Construction of a Nomogram Model for Predicting Pathologic Complete Response in Breast Cancer Neoadjuvant Chemotherapy Based on the Pan-Immune Inflammation Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection and Definition of Research Indicators
2.3. Determination of the Optimal PIV Cutoff Value
2.4. Evaluation of Efficacy of Neoadjuvant Chemotherapy
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
3.2. Association Analysis of PIV with Clinicopathological Characteristics and Chemotherapy Response
3.3. Identification of Independent Influencing Factors for pCR After NAC
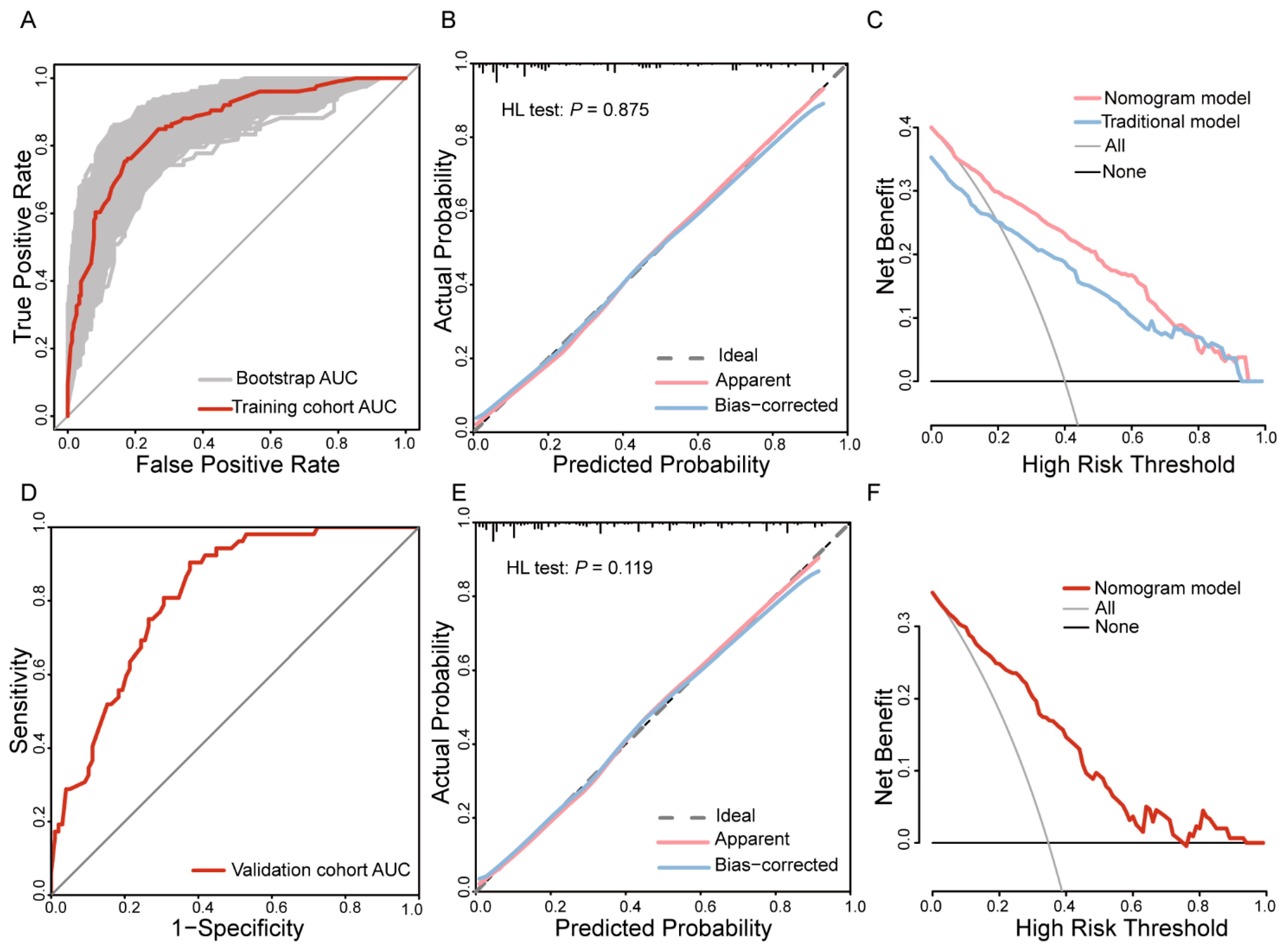
3.4. Nomogram Prediction Model Development and Evaluation
3.5. Validation of the Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| ALND | Axillary lymph node dissection |
| AR | Androgen receptor |
| AUC | Area under the curve |
| CA153 | Carbohydrate antigen 15-3 |
| CAFs | Cancer-associated fibroblasts |
| CEA | Carcinoembryonic antigen |
| CI | Confidence interval |
| cN | Clinical nodal stage |
| DCA | Decision curve analysis |
| DCs | Dendritic cells |
| DFS | Disease-free survival |
| ECM | Extracellular matrix |
| ECs | Endothelial cells |
| ER | Estrogen receptor |
| HDI | Human development index |
| Her2 | Human epidermal growth factor receptor-2 |
| HR | Hormone receptor |
| IDC | Invasive ductal carcinoma |
| IDI | Integrated discrimination improvement |
| IHC | Immunohistochemistry |
| IIBs | Immune-inflammatory biomarkers |
| ISH | In situ hybridization |
| ITC | Isolated tumor cells |
| MP | Miller–Payne |
| NAC | Neoadjuvant chemotherapy |
| NK cells | Natural killer cells |
| NLR | Neutrophil-to-lymphocyte ratio |
| NRI | Net reclassification improvement |
| OR | Odds ratio |
| OS | Overall survival |
| pCR | Pathologic complete response |
| PIV | Pan-immune inflammation value |
| PLR | Platelet-to-lymphocyte ratio |
| PR | Progesterone receptor |
| RCB | Residual cancer burden |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| ROC | Receiver operating characteristic |
| SII | Systemic immune inflammation index |
| SIRI | Systemic inflammation response index |
