A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening
Abstract
:1. Introduction
2. Materials and Methods
- 1.
- Studies that evaluated the screening of PC in comparison to no screening.
- 2.
- Studies investigating populations classified as at average or above-average risk for PC.
- 3.
- Studies that reported quality-adjusted life-years (QALY) or life-years saved (LYS) as patient outcomes.
- 4.
- Studies employing decision-analytic modelling to evaluate both the long-term effectiveness and cost-effectiveness of interventions aimed at early detection.
- 5.
- Studies that presented the Incremental Cost-Effectiveness Ratio (ICER) or offered sufficient data to calculate ICER.
- 6.
- Studies that specified cost per QALY, cost per LYS, or cost per utility gained.
- 7.
- Studies that included comprehensive economic evaluations.
- 8.
- Studies that were published in the English language.
- 1.
- Non-original studies.
- 2.
- Studies not published in the English language.
- 3.
- Grey literature.
- 4.
- Systematic reviews, editorials, letters, abstracts, and studies that do not constitute comprehensive health economic evaluations or focus exclusively on follow-up or treatment strategies.
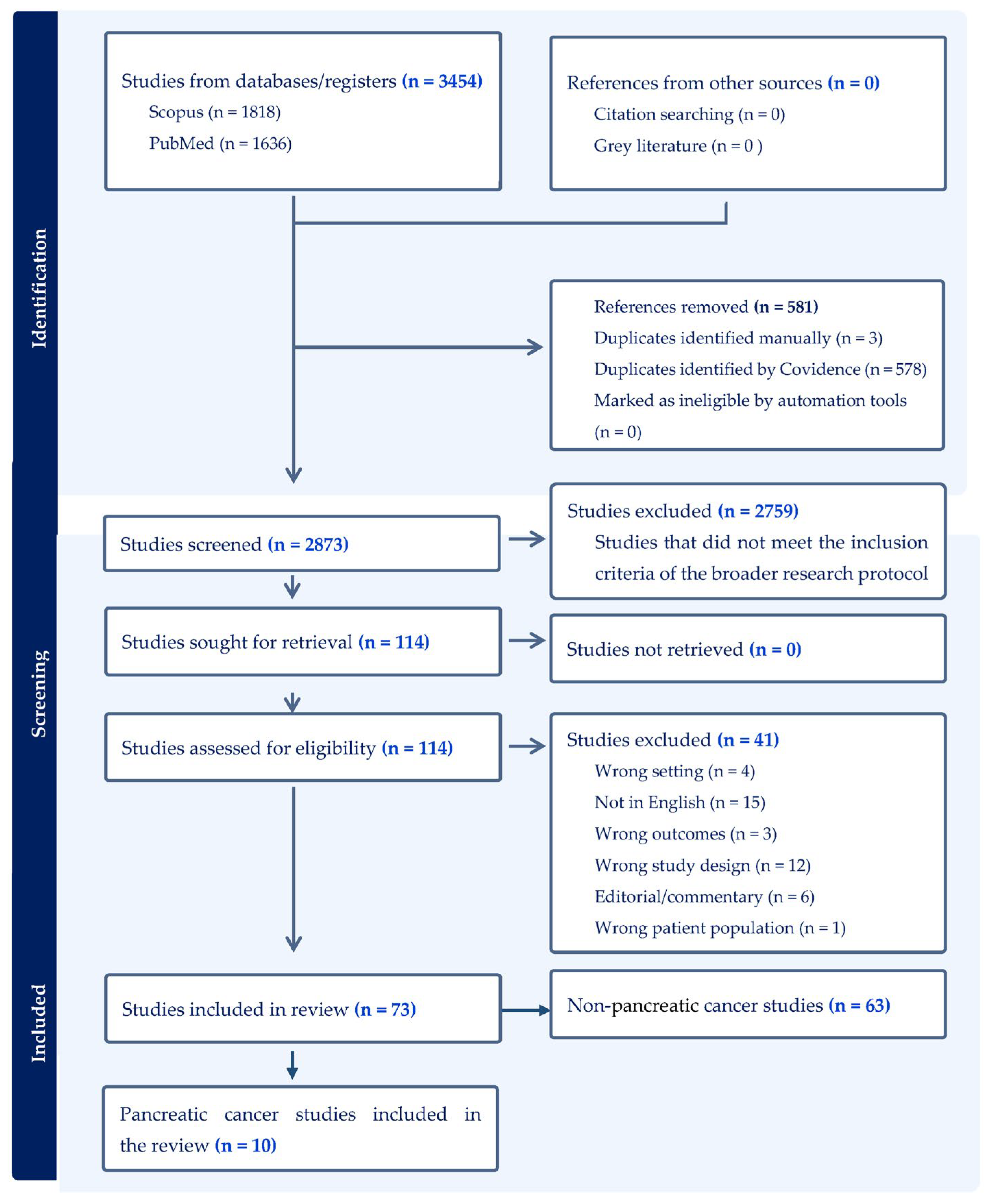
3. Results
| References | Country | Cancer Risk | Intervention Strategy | Reference Strategy | Study Objective |
|---|---|---|---|---|---|
| Wang et al., 2022 [25] | USA | High-risk | One-time screening using 3-year PDAC risk stratification | No screening | To evaluate the cost-effectiveness of this early detection strategy versus the current standard of care for sporadic PDAC among NOD patients and to determine the optimal risk threshold to identify high-risk NOD patients for targeted early detection efforts |
| Schwartz et al., 2022 [32] | USA | High-risk | One-time risk-based screening using standard-contrast CT | No screening | To develop an early-stage cost-effectiveness model to assess the potential value of risk-based CT pancreatic cancer screening using the END-PAC model in patients with NOD |
| Rulyak et al., 2003 [27] | USA | High-risk | One-time screening with EUS | No screening | To detect pancreatic dysplasia among members of high-risk familial pancreatic cancer kindreds might be cost-effective |
| Peters et al., 2024 [33] | USA | High-risk | MRI with EUS referral one-time, every 5 years, every 2 years, and annually | No screening | To evaluate the potential cost-effectiveness of combined MRI and EUS screening for pancreatic PDAC among high-risk populations |
| Kumar et al., 2021 [26] | USA | High-risk | One-time index EUS | No screening | To evaluate which factors make index EUS a potentially cost-effective strategy in identifying lesions in patients at high risk of developing PDAC, i.e., individuals with at least a 5% lifetime risk of pancreatic cancer |
