Mechanism of Methane Adsorption/Desorption in Low-Rank Vitrain and Durain Coal Affected by Pore Structure and Wettability: A Case Study in Dafosi Area, South Ordos Basin, China
Abstract
1. Introduction
2. Geological Settings
3. Samples and Experiments
3.1. Coal Composition
3.2. Characterization of Pore Structure
3.3. Wettability Test
3.4. Methane Adsorption and Desorption Experiment
- 1.
- The isothermal adsorption experiment was conducted at the Coalbed Methane Lab of the Xi’an University of Science and Technology. A CBM adsorption–desorption simulation instrument (AST-2000) was employed, with the temperature set at 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C, and the maximum adsorption pressure being 8 MPa and particle size being 60–80 mesh. Equilibrium water sample collections and experiments were based on the Experimental Methods of Coal’s High Pressure Isothermal Adsorption-Volumetric Method (GB/T19560-2008).
- 2.
- The process of methane adsorption and desorption involves a change in energy. The interaction between adsorbate and adsorbent can be measured by studying the same amount of adsorption heat in the process of methane adsorption and desorption (when the adsorption capacity on the adsorbent surface is constant, there is still an infinitesimal amount of heat released after adsorption, which is the change in enthalpy value at the moment of the adsorption process). In the process of methane adsorption by coal, the stronger the property of the coal adsorption performance is, the greater the enhancement in the interaction between the surface molecules of coal and methane molecules, and an increase in the adsorption heat can be detected, irrespectively. The isosteric heat of methane adsorption and desorption can be calculated indirectly using the Clausius Clapeyron Equation (3) [39]:
4. Results and Discussion
4.1. Differences between Vitrain and Durain
4.1.1. Coal Composition