| SLNB | Sentinel lymph node biopsy |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| ypN | Post-neoadjuvant pathological nodal staging |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- EBCTCG. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Cabioglu, N.; Koçer, H.B.; Karanlik, H.; Gülçelik, M.A.; Igci, A.; Müslümanoglu, M.; Uras, C.; Mantoglu, B.; Trabulus, D.C.; Akgül, G.; et al. De-Escalation of Nodal Surgery in Clinically Node-Positive Breast Cancer. JAMA Surg. 2025, 160, 257–266. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Shen, S.; Hou, Y. Inflammation and cancer: Paradoxical roles in tumorigenesis and implications in immunotherapies. Genes. Dis. 2023, 10, 151–164. [Google Scholar] [CrossRef]
- Tan, Y.G.; Eu, E.W.C.; Huang, H.H.; Lau, W.K.O. High neutrophil-to-lymphocyte ratio predicts worse overall survival in patients with advanced/metastatic urothelial bladder cancer. Int. J. Urol. 2018, 25, 232–238. [Google Scholar] [CrossRef]
- Xun, F.; Jiang, W.; Sha, M.; Wang, W.; Xia, Y.; Hu, H.; Liu, R.; Yu, H.; Wang, H. Neutrophil-to-lymphocyte ratio in colorectal tissue affects prognosis in patients with colorectal cancer. Pathology 2024, 56, 643–652. [Google Scholar] [CrossRef]
- Aoyama, T.; Ju, M.; Komori, K.; Tamagawa, H.; Tamagawa, A.; Onodera, A.; Morita, J.; Hashimoto, I.; Ishiguro, T.; Endo, K.; et al. The Platelet-to-Lymphocyte Ratio Is an Independent Prognostic Factor for Patients With Esophageal Cancer Who Receive Curative Treatment. In Vivo 2022, 36, 1916–1922. [Google Scholar] [CrossRef]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Litton, J.K.; Regan, M.M.; Pusztai, L.; Rugo, H.S.; Tolaney, S.M.; Garrett-Mayer, E.; Amiri-Kordestani, L.; Basho, R.K.; Best, A.F.; Boileau, J.F.; et al. Standardized Definitions for Efficacy End Points in Neoadjuvant Breast Cancer Clinical Trials: NeoSTEEP. J. Clin. Oncol. 2023, 41, 4433–4442. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef]
- He, X.Y.; Gao, Y.; Ng, D.; Michalopoulou, E.; George, S.; Adrover, J.M.; Sun, L.; Albrengues, J.; Daßler-Plenker, J.; Han, X.; et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell 2024, 42, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037. [Google Scholar] [CrossRef]
- Zheng, L.; Qin, S.; Si, W.; Wang, A.; Xing, B.; Gao, R.; Ren, X.; Wang, L.; Wu, X.; Zhang, J.; et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 2021, 374, abe6474. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef]
- Wang, T.C.; An, T.Z.; Li, J.X.; Pang, P.F. Systemic Inflammation Response Index is a Prognostic Risk Factor in Patients with Hepatocellular Carcinoma Undergoing TACE. Risk Manag. Healthc. Policy 2021, 14, 2589–2600. [Google Scholar] [CrossRef]
- Chen, L.; Kong, X.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J. Cell Mol. Med. 2020, 24, 2993–3021. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef]