| Kowada 2022 [34] | Japan | High-risk | Annual screening using either MicroRNA, CA 19-9, AU, MRI, EUS, CT, or PET | No screening | To assess the cost-effectiveness of microRNA compared with CA 19-9, AU, MRI, EUS, CT, PET, and no screening for pancreatic cancer screening in patients with diabetes |
| Kowada 2020 [35] | Japan | High-risk | One-time screening using either MRI, EU, CT, or PET, and no screening | One-time screening using AU | To assess the cost-effectiveness of AU, MRI, EUS, CT, PET, and no screening for pancreatic cancer screening in familial high-risk individuals |
| Joergensen et al., 2016 [36] | Denmark | High-risk | Annual EUS, MRI, MRCP, CT | No screening | To establish a screening program for high-risk groups (i.e., persons with hereditary pancreatitis (HP) or with a predisposition to HP and individuals with first-degree relatives with familial pancreatic cancer) and to evaluate its cost-effectiveness |
| Draus et al., 2023 [37] | Sweden | Average-risk and high-risk | One-time screening using a hypothetical blood-based biomarker test | Not reported | To evaluate the cost-effectiveness of screening for pancreatic cancer in Sweden using a hypothetical blood-based biomarker test with predetermined sensitivity and specificity in the general population and selected high-risk cohorts |
| Corral et al., 2019 [28] | USA | High-risk | Annual EUS or MRI | No screening | To perform an economic analysis to identify the different clinical, as well as cost determinants of pancreatic cancer surveillance in high-risk individuals, i.e., individuals with at least 5% lifetime risk of pancreatic cancer |
| References | Target Group: Age Range and Risk Condition | Analytical Model | Cycle Length | Time Horizon | Compliance | Perspective | Discount Rate | Source of Clinical Input |
|---|---|---|---|---|---|---|---|---|
| Wang et al., 2022 [25] | ≥50 years with NOD | Markov | 3 months | Lifetime | Not reported | Healthcare sector | 3% | Literature, published data, SEER |
| Schwartz et al., 2022 [32] | ≥50 years with NOD | Markov | Monthly | Lifetime | Not reported | Limited US healthcare payer | 3% | Literature (Enriching New-Onset Diabetes for Pancreatic Cancer (END-PAC) risk model validation study; Pancreatic Cancer Action Network (PanCAN) trial), SEER |
| Rulyak et al., 2003 [27] | 50-year-olds with FPC | Decision tree | Not applicable | Lifetime | Not reported | Third-party and societal | 3% | Literature, Familial Pancreatic Cancer Screening Program database, consensus of two experts |
| Peters et al., 2024 [33] | ≥40 years with germline mutations: PALB2, BRCA1, Lynch syndrome, ATM, BRCA2, TP53, CDKN2A, or STK11 | Markov mode | 1 month | Lifetime | 100% | Healthcare sector | 3% | SEER, 2019 Medicare and Medicaid data, literature |
| Kumar et al., 2021 [26] | 55-year-olds with FPC | Decision tree | Not applicable | Lifetime | Not reported | Third-party | 3% | Literature, Cancer of the Pancreas Screening (CAPS) trial, Centre for Medicare Services, expert opinion |