4.1.2. Pore Structure Characteristics
4.1.3. Wettability
4.2. Methane Adsorption and Desorption
4.2.1. Methane Adsorption Characteristics
4.2.2. Methane Desorption Characteristics
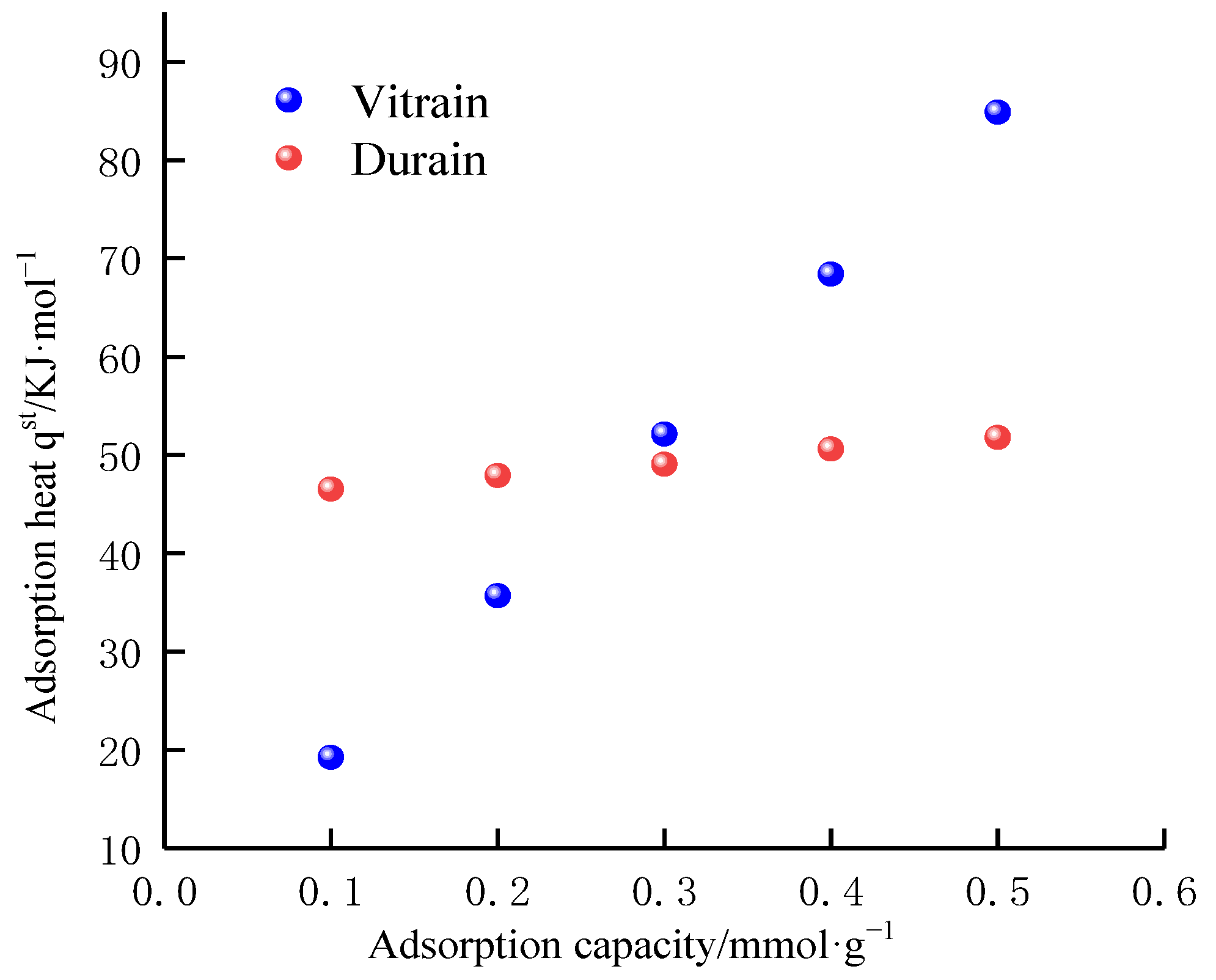
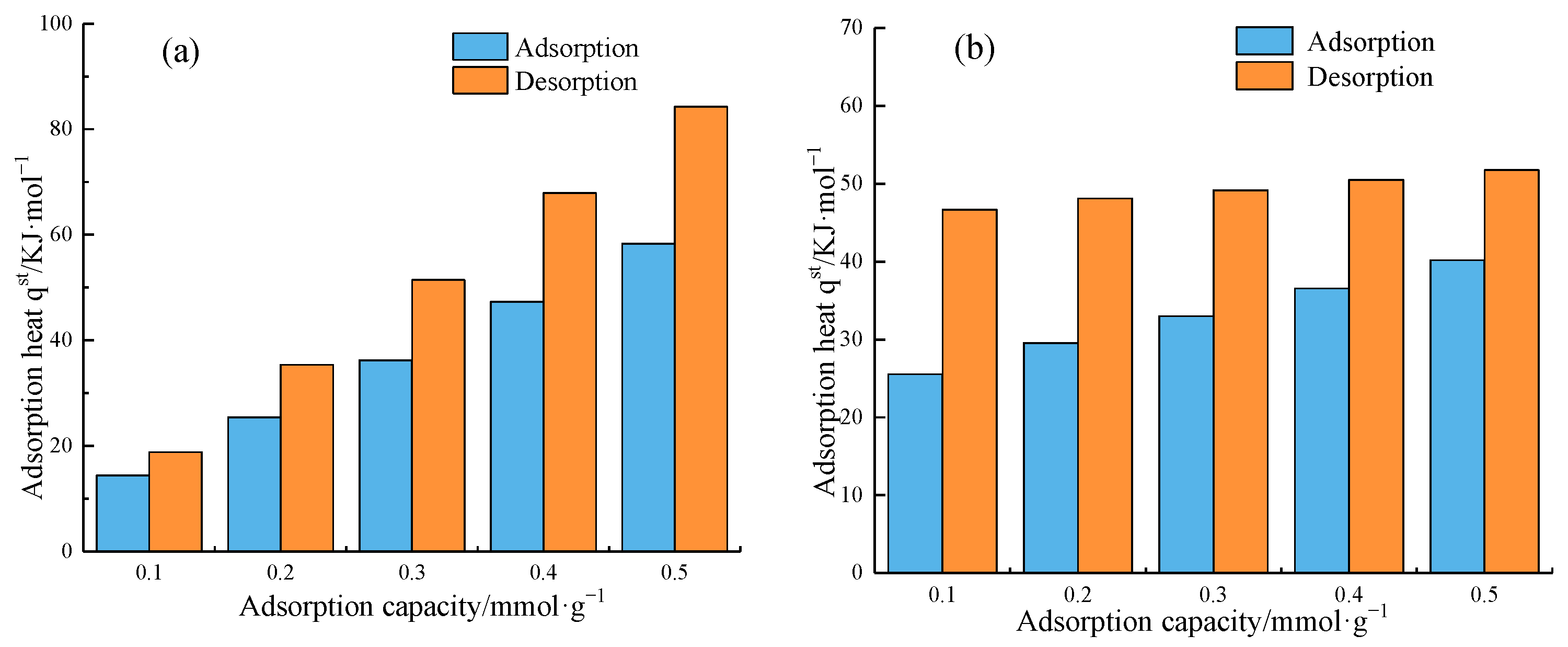
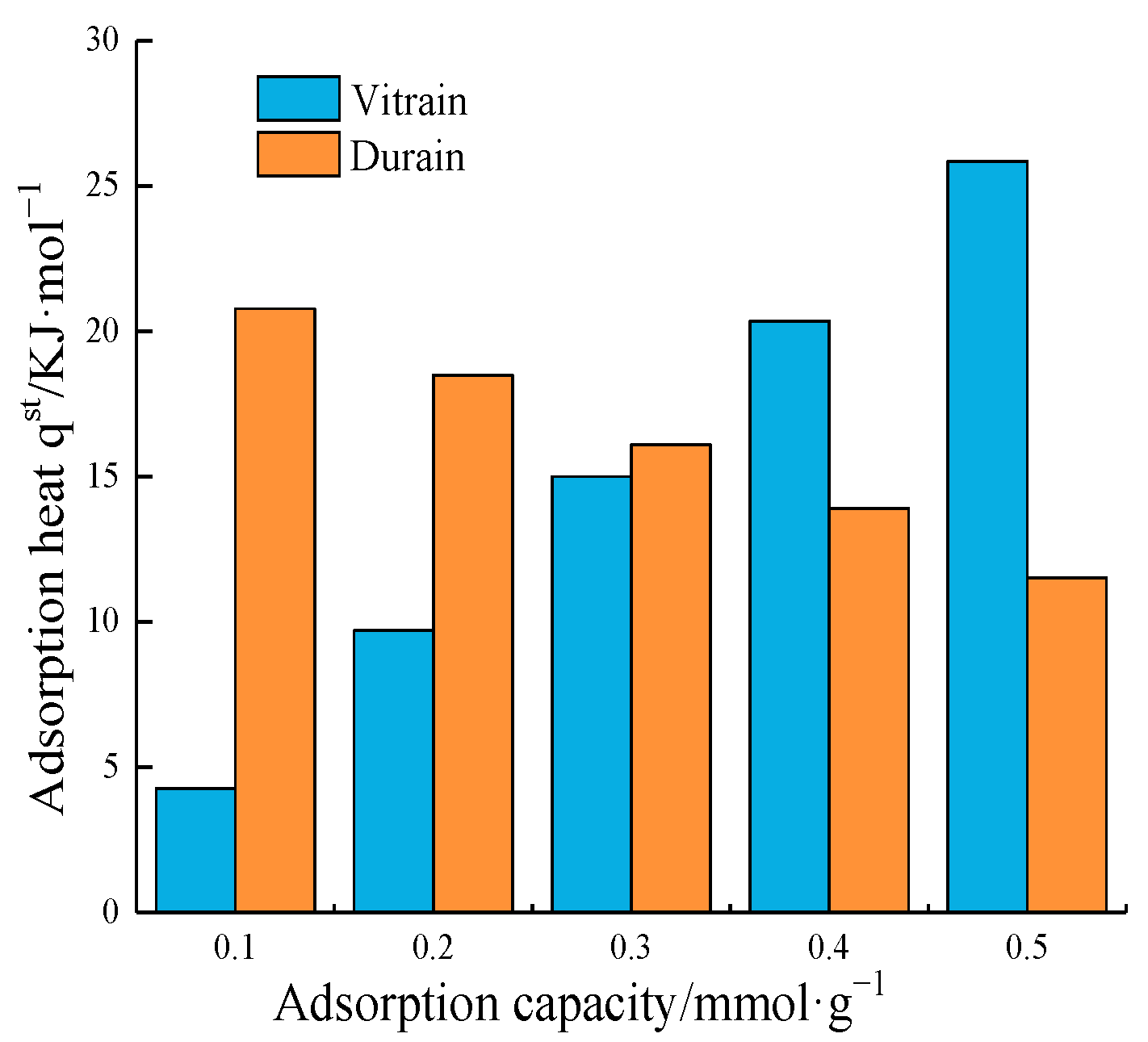
4.3. Adsorption Heat Analysis of Methane
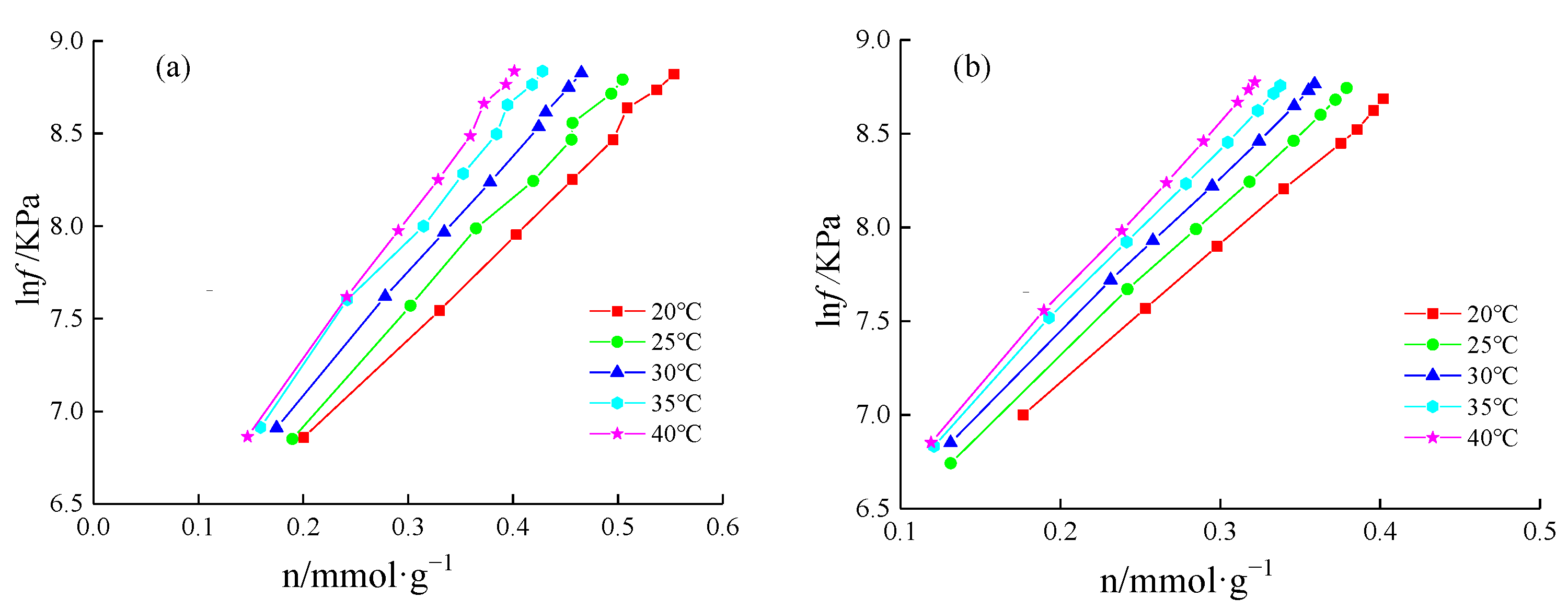
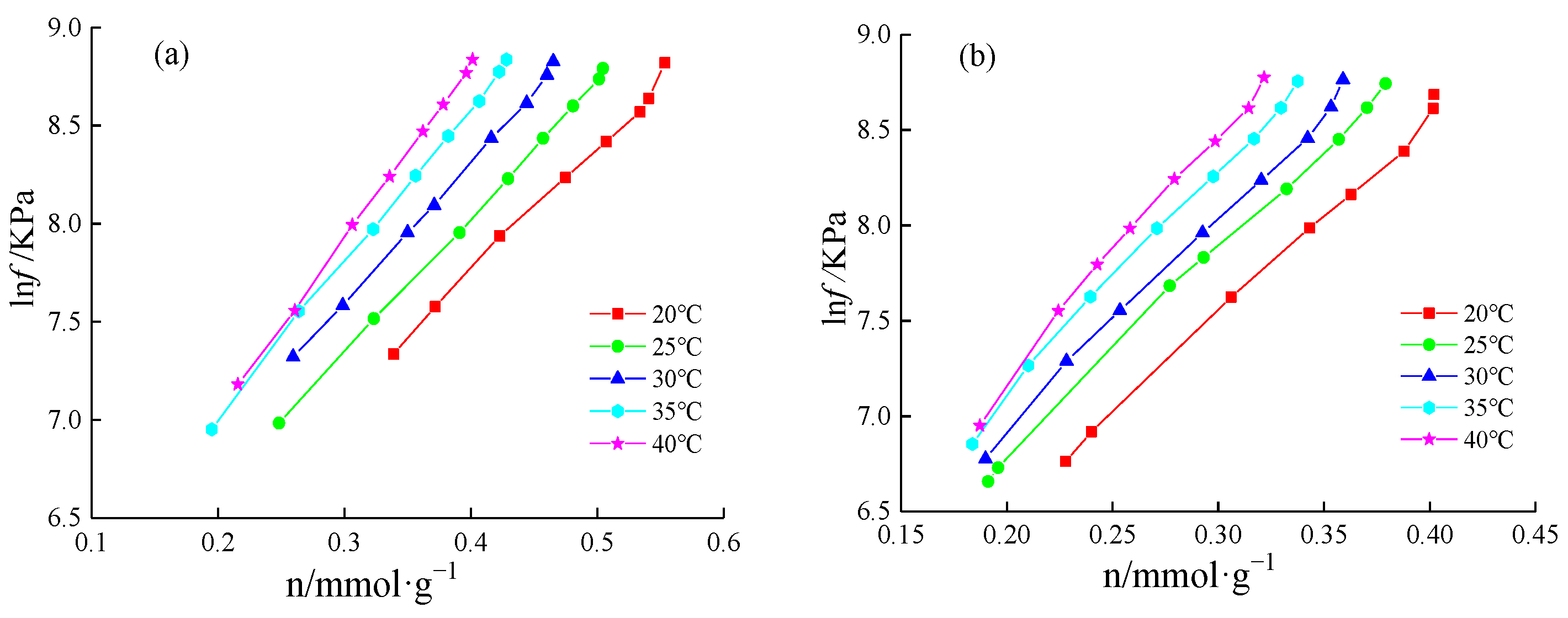
4.3.1. Adsorption Heat Calculation
- (1)
- Adsorption process
- (2)
- Desorption process
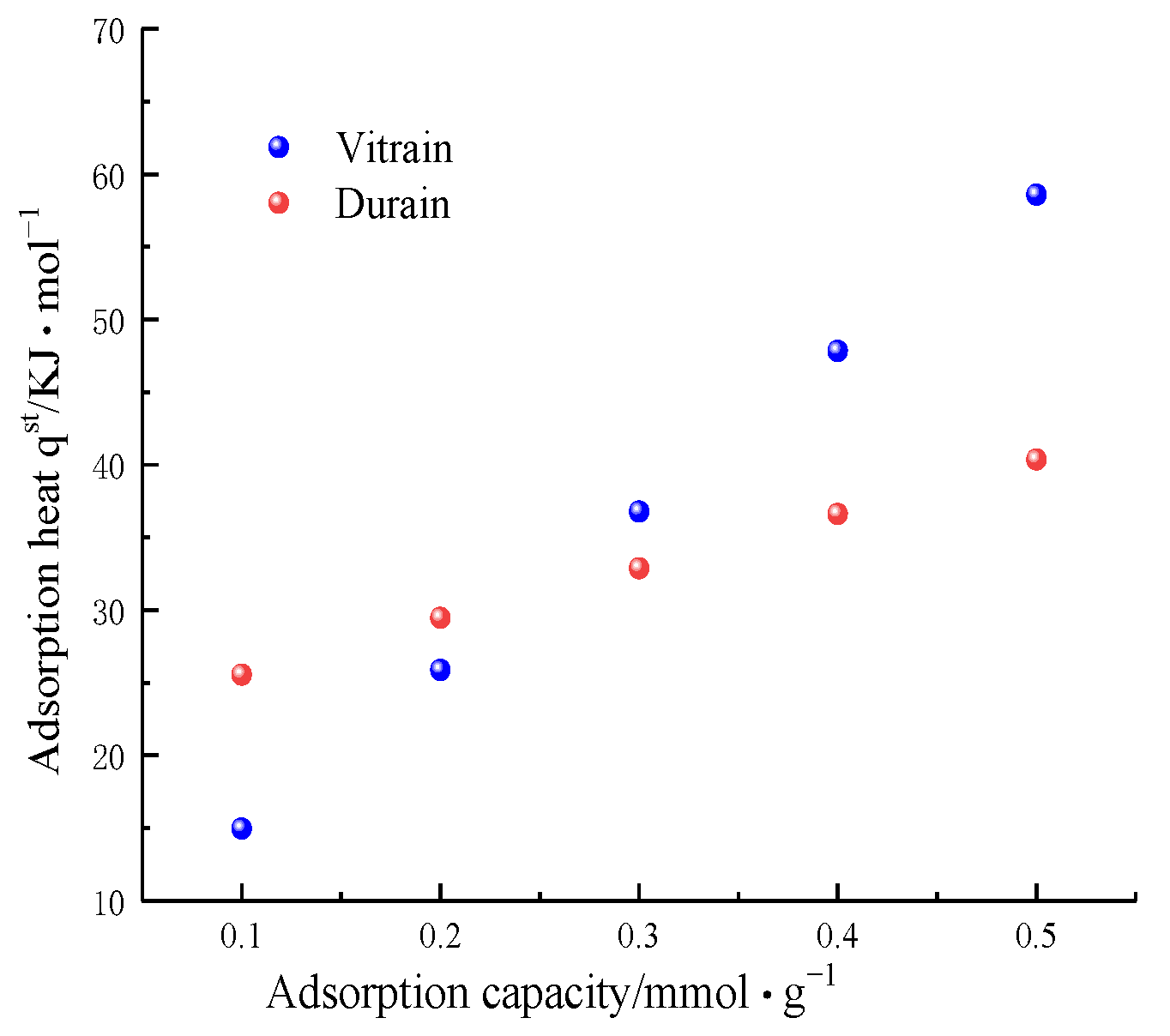
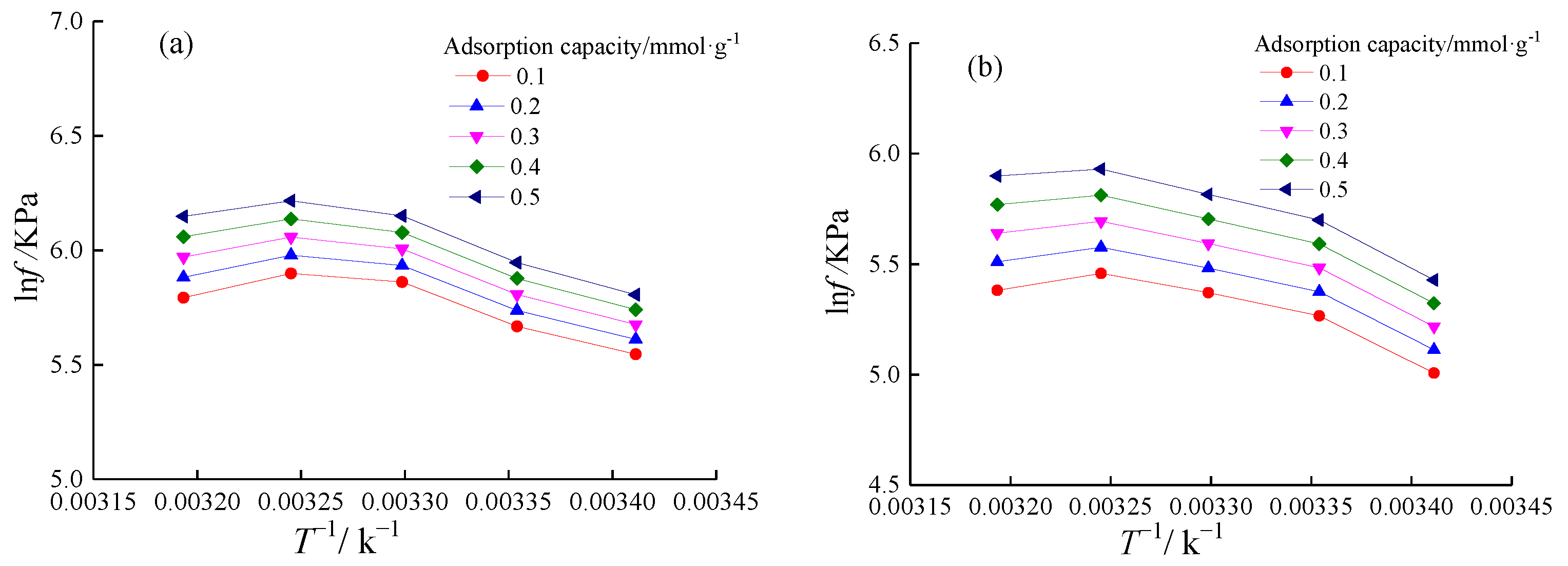
4.3.2. Adsorption Heat Variation