- Savioli, F.; Morrow, E.S.; Dolan, R.D.; Romics, L.; Lannigan, A.; Edwards, J.; McMillan, D.C. Prognostic role of preoperative circulating systemic inflammatory response markers in primary breast cancer: Meta-analysis. Br. J. Surg. 2022, 109, 1206–1215. [Google Scholar] [CrossRef]
- Corbeau, I.; Jacot, W.; Guiu, S. Neutrophil to Lymphocyte Ratio as Prognostic and Predictive Factor in Breast Cancer Patients: A Systematic Review. Cancers 2020, 12, 958. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Yang, X.; Chen, Q.; Cheng, X. Pretreatment Pan-Immune-Inflammation Value (PIV) in Predicting Therapeutic Response and Clinical Outcomes of Neoadjuvant Immunochemotherapy for Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2024, 31, 272–283. [Google Scholar] [CrossRef]
- Zhai, W.Y.; Duan, F.F.; Lin, Y.B.; Lin, Y.B.; Zhao, Z.R.; Wang, J.Y.; Rao, B.Y.; Zheng, L.; Long, H. Pan-Immune-Inflammatory Value in Patients with Non-Small-Cell Lung Cancer Undergoing Neoadjuvant Immunochemotherapy. J. Inflamm. Res. 2023, 16, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Albasini, S.; Truffi, M.; Favilla, K.; Tagliaferri, B.; Piccotti, F.; Bossi, D.; Armatura, G.; Calcinotto, A.; Chiappa, C.; et al. Low neutrophil-to-lymphocyte ratio and pan-immune-inflammation-value predict nodal pathologic complete response in 1274 breast cancer patients treated with neoadjuvant chemotherapy: A multicenter analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359231193732. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, J.; Meng, C.; Liu, B.; Liao, J. Pan-immune-inflammation value as a novel prognostic biomarker for digestive system cancers: A meta-analysis. World J. Surg. Oncol. 2024, 22, 306. [Google Scholar] [CrossRef]
- Topkan, E.; Selek, U.; Ozturk, D.; Şenyürek, Ş.; Kılıç Durankuş, N. Prognostic Value of Pre-Chemoradiotherapy Pan-Immune-Inflammation Value (PIV) in Locally Advanced Nasopharyngeal Cancers. Cancer Control 2024, 31, 10732748241290746. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Verma, A.; Marderstein, A.R.; White, J.; Bhinder, B.; Garcia Medina, J.S.; Elemento, O. Pan-cancer analysis reveals molecular patterns associated with age. Cell Rep. 2021, 37, 110100. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (n = 507), n (%) | Training Cohort (n = 357), n (%) | Validation Cohort (n = 150), n (%) | p Value |
|---|---|---|---|---|
| CA153 (U/mL) | 0.324 | |||