| Kowada 2022 [34] | 40–70 years with either NOD, LSD, or LSD with IPMN | Markov | 1 year | Lifetime | 100% | Healthcare payer | 3% | Literature, MEDLINE, Japanese statistics |
| Kowada 2020 [35] | 50-year-olds with FPC | Markov | 1 year | Lifetime | Not reported | Healthcare sector | 3% | Literature, MEDLINE, Japanese statistics |
| Joergensen et al., 2016 [36] | >30 years with HP and >50 years with FPC patients | Prospective cohort analysis | Not applicable | 2006–2014 | 100% | Not reported (health program intervention) | 4% | Danish Diagnose Related Group, study data |
| Draus et al., 2023 [37] | General population 50–79 years, daily smokers 50–79 years, FPC or hereditary PC 40–79 years, NOD 50–79 years, early symptoms of PC 40–79 years | Not reported | Not reported | Not reported | Not reported | Social economy | 3% | Literature, databases |
| Corral et al., 2019 [28] | 40-year-olds with either FPC, genetic syndromes (BRCA 1 and 2), PJS, HP, FAMMM, Lynch syndrome, Li Fraumeni, Familial adenomatous polyposis, or NOD | Markov | 1 year | Lifetime | Not reported | Third-party payer | 3% | Literature, life tables, SEER |
| References | Intervention Strategy | Reference Strategy | Year/Currency | Incremental Cost-Effectiveness Ratio | Willingness-to-Pay | Sensitivity Analysis | Sensitive Variables-1-Way SA | Probabilistic Sensitivity Analysis | CHEERS Checklist |
|---|---|---|---|---|---|---|---|---|---|
| Wang et al., 2022 [25] | Screening through 3-year PDAC risk stratification | No screening | 2020 USD | Riskthreshold-1%: $116,910.97/QALY, Threshold-2%: $63,045.49/QALY, Threshold-3%: $47,948.20, Threshold-4%: $31,389.02, Threshold-5%: $5407.44, | $100,000/QALY and $150,000/QALY | 1-way SA and PSA | The proportion of PDACs detected at local stage, costs of treatment for metastatic PDAC, utilities of local and regional cancers, and sensitivity of screening | 1% and 2% risk thresholds CE 30.6% and 20.4% of the times at WTP threshold of $150,000/QALY and 27.3% and 22.8% of the times at WTP threshold of $100,000/QALY | Not reported |
| Schwartz et al., 2022 [32] | Risk-based screening of patients with NOD using standard-contrast CT | No screening | 2020 USD | Screening: $65,076/QALY | $100,000/QALY | 1-way SA and PSA | The proportion of screen-detected PAC cases that are resectable, the health state utility for resectable PAC from 6 months after surgery to progression, and the proportion of clinically detected PAC cases with distant-stage disease | Screening CE > 99% of the time | Not reported |
| Rulyak et al., 2003 [27] | One-time screening with EUS | No screening | 2000 USD | Screening: $16,885/LYS | Not reported | 1-way SA and 2-way SA | Prevalence of dysplasia, sensitivity of EUS and ERCP, life expectancy (after pancreatectomy) | Not reported | Not reported |