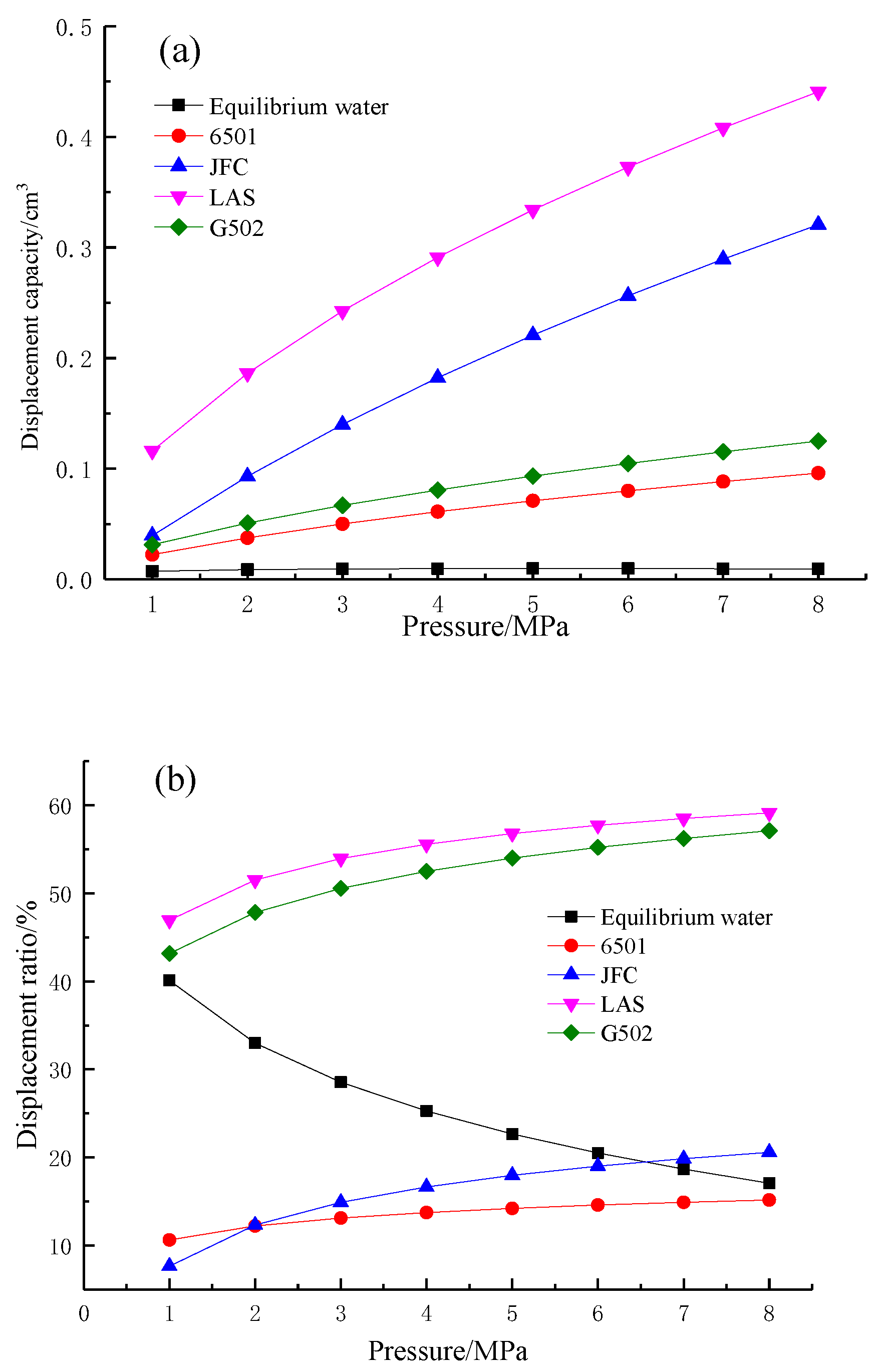
4.4. Effects of Wettability on Methane Adsorption and Desorption
4.4.1. Characteristics of Wettability on Adsorption and Desorption
4.4.2. Effects of Wettability on Desorption
- (1)
- Methane desorption capacity
- (2)
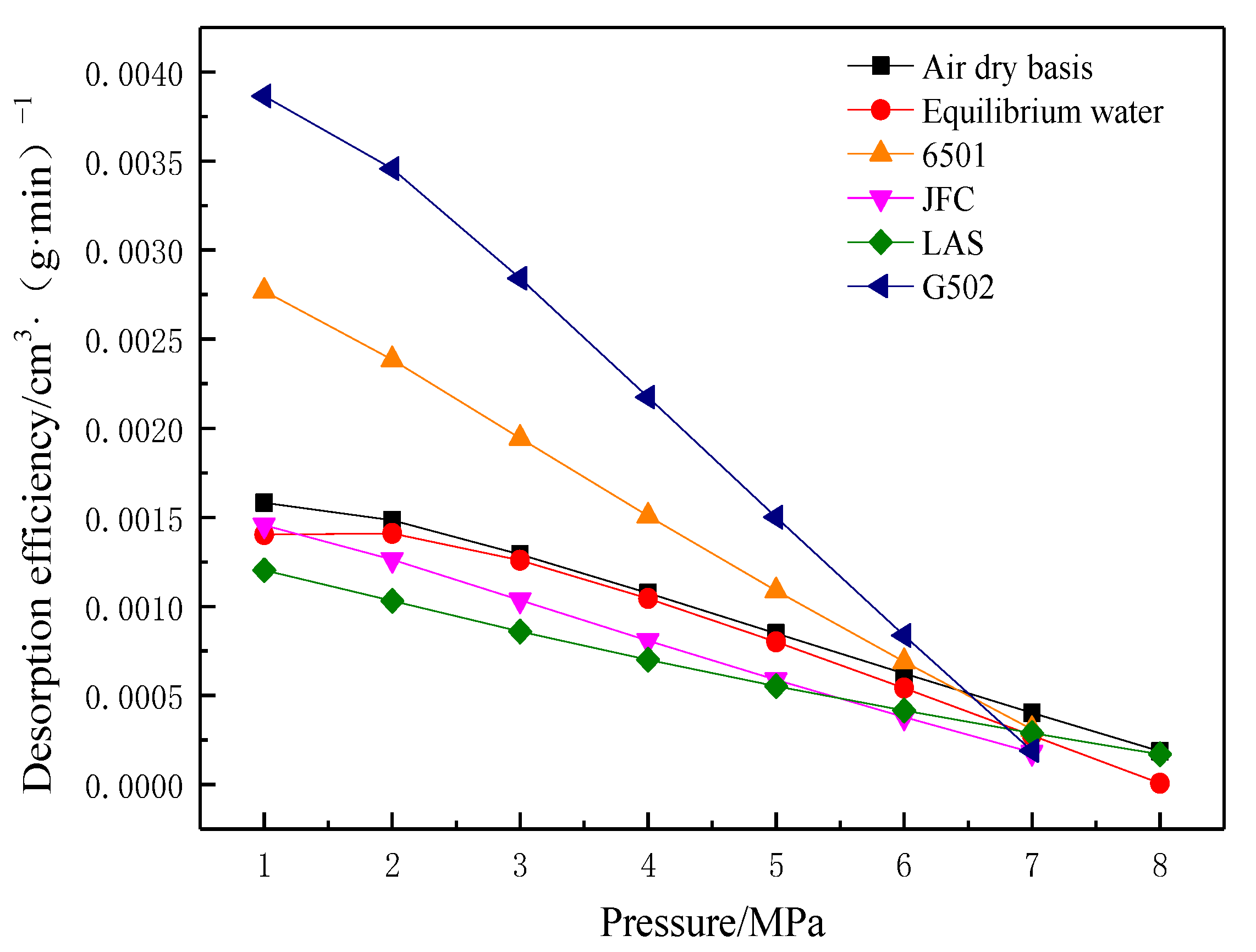
- Methane desorption efficiency
- (3)
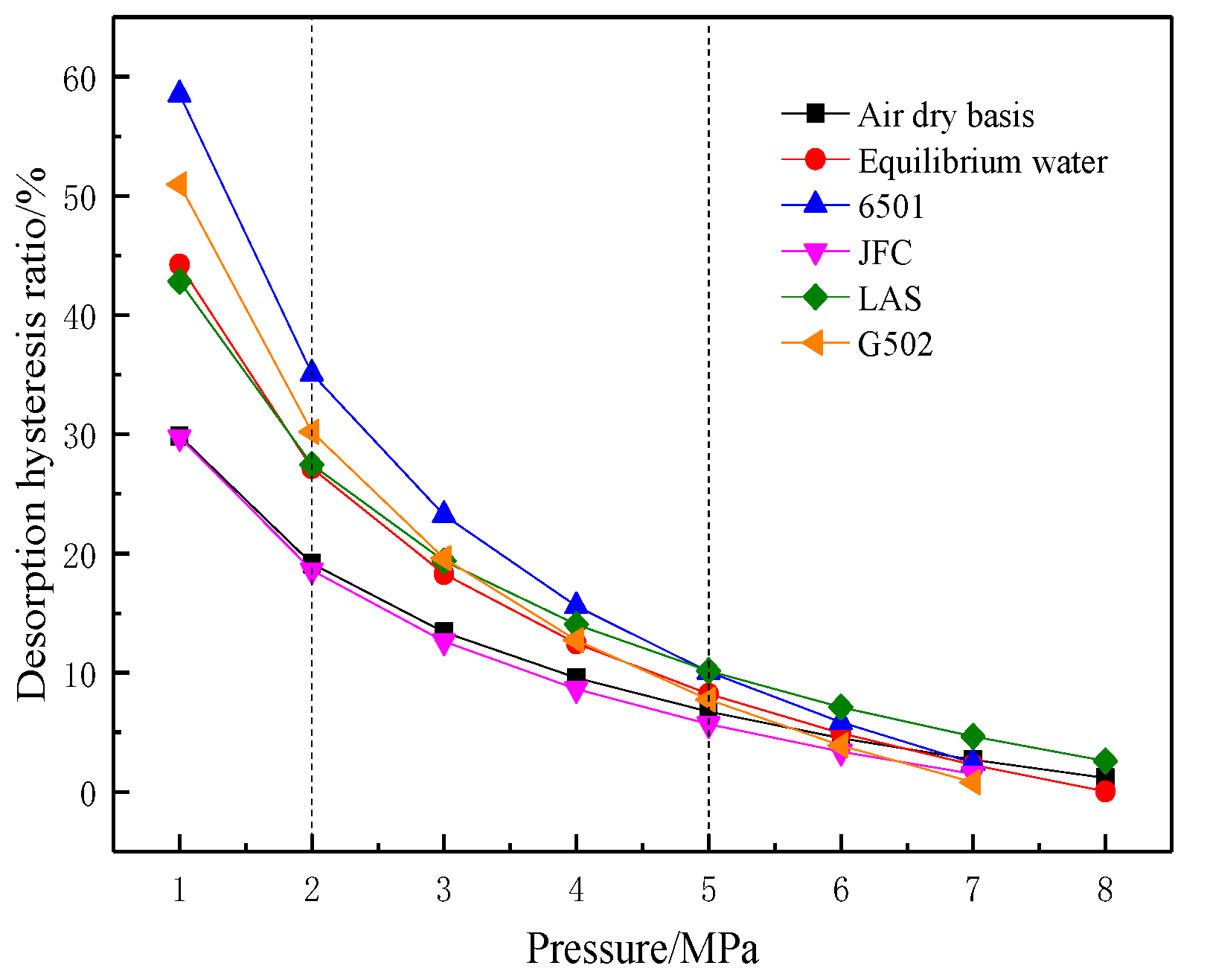
- Desorption hysteresis ratio
4.5. Mechanism of CBM Desorption in Coal Covered with Water
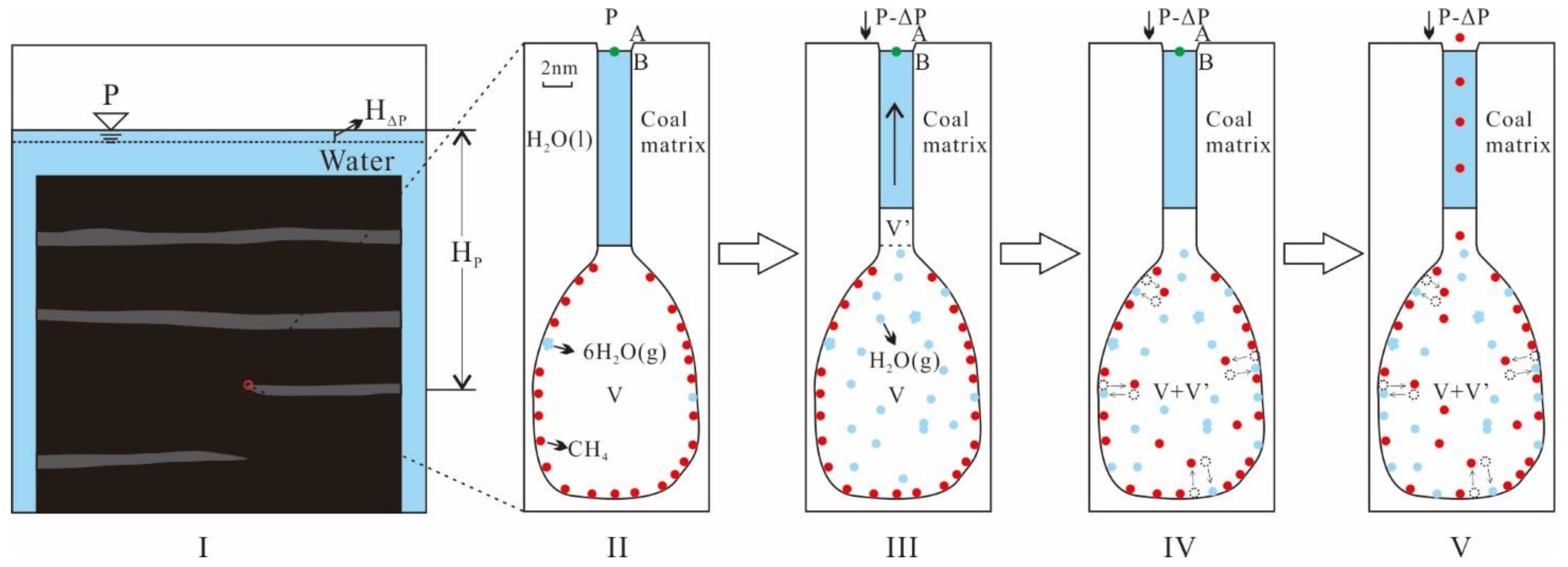
4.5.1. Vapor Displacement
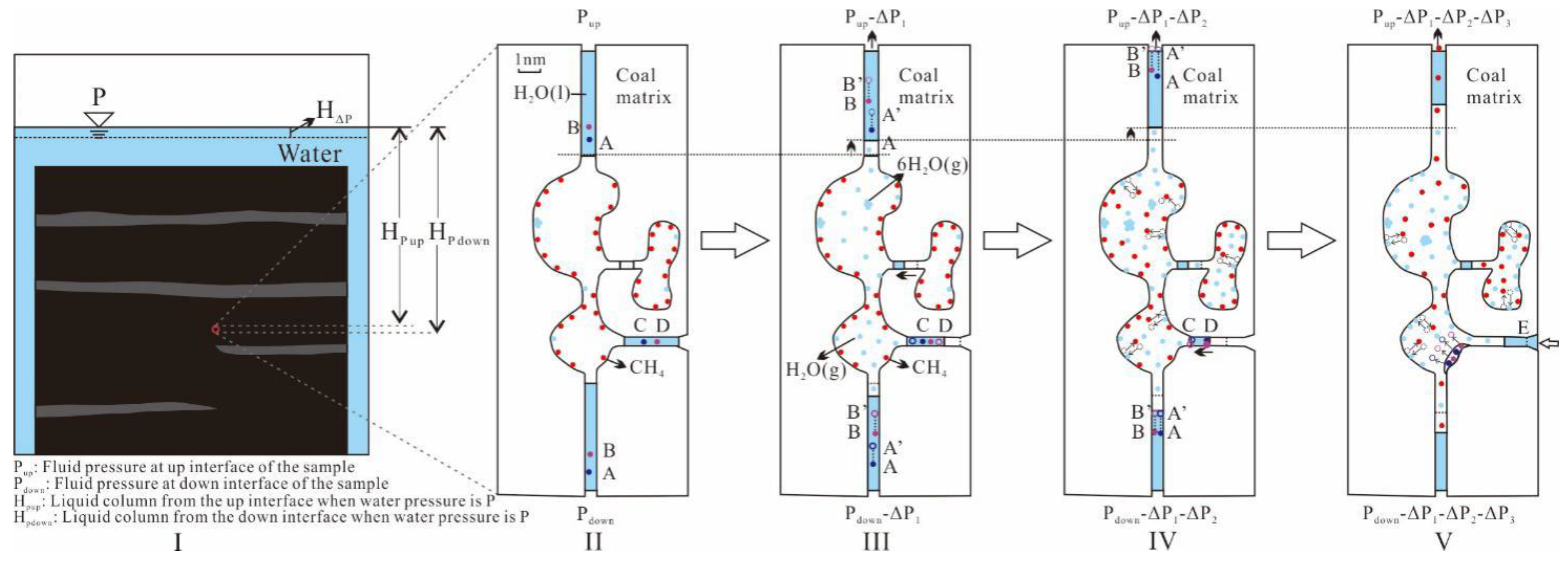
4.5.2. CBM Desorption Models
- CBM desorption in semi-open pores
- 2.