| ≤28 | 409 (80.67) | 292 (81.79) | 117 (78.00) | |
| >28 | 98 (19.33) | 65 (18.21) | 33 (22.00) | |
| CEA (ng/mL) | 0.805 | |||
| ≤5 | 433 (85.40) | 304 (85.15) | 129 (86.00) | |
| >5 | 74 (14.60) | 53 (14.85) | 21 (14.00) | |
| PIV | 0.346 | |||
| Low | 389 (76.73) | 278 (77.87) | 111 (74.00) | |
| High | 118 (23.27) | 79 (22.13) | 39 (26.00) | |
| Age (years) | 0.947 | |||
| ≤50 | 276 (54.44) | 194 (54.34) | 82 (54.67) | |
| >50 | 231 (45.56) | 163 (45.66) | 68 (45.33) | |
| Menstrual status | 0.666 | |||
| Pre-menopausal | 261 (51.48) | 186 (52.10) | 75 (50.00) | |
| Post-menopausal | 246 (48.52) | 171 (47.90) | 75 (50.00) | |
| Lesion number | 0.676 | |||
| Single lesion | 355 (70.02) | 248 (69.47) | 107 (71.33) | |
| Multiple lesions | 152 (29.98) | 109 (30.53) | 43 (28.67) | |
| Grade | 0.213 | |||
| Grades I–II | 324 (63.91) | 222 (62.18) | 102 (68.00) | |
| Grade III | 183 (36.09) | 135 (37.82) | 48 (32.00) | |
| Pathological type | 0.518 | |||
| IDC | 437 (86.19) | 310 (86.83) | 127 (84.67) | |
| Others | 70 (13.81) | 47 (13.17) | 23 (15.33) | |
| Chemotherapy cycles | 0.134 | |||
| ≤4 | 73 (14.40) | 46 (12.89) | 27 (18.00) | |
| >4 | 434 (85.60) | 311 (87.11) | 123 (82.00) | |
| AR | 0.897 | |||
| Negative | 93 (18.34) | 66 (18.49) | 27 (18.00) | |
| Positive | 414 (81.66) | 291 (81.51) | 123 (82.00) | |
| ER | 0.593 | |||
| Negative | 224 (44.18) | 155 (43.42) | 69 (46.00) | |
| Positive | 283 (55.82) | 202 (56.58) | 81 (54.00) | |
| PR | 0.323 | |||
| Negative | 274 (54.04) | 198 (55.46) | 76 (50.67) | |
| Positive | 233 (45.96) | 159 (44.54) | 74 (49.33) | |
| Her2 | 0.111 | |||
| Negative | 271 (53.45) | 199 (55.74) | 72 (48.00) | |
| Positive | 236 (46.55) | 158 (44.26) | 78 (52.00) | |
| Ki67 | 0.598 | |||
| ≤20% | 91 (17.95) | 62 (17.37) | 29 (19.33) | |
| >20% | 416 (82.05) | 295 (82.63) | 121 (80.67) | |
| Location | 0.359 | |||
| Central region | 66 (13.02) | 49 (13.73) | 17 (11.33) | |
| Upper outer quadrant | 235 (46.35) | 169 (47.34) | 66 (44.00) | |
| Upper inner quadrant | 90 (17.75) | 56 (15.69) | 34 (22.67) | |
| Lower inner quadrant | 39 (7.69) | 26 (7.28) | 13 (8.67) | |
| Lower outer quadrant | 77 (15.19) | 57 (15.97) | 20 (13.33) | |
| Tumor diameter | 0.846 | |||
| ≤2 cm | 51 (10.06) | 37 (10.36) | 14 (9.33) | |
| >2 cm, ≤5 cm | 322 (63.51) | 228 (63.87) | 94 (62.67) | |
| >5 cm | 134 (26.43) | 92 (25.77) | 42 (28.00) | |
| Clinical nodal stage | 0.202 | |||
| cN0 | 88 (17.36) | 59 (16.53) | 29 (19.33) | |