| Peters et al., 2024 [33] | MRI with EUS referral one-time, every 5 years, every 2 years, and annually | No screening | 2019 USD | Men: -STK11 (RR 28) annual screening from 40 years: $69,000/QALY -CDKN2A (RR 12.33) annual screening from 55 years: $82,000/QALY Women: -STK11 (RR 28) annual screening from 45 years: $45,000/QALY | $100,000/QALY | 1-way SA | Specificity of screening, screening cost | Not reported | Not reported |
| Kumar et al., 2021 [26] | One index EUS | No screening | 2018 USD | Screening: $82,669/QALY | $100,000/QALY | 1-way SA and 2-way SA | Lifetime risk of PDAC, probability of future PDAC after normal index EUS, and probability of a missed lesion, length of survival after resection | Not reported | Not reported |
| Kowada 2022 [34] | MicroRNA, CA 19-9, AU, MRI, EUS, CT, and PET | No screening | 2020 USD | LSD: AU: $14,968–19,540/QALY, NOD: MicroRNA: $52,611–$68,752/QALY, LSD with IPMN: MicroRNA: $10,130–12,911/QALY | $100,000/QALY | 1-way SA, 2-way SA, PSA | Cost of microRNA in patients with LSD, pancreatic cancer incidence in all diabetic patients | AU was 60% to 76% cost-effective in patients with LSD, and microRNA was 42% to 54% cost-effective for NOD and 76% to 78% cost-effective for LSD having IPMN at a WTP threshold of $100,000/QALY | Not reported |
| Kowada 2020 [35] | MRI, EUS, CT, PET, and no screening | AU | 2018 USD | AU is cost-effective (USD 11,035, 17.4875 QALY) | $50,000/QALY | 1-way SA, PSA | Incidence of pancreatic cancer | AU is cost-effective 76% of the time at a WTP threshold of $50,000/QALY | Not reported |
| Joergensen et al., 2016 [36] | Annual EUS or MRI, MRCP or CT, if EUS is not possible | No screening | 2015 USD | FPC: $38,785/QALY, HP: $58,647/QALY, Total: $42,128/QALY | $50,000/QALY | Not reported | Risk stratification of the patients offered screening, the performance characteristics of EUS and EUS with FNA or other modalities used as well as the mortality rate after a total pancreatectomy | Not reported | Not reported |
| Draus et al., 2023 [37] | One-time screening using a hypothetical blood-based biomarker test | Not reported | 2018 EUR | Not reported | EUR100,000/QALY | Not reported | Not reported | Not reported | Not reported |
| Corral et al., 2019 [28] | EUS, MRI | No screening | Annual screening of (1) high-risk individuals: EUS: $13,200/QALY, MRI: dominant strategy; and (2) highest-risk individuals: EUS: dominant strategy, MRI: $7847/QALY | $100,000/QALY | 1-way SA, PSA | Age, diagnostic performance of imaging tools | MRI CE > 50% of the time, EUS CE more than 45% of the time | Not reported |
3.1. Overview of Selected Studies: Target Population and Screening Strategies
3.2. Cost-Effectiveness of Studies Focusing on Screening Patients with Diabetes
3.3. Cost-Effectiveness of Studies Focusing on Screening Individuals with FPC
- 1.