- CBM Desorption in Interconnected Pores
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Pan, J.; Hou, Q.; Yu, B.; Li, M.; Niu, Q. Anisotropic characteristics of low-rank coal fractures in the Fukang mining area, China. Fuel 2018, 211, 182–193. [Google Scholar] [CrossRef]
- Fu, H.; Tang, D.; Xu, T.; Xu, H.; Tao, S.; Li, S.; Yin, Z.; Chen, B.; Zhang, C.; Wang, L. Characteristics of pore structure and fractal dimension of low-rank coal: A case study of Lower Jurassic Xishanyao coal in the southern Junggar Basin, NW China. Fuel 2017, 193, 254–264. [Google Scholar] [CrossRef]
- Qin, Y.; Moore, T.A.; Shen, J.; Yang, Z.; Shen, Y.; Wang, G. Resources and geology of coalbed methane in China: A review. Int. Geol. Rev. 2017, 60, 777–812. [Google Scholar] [CrossRef]
- Fu, H.; Tang, D.; Xu, H.; Xu, T.; Chen, B.; Hu, P.; Yin, Z.; Wu, P.; He, G. Geological characteristics and CBM exploration potential evaluation: A case study in the middle of the southern Junggar Basin, NW China. J. Nat. Gas Sci. Eng. 2016, 30, 557–570. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, D.; Cai, Y.; Yao, Y. Fractal characterization of pore–fracture in low-rank coals using a low-field NMR relaxation method. Fuel 2016, 181, 218–226. [Google Scholar] [CrossRef]
- Ou, C.H.; Li, C.C.; He, J.; Hou, S.Y. Low-Rank Coalbed Methane Enrichment Characteristics in the Eastern Zone of Junggar Basin. Adv. Mater. Res. 2015, 1092, 1416–1419. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Cao, D.; Zhang, A.; Wang, A.; Xiang, X. New Progress on the Coal Fines Affecting the Development of Coalbed Methane. Acta Geol. Sinca 2018, 92, 2060–2062. [Google Scholar] [CrossRef]
- Flores, R.M. Coal and Coalbed Gas: Fueling the Future. Int. J. Coal Geol. 2014, 127, 1–2. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Bai, J.; Zhang, H. Thermodynamic Characteristics of Adsorption-Desorption of Methane in 3# Coal Seam of Sihe. Nat. Resour. 2014, 05, 782–794. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Zhang, S.; Sang, S. Physical chemistry mechanism of influence of liquid water on coalbed methane adsorption. Procedia Earth Planet. Sci. 2009, 1, 263–268. [Google Scholar] [CrossRef]
- Sang, S.; Zhu, Y.; Zhang, J.; Zhang, X.; Zhang, S. Influence of liquid water on coalbed methane adsorption An experimental research on coal reservoirs in the south of Qinshui Basin. Chin. Sci. Bull. 2005, 50, 79–85. [Google Scholar] [CrossRef]
- Bustin, R.M.; Clarkson, C.R. Geological controls on coalbed methane reservoir capacity and gas content. Coal Geol. 1998, 38, 3–26. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, P.; Qin, Y.; Wei, C.; Wang, L. New discovery on methane adsorption change of coal due to Sc-CO2 extraction during CO2-ECBM. Acta Geol. Sinca 2018, 92, 2438–2439. [Google Scholar]
- Zhang, Z.; Qin, Y.; Zhuang, X.; Li, G.; Liu, D. Geological Controls on the CBM Productivity of No.15 Coal Seam of Carboniferous–Permian Taiyuan Formation in Southern Qinshui Basin and Prediction for CBM High-yield Potential Regions. Acta Geol. Sinca 2018, 92, 2310–2332. [Google Scholar] [CrossRef]
- Wang, K.; Wang, G.; Ren, T.; Cheng, Y. Methane and CO2 sorption hysteresis on coal: A critical review. Int. J. Coal Geol. 2014, 132, 60–80. [Google Scholar] [CrossRef]
- Ding, G.; Rice, J.A. Effect of lipids on sorption/desorption hysteresis in natural organic matter. Chemosphere 2011, 84, 519–526. [Google Scholar] [CrossRef]
- Ran, Y.; Xing, B.; Rao, P.S.; Fu, J. Importance of Adsorption (Hole-Filling) Mechanism for Hydrophobic Organic Contaminants on an Aquifer Kerogen Isolate. Environ. Sci. Technol. 2004, 38, 4340–4348. [Google Scholar] [CrossRef]
- Braida, W.J.; Pignatello, J.J.; Lu, Y.; Ravikovitch, P.I.; Neimark, A.V.; Xing, B. Sorption Hysteresis of Benzene in Charcoal Particles. Environ. Sci. Technol. 2003, 37, 409–417. [Google Scholar] [CrossRef]
- Wu, W.; Sun, H. Sorption-desorption hysteresis of phenanthrene-effect of nanopores, solute concentration, and salinity. Chemosphere 2010, 81, 961–967. [Google Scholar] [CrossRef]
- Zhu, H.; Selim, H.M. Hysteretic Behavior of Metolachlor Adsorption-Desorption in Soils. Soil Sci. 2000, 165, 632–645. [Google Scholar] [CrossRef]
- Wang, K.; Fu, X.; Qin, Y.; Sesay, S.K. Adsorption characteristics of lignite in China. J. Earth Sci. 2011, 22, 371–376. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, W.; Liu, D.; Chu, W. Surface Modification of Bituminous Coal and Its Effects on Methane Adsorption. Chin. J. Chem. 2013, 31, 1102–1108. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, W.; Chu, W. Coalbed methane adsorption and desorption characteristics related to coal particle size. Chin. Phys. B 2016, 25, 068102. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Yao, Y.; Li, J.; Liu, J. Fractal Characteristics of Coal Pores Based on Classic Geometry and Thermodynamics Models. Acta Geol. Sinca 2011, 85, 1150–1162. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Wang, G.; Booth, P.; Nemcik, J. Experimental study of the adsorption-induced coal matrix swelling and its impact on ECBM. J. Earth Sci. 2017, 28, 917–925. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, T.; Liang, X.; Shu, D.; Zhang, Z.; Wei, X.; Zhang, K.; Feng, X.; Zhu, H.; Wang, S.; et al. Effects of nano-pore system characteristics on CH4 adsorption capacity in anthracite. Front. Earth Sci. 2018, 13, 75–91. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, C.; Liu, D.; Chu, W. Microstructure and its influence on CH4 adsorption behavior of deep coal. Chin. Phys. B 2014, 23, 028201. [Google Scholar] [CrossRef]
- Han, X.; Zhang, B.; Ye, J. Analysis and Application of CO2 Phase Change During CO2-ECBM Injection in CBM-Taking the study of Shizhuang North Block in Qinshui Basin as An Example. Unconventonaloil Gas 2018, 5, 80–85. [Google Scholar]