| cN1-2 | 302 (59.57) | 208 (58.26) | 94 (62.67) | |
| cN3 | 117 (23.08) | 90 (25.21) | 27 (18.00) | |
| Chemotherapy regimen | 0.157 | |||
| Anthracycline–taxane combination | 307 (60.55) | 223 (62.46) | 84 (56.00) | |
| Taxane-based | 149 (29.39) | 96 (26.89) | 53 (35.33) | |
| Anthracycline-based | 51 (10.06) | 38 (10.64) | 13 (8.67) | |
| pCR status | 0.893 | |||
| pCR | 178 (35.11) | 126 (35.29) | 52 (34.67) | |
| Non-pCR | 329 (64.89) | 231 (64.71) | 98 (65.33) | |
| ypN status | 0.553 | |||
| ypN-negative | 284 (56.02) | 203 (56.86) | 81 (54.00) | |
| ypN-positive | 223 (43.98) | 154 (43.14) | 69 (46.00) | |
| Breast surgery | 0.919 | |||
| Breast-conserving | 53 (10.45) | 37 (10.36) | 16 (10.67) | |
| Mastectomy | 454 (89.55) | 320 (89.64) | 134 (89.33) | |
| Axillary surgery | 0.268 | |||
| SLNB | 56 (11.05) | 43 (12.04) | 13 (8.67) | |
| ALND | 451 (88.95) | 314 (87.96) | 137 (91.33) |
| Characteristics | Training Cohort (n = 357), n (%) | Low PIV (n = 278), n (%) | High PIV (n = 79), n (%) | p-Value |
|---|---|---|---|---|
| CA153 (U/mL) | <0.001 *** | |||
| ≤28 | 292 (81.79) | 238 (85.61) | 54 (68.35) | |
| >28 | 65 (18.21) | 40 (14.39) | 25 (31.65) | |
| CEA (ng/mL) | 0.794 | |||
| ≤5 | 304 (85.15) | 236 (84.89) | 68 (86.08) | |
| >5 | 53 (14.85) | 42 (15.11) | 11 (13.92) | |
| Age (years) | 0.039 * | |||
| ≤50 | 194 (54.34) | 143 (51.44) | 51 (64.56) | |
| >50 | 163 (45.66) | 135 (48.56) | 28 (35.44) | |
| Menstrual status | 0.006 ** | |||
| Pre-menopausal | 186 (52.10) | 134 (48.20) | 52 (65.82) | |
| Post-menopausal | 171 (47.90) | 144 (51.80) | 27 (34.18) | |
| Lesion number | 0.090 | |||
| Single lesion | 248 (69.47) | 187 (67.27) | 61 (77.22) | |
| Multiple lesions | 109 (30.53) | 91 (32.73) | 18 (22.78) | |
| Grade | 0.767 | |||
| Grades I–II | 222 (62.18) | 174 (62.59) | 48 (60.76) | |
| Grade III | 135 (37.82) | 104 (37.41) | 31 (39.24) | |
| Pathological type | 0.035 * | |||
| IDC | 310 (86.83) | 247 (88.85) | 63 (79.75) | |
| Others | 47 (13.17) | 31 (11.15) | 16 (20.25) | |
| AR | 0.265 | |||
| Negative | 66 (18.49) | 48 (17.27) | 18 (22.78) | |
| Positive | 291 (81.51) | 230 (82.73) | 61 (77.22) | |
| ER | 0.173 | |||
| Negative | 155 (43.42) | 126 (45.32) | 29 (36.71) | |
| Positive | 202 (56.58) | 152 (54.68) | 50 (63.29) | |
| PR | 0.217 | |||
| Negative | 198 (55.46) | 159 (57.19) | 39 (49.37) | |
| Positive | 159 (44.54) | 119 (42.81) | 40 (50.63) | |
| Her2 | 0.005 ** | |||
| Negative | 199 (55.74) | 144 (51.80) | 55 (69.62) | |
