumors. Curr. Oncol. 2025, 32, 185. https://doi.org/10.3390/curroncol32040185
Rousseau J, Lapointe S, Roberge D. Tumor-Treating Fields and Related Treatments in the Management of Pediatric Brain Tumors. Current Oncology. 2025; 32(4):185. https://doi.org/10.3390/curroncol32040185
Chicago/Turabian StyleRousseau, Julien, Sarah Lapointe, and David Roberge. 2025. "Tumor-Treating Fields and Related Treatments in the Management of Pediatric Brain Tumors" Current Oncology 32, no. 4: 185. https://doi.org/10.3390/curroncol32040185
APA StyleRousseau, J., Lapointe, S., & Roberge, D. (2025). Tumor-Treating Fields and Related Treatments in the Management of Pediatric Brain Tumors. Current Oncology, 32(4), 185. https://doi.org/10.3390/curroncol32040185