- An individual who has two or more first-degree relatives (FDRs) with PC.
- 2.
- An individual who has one first-degree relative (FDR) diagnosed with PC at an early age (≤50).
- 3.
- An individual who has two or more second-degree relatives with PC, one of whom developed it at an early age [38].
3.4. Cost-Effectiveness of Studies Focusing on Multiple Risk Factors
3.5. Study Type, Models, Input Parameters, and Sensitivity Analysis
3.6. Results on Quality of Reporting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AU | Abdominal ultrasound |
| CA 19-9 | Carbohydrate antigen 19-9 |
| CAPS | Cancer of the Pancreas Screening Consortium |
| CT | Computed tomography |
| CHEERS | Consolidated Health Economic Evaluation Reporting Standards |
| CE | Cost-effectiveness |
| END-PAC | Enriching New-Onset Diabetes for Pancreatic Cancer |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EU | Endoscopic ultrasound |
| FAMMM | Familial atypical mole melanoma |
| FPC | Familial pancreatic cancer |
| FNA | Fine needle aspiration |
| FDR | First-degree relative |
| HP | Hereditary pancreatitis |
| ICER | Incremental cost-effectiveness ratio |
| IPMN | Intraductal papillary mucinous neoplasm |
| LSD | Long-standing type 2 diabetes |
| LYS | Life-years saved |
| MRCP | Magnetic resonance cholangiopancreatography |
| MRI | Magnetic resonance imaging |
| NOD | New-onset diabetes |
| PC | Pancreatic cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| PJS | Peutz–Jeghers syndrome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| PSA | Probabilistic sensitivity analysis |
| PET | Positron emission tomography |
| QALY | Quality-adjusted life-years |
| SEER | Surveillance, Epidemiology, and End Results |
| THIN | The Health Improvement Network |
| WTP | Willingness to pay |
| 1-way SA | one-way sensitivity analysis |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I. Bray Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 17 February 2024).
- Kenner, B.J.; Chari, S.T.; Maitra, A.; Srivastava, S.; Cleeter, D.F.; Go, V.L.W.; Rothschild, L.J.; Goldberg, A.E. Early Detection of Pancreatic Cancer—A Defined Future Using Lessons from Other Cancers. Pancreas 2016, 45, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, J.K.; Mortensen, M.B.; Schønnemann, K.R.; Pfeiffer, P. Characteristics, Therapy and Outcome in an Unselected and Prospectively Registered Cohort of Pancreatic Cancer Patients. Eur. J. Cancer 2013, 49, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jansen, L.; Balavarca, Y.; Molina-Montes, E.; Babaei, M.; van der Geest, L.; Lemmens, V.; Van Eycken, L.; De Schutter, H.; Johannesen, T.B.; et al. Resection of Pancreatic Cancer in Europe and USA: An International Large-Scale Study Highlighting Large Variations. Gut 2019, 68, 130–139. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute SEER Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 22 September 2024).
- American Cancer Society Cancer Facts & Figures 2024. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer.html (accessed on 22 August 2024).