| Positive | 158 (44.26) | 134 (48.20) | 24 (30.38) | |
| Ki67 | 0.443 | |||
| ≤20% | 62 (17.37) | 46 (16.55) | 16 (20.25) | |
| >20% | 295 (82.63) | 232 (83.45) | 63 (79.75) | |
| Location | 0.919 | |||
| Central region | 49 (13.73) | 36 (12.95) | 13 (16.46) | |
| Upper outer quadrant | 169 (47.34) | 133 (47.84) | 36 (45.57) | |
| Upper inner quadrant | 56 (15.69) | 43 (15.47) | 13 (16.46) | |
| Lower inner quadrant | 26 (7.28) | 20 (7.19) | 6 (7.59) | |
| Lower outer quadrant | 57 (15.97) | 46 (16.55) | 11 (13.92) | |
| Tumor diameter | 0.038 * | |||
| ≤2 cm | 37 (10.36) | 31 (11.15) | 6 (7.59) | |
| >2 cm, ≤5 cm | 228 (63.87) | 184 (66.19) | 44 (55.70) | |
| >5 cm | 92 (25.77) | 63 (22.66) | 29 (36.71) | |
| Clinical nodal stage | 0.260 | |||
| cN0 | 59 (16.53) | 50 (17.99) | 9 (11.39) | |
| cN1-2 | 208 (58.26) | 162 (58.27) | 46 (58.23) | |
| cN3 | 90 (25.21) | 66 (23.74) | 24 (30.38) | |
| pCR status | <0.001 *** | |||
| pCR | 126 (35.29) | 112 (40.29) | 14 (17.72) | |
| Non-pCR | 231 (64.71) | 166 (59.71) | 65 (82.28) | |
| ypN status | 0.011 * | |||
| ypN-negative | 203 (56.86) | 168 (60.43) | 35 (44.30) | |
| ypN-positive | 154 (43.14) | 110 (39.57) | 44 (55.70) |
| Characteristics | Univariable | Multivariable | ||
|---|---|---|---|---|
| Odds Ratio (95%CI) | p Value | Odds Ratio (95%CI) | p Value | |
| CA153 (U/mL) | ||||
| >28 vs. ≤28 | 0.441 (0.226–0.814) | 0.012 * | 0.849 (0.366–1.918) | 0.696 |
| CEA (ng/mL) | ||||
| >5 vs. ≤5 | 0.549 (0.272–1.045) | 0.079 | ||
| PIV | ||||
| High vs. low | 0.319 (0.165–0.581) | <0.001 *** | 0.349 (0.149–0.778) | 0.012 * |
| Age (years) | ||||
| >50 vs. ≤50 | 0.839 (0.541–1.298) | 0.433 | ||
| Menstrual status | ||||
| Post-menopausal vs. pre-menopausal | 1.032 (0.668–1.594) | 0.886 | ||
| Lesion number | ||||
| Multiple lesions vs. single lesion | 1.533 (0.962–2.438) | 0.071 | ||
| Grade | ||||
| Grade III vs. grades I-II | 1.891 (1.212–2.956) | 0.005 ** | 1.835 (0.990–3.444) | 0.055 |
| Pathological type | ||||
| Others vs. IDC | 0.452 (0.206–0.910) | 0.034 * | 0.712 (0.260–1.840) | 0.494 |
| Chemotherapy cycles | ||||
| >4 vs. ≤4 | 2.904 (1.375–6.898) | 0.009 ** | 2.255 (0.703–7.604) | 0.177 |
| AR | ||||
| Positive vs. negative | 0.944 (0.545–1.666) | 0.840 | ||
| ER | ||||
| Positive vs. negative | 0.210 (0.131–0.332) | <0.001 *** | 0.467 (0.220–0.972) | 0.044 * |
| PR | ||||
| Positive vs. negative | 0.247 (0.150–0.398) | <0.001 *** | 0.473 (0.214–1.041) | 0.062 |
| Her2 | ||||