- Yao, Y.; Liu, D.; Tang, D.; Huang, W.; Tang, S.; Che, Y. A Comprehensive Model for Evaluating Coalbed Methane Reservoirs in China. Acta Geol. Sin.-Engl. Ed. 2008, 82, 1253–1270. [Google Scholar] [CrossRef]
- Chen, P.; Tang, X. The research on the adsorption of nitrogen in low temperature and micro-pore properties in coal. J. China Coal Soc. 2001, 26, 2438–2439. [Google Scholar]
- Harpalani, S.; Pariti, U.M. Study of coal sorption isotherms using amulticomponents gas mixture. Int. Coalbed Methane Symp. 1993, 31, 151–160. [Google Scholar] [CrossRef]
- Greaves, K.H.; Owen, L.B.; McLennan, J.D.; Olszcwski, A. Multicomponent gas adsorption-desorption behavior of coal. Int. Coalbed Methane Symp. 1993, 1, 197–205. [Google Scholar]
- Busch, A.; Gensterblum, Y.; Krooss, B.M. Methane and CO2 sorption and desorption measurements on dry Argonne premium coals: Pure components and mixtures. Int. J. Coal Geol. 2003, 55, 205–224. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, S.; Lin, Y. Isothermal adsorption and desorption experiment of coal and experimental results accuracy fitting. J. China Coal Soc. 2011, 36, 477–480. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, S.; Wang, P.; Lin, Y.; Wang, C. Mechanism of coalbed methane desorption at different temperatures. Coal Geol Explor. 2011, 39, 20–23. [Google Scholar] [CrossRef]
- Ma, D.; Cao, S.; Li, P.; Zhang, H.; Wu, J.; Hao, C.; Wang, L. Comparison on adsorption and desorption thermodynamics features between shale gas and coalbed methane. Coal Sci. Technol. 2015, 43, 64–67. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, L.; Wang, Y. Reasearch on CO2, CH4 Competitive Adsorption, Desorption and Replacement Effect of High and Low Rank Coal. Unconventonaloil Gas 2018, 5, 46–51. [Google Scholar]
- Gao, Z.; Ma, D.; Chen, Y. Hysteresis of methane desorption from coal seams in the Huanglong coalfield and quantitative evaluation of desorption hysteresis. J. Henan Univ. Sci. Technol. 2021, 40, 16–22+79. [Google Scholar] [CrossRef]
- Zhang, D.; Xian, X.; Tan, X. The Experimental Study of the Relation Between the Macromolecular Stucture of Coal and Its Bank. J. Chong Qing Univ. 1990, 13, 78–83. [Google Scholar]
- Nie, B.; He, X.; Wang, E. Surface free energy of coal and its calculation. J. Taiyuan Univ. Technol. 2000, 4, 346–348. [Google Scholar] [CrossRef]
- Nie, B.; He, X.; Wang, E.; Zhang, L. Micro-mechanism of coal adsorbing water. J. China Univ. Min. Technol. 2004, 33, 379–383. [Google Scholar]
- Li, Z. Experimental Study on Migration Characteristics of Pulverized Coal in Horizontal Wellbore of Coalbed Methane. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2017. [Google Scholar]
- Su, X.; Chen, R.; Lin, X.; Song, Y. Application of adsorption potential theory in the fractionation of coalbed gas during the process of adsorption/desorption. Acta Geol. Sinica 2008, 82, 1382–1389. [Google Scholar]
- Duan, L.; Tang, S.; Liu, H.; Zhen, G. Implication of canister desorption of coal for coalbed methane production. Beijing Geol. Publ. House 2008, 182–187. [Google Scholar]
- Pang, X.; Jing, X.; Wang, W.; Li, B.; Cheng, L.; Wang, Y. Coalbed gas content experiment research based on desorption by raising temperature. Coal Mine Saf. 2010, 41, 1–3. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, Q.; Qin, Y. Thermodynamic analysis of competitive adsorption of CO2 and CH4 on coal matrix. J. China Coal Soc. 2011, 36, 1307–1311. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Zhang, X.; Yang, X. Adsorption-disorption experiments of CH4 and CO2 with different consistency. J. China Coal Soc. 2009, 34, 551–555. [Google Scholar] [CrossRef]
- Yi, J.; Jiang, Y.; Xian, X. An experimental research on the characters of methane seepage in stress field and temperature field. China Min. Mag. 2007, 16, 113–116. [Google Scholar]
- Yi, J.; Jiang, Y.; Xian, X.; Luo, Y.; Zhang, Y. A liquid-solid dynamic coupling model of ultrasound enhanced coalbed gas desorption and flow. Rock Soil Mech. 2009, 30, 2945–2950. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Q.; Cui, Y. Quantum chemistry characteristics of coal adsorbing gas and their applications. Nat. Gas Geosci. 2014, 25, 444–452. [Google Scholar] [CrossRef]
- Nie, B.; He, X.; Wang, E.; Zhang, L. Research on the mechanisms of the influence of electromagnetic field on coalbed methane adsorption. Nat. Gas Ind. 2004, 24, 32–34. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Qin, Y.; Wang, L.; Wei, C.; Zhang, P. New Discovery on Supercritical CO2-H2O Treated Coal Pore Structure and Methane Adsorption. Acta Geol. Sinca 2017, 91, 1509–1510. [Google Scholar] [CrossRef]
- Xiang, J.; Zeng, F.; Liang, H.; Li, B.; Song, X. Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci. China Earth Sci. 2014, 57, 1749–1759. [Google Scholar] [CrossRef]
- Blake, J.R.; Gibson, D.C. Growth and collapse of a vapor cavity near a free surface. J. Fluid Mech. 1981, 111, 123–140. [Google Scholar] [CrossRef]
- Cui, J.; Li, P.; Ma, D.; Li, Z.; Zhang, K.; Meng, F. Experiment study on migration law of pulverized coal in borehole of coalbed methane horizontal well. Coal Sci. Technol. 2016, 44, 74–78. [Google Scholar] [CrossRef]
- Xu, M. Visual Experiment Research of the Bubble near the Wall; Zhejiang University: Zhejiang, China, 2013. [Google Scholar]
- Xu, L.; Xian, X.; Yang, M. Basic research on CMM concentration and purification. China Coalbed Methane 2005, 2, 11–15. [Google Scholar]









| Full Name | Principal Component | Code |
|---|---|---|
| Twelve fluoroalkyl trimethoxy silane | C14F12H2OSiO3 | G502 |
| Cocoanut fatty acid diethanolamide | C16H33O3N | 6501 |
| Fatty alcohol-polyoxyethylene ether | CmH2m+1(EO)nH | JFC |
| Sodium dodecyl benzene sulfonate | C18H29NaO3S | LAS |
| Distilled water | H2O | DW |
| Production water | H2O and Minerals | PW |