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early Detection of Pancreatic Cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Lu, C.; Xu, C.-F.; Wan, X.-Y.; Zhu, H.-T.; Yu, C.-H.; Li, Y.-M. Screening for Pancreatic Cancer in Familial High-Risk Individuals: A Systematic Review. World J. Gastroenterol. 2015, 21, 8678–8686. [Google Scholar] [CrossRef]
- Vanek, P.; Urban, O.; Zoundjiekpon, V.; Falt, P. Current Screening Strategies for Pancreatic Cancer. Biomedicines 2022, 10, 2056. [Google Scholar] [CrossRef]
- Lucas, A.L.; Kastrinos, F. Screening for Pancreatic Cancer. JAMA 2019, 322, 407. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Epling, J.W.; Kubik, M.; et al. Screening for Pancreatic Cancer. JAMA 2019, 322, 438. [Google Scholar] [CrossRef]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.-W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) Consortium Summit on the Management of Patients with Increased Risk for Familial Pancreatic Cancer. Gut 2013, 62, 339–347. [Google Scholar] [CrossRef]
- Cid-Arregui, A. Perspectives in the Treatment of Pancreatic Adenocarcinoma. World J. Gastroenterol. 2015, 21, 9297. [Google Scholar] [CrossRef]
- Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. C-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-Term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ohtsuka, T.; Matsunaga, T.; Kimura, H.; Tamura, K.; Ideno, N.; Aso, T.; Miyasaka, Y.; Ueda, J.; Takahata, S.; et al. Long-term Outcomes after Total Pancreatectomy: Special Reference to Survivors’ Living Conditions and Quality of Life. World J. Surg. 2015, 39, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, T.A.; Bronner, M.P.; Byrd, D.R.; Haggitt, R.C.; Kimmey, M.B. Early Diagnosis and Treatment of Pancreatic Dysplasia in Patients with a Family History of Pancreatic Cancer. Ann. Intern. Med. 1999, 131, 247. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating Biomarkers for Early Diagnosis of Pancreatic Cancer: Facts and Hopes. Am. J. Cancer Res. 2018, 8, 332–353. [Google Scholar]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Toft, J.; Hadden, W.J.; Laurence, J.M.; Lam, V.; Yuen, L.; Janssen, A.; Pleass, H. Imaging Modalities in the Diagnosis of Pancreatic Adenocarcinoma: A Systematic Review and Meta-Analysis of Sensitivity, Specificity and Diagnostic Accuracy. Eur. J. Radiol. 2017, 92, 17–23. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of Patients with Increased Risk for Familial Pancreatic Cancer: Updated Recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Boursi, B.; Finkelman, B.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.-X. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer among Patients with New-Onset Diabetes. Gastroenterology 2017, 152, 840–850.e3. [Google Scholar] [CrossRef]
- Boursi, B.; Finkelman, B.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.-X. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients with Prediabetes. Eur. J. Gastroenterol. Hepatol. 2022, 34, 33–38. [Google Scholar] [CrossRef]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients with New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Scott, F.I.; Boursi, B.; Reiss, K.A.; Williams, S.; Glick, H.; Yang, Y.-X. Cost-Effectiveness of a Risk-Tailored Pancreatic Cancer Early Detection Strategy among Patients with New-Onset Diabetes. Clin. Gastroenterol. Hepatol. 2022, 20, 1997–2004.e7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saumoy, M.; Oh, A.; Schneider, Y.; Brand, R.E.; Chak, A.; Ginsberg, G.G.; Kochman, M.L.; Canto, M.I.; Goggins, M.G.; et al. Threshold Analysis of the Cost-Effectiveness of Endoscopic Ultrasound in Patients at High Risk for Pancreatic Ductal Adenocarcinoma. Pancreas 2021, 50, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Rulyak, S.J.; Kimmey, M.B.; Veenstra, D.L.; Brentnall, T.A. Cost-Effectiveness of Pancreatic Cancer Screening in Familial Pancreatic Cancer Kindreds. Gastrointest. Endosc. 2003, 57, 23–29. [Google Scholar] [CrossRef]