| Positive vs. negative | 7.665 (4.724–12.710) | <0.001 *** | 4.529 (2.252–9.304) | <0.001 *** |
| Ki67 | ||||
| >20% vs. ≤20% | 1.890 (1.031–3.643) | 0.047 * | 1.585 (0.680–3.822) | 0.293 |
| Location | ||||
| Upper outer quadrant vs. central region | 1.646 (0.828–3.437) | 0.167 | ||
| Upper inner quadrant vs. central region | 1.312 (0.565–3.104) | 0.530 | ||
| Lower inner quadrant vs. central region | 1.731 (0.622–4.792) | 0.289 | ||
| Lower outer quadrant vs. central region | 1.741 (0.767–4.062) | 0.190 | ||
| Tumor diameter | ||||
| >2 cm, ≤5 cm vs. ≤2 cm | 0.618 (0.306–1.244) | 0.176 | 0.639 (0.249–1.627) | 0.347 |
| >5 cm vs. ≤2 cm | 0.215 (0.092–0.489) | <0.001 *** | 0.238 (0.077–0.715) | 0.011 * |
| Clinical nodal stage | ||||
| cN1-2 vs. cN0 | 0.624 (0.348–1.123) | 0.114 | 0.577 (0.264–1.246) | 0.163 |
| cN3 vs. cN0 | 0.380 (0.188–0.759) | 0.007 ** | 0.269 (0.105–0.665) | 0.005 ** |
| Chemotherapy regimen | ||||
| Taxane-based vs. anthracycline–taxane combination | 9.080 (5.313–15.939) | <0.001 *** | 3.841 (1.848–8.215) | <0.001 *** |
| Anthracycline-based vs. anthracycline–taxane combination | 0.511 (0.168–1.272) | 0.184 | 1.394 (0.367–4.739) | 0.605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Xi, Y.; Chen, M.; Hu, M.; Chen, F.; Wei, L.; Zhang, J. Construction of a Nomogram Model for Predicting Pathologic Complete Response in Breast Cancer Neoadjuvant Chemotherapy Based on the Pan-Immune Inflammation Value. Curr. Oncol. 2025, 32, 194. https://doi.org/10.3390/curroncol32040194
Tian Z, Xi Y, Chen M, Hu M, Chen F, Wei L, Zhang J. Construction of a Nomogram Model for Predicting Pathologic Complete Response in Breast Cancer Neoadjuvant Chemotherapy Based on the Pan-Immune Inflammation Value. Current Oncology. 2025; 32(4):194. https://doi.org/10.3390/curroncol32040194
Chicago/Turabian StyleTian, Zhuowan, Yiqing Xi, Mengting Chen, Meishun Hu, Fangfang Chen, Lei Wei, and Jingwei Zhang. 2025. "Construction of a Nomogram Model for Predicting Pathologic Complete Response in Breast Cancer Neoadjuvant Chemotherapy Based on the Pan-Immune Inflammation Value" Current Oncology 32, no. 4: 194. https://doi.org/10.3390/curroncol32040194
APA StyleTian, Z., Xi, Y., Chen, M., Hu, M., Chen, F., Wei, L., & Zhang, J. (2025). Construction of a Nomogram Model for Predicting Pathologic Complete Response in Breast Cancer Neoadjuvant Chemotherapy Based on the Pan-Immune Inflammation Value. Current Oncology, 32(4), 194. https://doi.org/10.3390/curroncol32040194