| Samples | Mad/% | Aad/% | Vdaf/% | FCad% | C/% | H/% | N/% | O/% | C/H | C/O |
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrain | 3.90 | 1.96 | 37.48 | 58.86 | 83.60 | 4.80 | 0.86 | 10.73 | 17.42 | 7.79 |
| Durain | 2.94 | 9.75 | 31.46 | 59.83 | 82.4 | 4.68 | 0.89 | 12.03 | 17.61 | 6.85 |
| Coal Sample | Pore Volume/cm3·g−1 | Pore Throat Radius/μm | Pore Sorting | Pore Connectivity | |||
|---|---|---|---|---|---|---|---|
| Sp | Skp | Pd/MPa | SHG/% | WE/% | |||
| Vitrain | 0.04154 | 3.18 | 4.98 | 1.42 | 0.03 | 60.2 | 63.88 |
| Durain | 0.03038 | 4.83 | 3.39 | −1.37 | 0.02 | 98.53 | 36.56 |
| Coal Sample | SSA/m2·g−1 | TPV/cm3·g−1 | APZ/nm | MTV/cm3·g−1 | MPPZ/nm |
|---|---|---|---|---|---|
| Vitrain | 22.922 | 0.0257 | 4.4841 | 0.0074 | 1.09676 |
| Durain | 15.668 | 0.0194 | 4.9394 | 0.0052 | 1.02288 |
| Coal Sample | Coal–Water Interface Contact Angle/(°) | |||||
|---|---|---|---|---|---|---|
| Distilled Water | JFC | G502 | LAS | Stratum Water | 6501 | |
| Vitrain | 66.9° | 33.8° | 98.8° | 39.9° | 93.2° | 49.9° |
| Durain | 61.2° | 32.5° | 90.3° | 36.7° | 91.3° | 42.1° |
| Coal Sample | T/°C | Adsorption Process | Desorption Process | |||||
|---|---|---|---|---|---|---|---|---|
| VL/ cm3·g−1 | PL/ MPa | R2 | VL/ cm3·g−1 | PL/ MPa | c/ cm3·g−1 | R2 | ||
| Vitrain | 20 | 16.04 | 2.37 | 0.9988 | 13.49 | 2.75 | 2.61 | 0.9944 |
| 25 | 14.94 | 2.46 | 0.9989 | 13.03 | 2.34 | 1.40 | 0.9996 | |
| 30 | 13.92 | 2.60 | 0.9999 | 12.51 | 2.44 | 0.97 | 0.9993 | |
| 35 | 12.98 | 2.73 | 0.9988 | 11.52 | 2.74 | 1.14 | 0.9996 | |
| 40 | 11.87 | 2.51 | 0.9995 | 10.09 | 2.91 | 1.69 | 0.9990 | |
| Durain | 20 | 12.18 | 2.33 | 0.9999 | 8.68 | 1.99 | 2.42 | 0.9973 |
| 25 | 11.50 | 2.50 | 0.9999 | 8.58 | 2.65 | 2.30 | 0.9993 | |
| 30 | 11.00 | 2.63 | 0.9999 | 8.20 | 2.36 | 1.97 | 0.9977 | |
| 35 | 10.50 | 2.70 | 0.9999 | 7.78 | 3.23 | 2.31 | 0.9988 | |
| 40 | 9.76 | 2.55 | 1.0000 | 7.13 | 3.94 | 2.67 | 0.9980 | |
| Coal Sample | Temperature/°C | A | B | R2 |
|---|---|---|---|---|
| Vitrain | 20 | 5.6289 | 5.7064 | 0.9977 |
| 25 | 6.1218 | 5.7096 | 0.9974 | |
| 30 | 6.5486 | 5.7765 | 0.9995 | |
| 35 | 7.0420 | 5.8226 | 0.9959 | |
| 40 | 7.7446 | 5.7296 | 0.9877 | |
| Durain | 20 | 7.3758 | 5.6974 | 0.9995 |
| 25 | 7.9973 | 5.7063 | 0.9995 | |
| 30 | 8.3284 | 5.7702 | 0.9997 | |
| 35 | 8.7543 | 5.7979 | 0.9994 | |
| 40 | 9.4074 | 5.7441 | 0.9996 |
| Adsorbing Capacity/mmol·g−1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
|---|---|---|---|---|---|---|
| Adsorption heat/kJ·mol−1 | Vitrain | 14.88 | 25.80 | 36.72 | 47.65 | 58.57 |
| Durain | 25.79 | 29.40 | 33.01 | 36.62 | 40.23 | |
| Coal Sample | Temperature/°C | A | B | R2 |
|---|---|---|---|---|
| Vitrain | 20 | 6.4847 | 5.1573 | 0.9944 |
| 25 | 6.9706 | 5.2496 | 0.9995 | |
| 30 | 7.2328 | 5.4274 | 0.9988 | |
| 35 | 7.9271 | 5.4237 | 0.9999 | |
| 40 | 8.8738 | 5.2608 | 0.9996 | |
| Durain | 20 | 10.536 | 4.3749 | 0.9965 |
| 25 | 10.845 | 4.6156 | 0.9980 | |
| 30 | 11.130 | 4.7030 | 0.9961 | |
| 35 | 11.809 | 4.7496 | 0.9964 | |
| 40 | 12.940 | 4.6052 | 0.9936 |
| Adsorbing Capacity/mmol·g−1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
|---|---|---|---|---|---|---|
| Adsorption heat/kJ·mol−1 | Vitrain | 19.27 | 35.57 | 51.87 | 68.16 | 84.46 |
| Durain | 46.73 | 48.02 | 49.30 | 50.59 | 51.87 | |
| qst/kJ·mol−1 | Adsorption Process | Desorption Process | Desorption Heat Supplement | ||||
|---|---|---|---|---|---|---|---|
| Adsorption/mmol·g−1 | Vitrain | Durain | Vitrain | Durain | Vitrain | Durain | |
| 0.1 | −14.877 | −25.793 | 19.270 | 46.734 | 4.393 | 20.941 | |
| 0.2 | −25.800 | −29.402 | 35.568 | 48.018 | 9.768 | 18.616 | |
| 0.3 | −36.723 | −33.011 | 51.866 | 49.302 | 15.143 | 16.291 | |
| 0.4 | −47.645 | −36.620 | 68.164 | 50.585 | 20.519 | 13.965 | |
| 0.5 | −58.568 | −40.229 | 84.462 | 51.869 | 25.894 | 11.648 | |
| Coal Sample | Adsorption Process | Desorption Process | |||||
|---|---|---|---|---|---|---|---|
| VL/cm3·g−1 | PL/MPa | R2 | VL/cm3·g−1 | PL/MPa | c/cm3·g−1 | R2 | |
| Air dry basis | 16.511 | 6.536 | 0.999 | 13.835 | 5.376 | 1.014 | 0.992 |
| Equilibrium water | 15.462 | 3.344 | 0.996 | 13.832 | 2.101 | 0.138 | 0.997 |
| G502 | 26.984 | 4.444 | 0.994 | 22.745 | 6.944 | 5.569 | 0.997 |
| 6501 | 12.459 | 2.841 | 0.999 | 8.905 | 2.604 | 2.790 | 0.984 |
| JFC | 11.516 | 2.577 | 0.992 | 9.431 | 3.546 | 2.423 | 0.994 |
| LAS | 5.626 | 2.020 | 0.985 | 4.273 | 1.764 | 1.204 | 0.983 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.; Chen, Y.; Wang, L.; Ma, D.; Li, G.; Li, W.; Zheng, C.; Ji, Y.; Ma, Z.; Hui, P.; et al. Mechanism of Methane Adsorption/Desorption in Low-Rank Vitrain and Durain Coal Affected by Pore Structure and Wettability: A Case Study in Dafosi Area, South Ordos Basin, China. Energies 2022, 15, 5094. https://doi.org/10.3390/en15145094
Peng T, Chen Y, Wang L, Ma D, Li G, Li W, Zheng C, Ji Y, Ma Z, Hui P, et al. Mechanism of Methane Adsorption/Desorption in Low-Rank Vitrain and Durain Coal Affected by Pore Structure and Wettability: A Case Study in Dafosi Area, South Ordos Basin, China. Energies. 2022; 15(14):5094. https://doi.org/10.3390/en15145094
Chicago/Turabian StylePeng, Tao, Yue Chen, Liya Wang, Dongmin Ma, Guofu Li, Weibo Li, Chao Zheng, Yusong Ji, Zhuoyuan Ma, Peng Hui, and et al. 2022. "Mechanism of Methane Adsorption/Desorption in Low-Rank Vitrain and Durain Coal Affected by Pore Structure and Wettability: A Case Study in Dafosi Area, South Ordos Basin, China" Energies 15, no. 14: 5094. https://doi.org/10.3390/en15145094
APA StylePeng, T., Chen, Y., Wang, L., Ma, D., Li, G., Li, W., Zheng, C., Ji, Y., Ma, Z., Hui, P., & Wang, X. (2022). Mechanism of Methane Adsorption/Desorption in Low-Rank Vitrain and Durain Coal Affected by Pore Structure and Wettability: A Case Study in Dafosi Area, South Ordos Basin, China. Energies, 15(14), 5094. https://doi.org/10.3390/en15145094