- Corral, J.E.; Das, A.; Bruno, M.J.; Wallace, M.B. Cost-Effectiveness of Pancreatic Cancer Surveillance in High-Risk Individuals. Pancreas 2019, 48, 526–536. [Google Scholar] [CrossRef]
- Wang, L.; Levinson, R.; Mezzacappa, C.; Katona, B.W. Review of the Cost-Effectiveness of Surveillance for Hereditary Pancreatic Cancer. Fam. Cancer 2024, 23, 351–360. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. BMJ 2022, 376, e067975. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Schwartz, N.R.M.; Matrisian, L.M.; Shrader, E.E.; Feng, Z.; Chari, S.; Roth, J.A. Potential Cost-Effectiveness of Risk-Based Pancreatic Cancer Screening in Patients with New-Onset Diabetes. J. Natl. Compr. Cancer Netw. 2022, 20, 451–459. [Google Scholar] [CrossRef]
- Peters, M.L.B.; Eckel, A.; Seguin, C.L.; Davidi, B.; Howard, D.H.; Knudsen, A.B.; Pandharipande, P.V. Cost-Effectiveness Analysis of Screening for Pancreatic Cancer Among High-Risk Populations. JCO Oncol. Pract. 2024, 20, 278–290. [Google Scholar] [CrossRef]
- Kowada, A. Cost-Effectiveness of MicroRNA for Pancreatic Cancer Screening in Patients with Diabetes. Pancreas 2022, 51, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. Cost-Effectiveness of Abdominal Ultrasound versus Magnetic Resonance Imaging for Pancreatic Cancer Screening in Familial High-Risk Individuals in Japan. Pancreas 2020, 49, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Joergensen, M.T.; Gerdes, A.-M.; Sorensen, J.; Schaffalitzky de Muckadell, O.; Mortensen, M.B. Is Screening for Pancreatic Cancer in High-Risk Groups Cost-Effective?–Experience from a Danish National Screening Program. Pancreatology 2016, 16, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Draus, T.; Ansari, D.; Andersson, R. Model-Based Screening for Pancreatic Cancer in Sweden. Scand. J. Gastroenterol. 2023, 58, 534–541. [Google Scholar] [CrossRef]
- Brentnall, T.A. Cancer Surveillance of Patients from Familial Pancreatic Cancer Kindreds. Med. Clin. N. Am. 2000, 84, 707–718. [Google Scholar] [CrossRef]
- Pannala, R.; Basu, A.; Petersen, G.M.; Chari, S.T. New-Onset Diabetes: A Potential Clue to the Early Diagnosis of Pancreatic Cancer. Lancet Oncol. 2009, 10, 88–95. [Google Scholar] [CrossRef]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S.; Cho, C.; Macgregor-Das, A.; Siddiqui, A.; Witmer, P.D.; Tamura, K.; Song, T.J.; et al. Deleterious Germline Mutations in Patients with Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017, 35, 3382–3390. [Google Scholar] [CrossRef]
- Grant, R.C.; Selander, I.; Connor, A.A.; Selvarajah, S.; Borgida, A.; Briollais, L.; Petersen, G.M.; Lerner-Ellis, J.; Holter, S.; Gallinger, S. Prevalence of Germline Mutations in Cancer Predisposition Genes in Patients with Pancreatic Cancer. Gastroenterology 2015, 148, 556–564. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, D.; Jiménez, L.; Chan, K.K.; Horton, S.; Wong, W.W.L. A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening. Curr. Oncol. 2025, 32, 225. https://doi.org/10.3390/curroncol32040225
Lewis D, Jiménez L, Chan KK, Horton S, Wong WWL. A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening. Current Oncology. 2025; 32(4):225. https://doi.org/10.3390/curroncol32040225
Chicago/Turabian StyleLewis, Diedron, Laura Jiménez, Kelvin K. Chan, Susan Horton, and William W. L. Wong. 2025. "A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening" Current Oncology 32, no. 4: 225. https://doi.org/10.3390/curroncol32040225
APA StyleLewis, D., Jiménez, L., Chan, K. K., Horton, S., & Wong, W. W. L. (2025). A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening. Current Oncology, 32(4), 225. https://doi.org/10.3390/curroncol32040225





