Incorporation of Manganese (II) in Beta-Tricalcium Phosphate from EPR and ENDOR Measurements for Powders
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
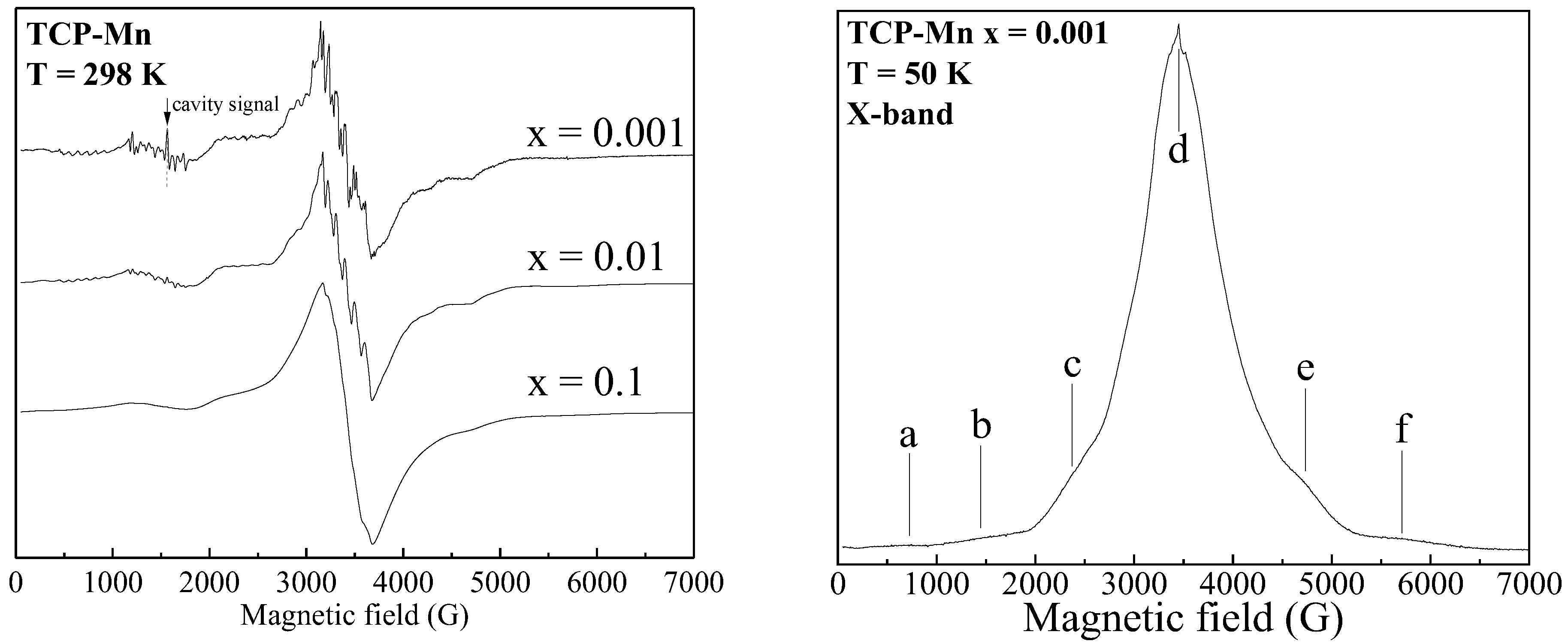
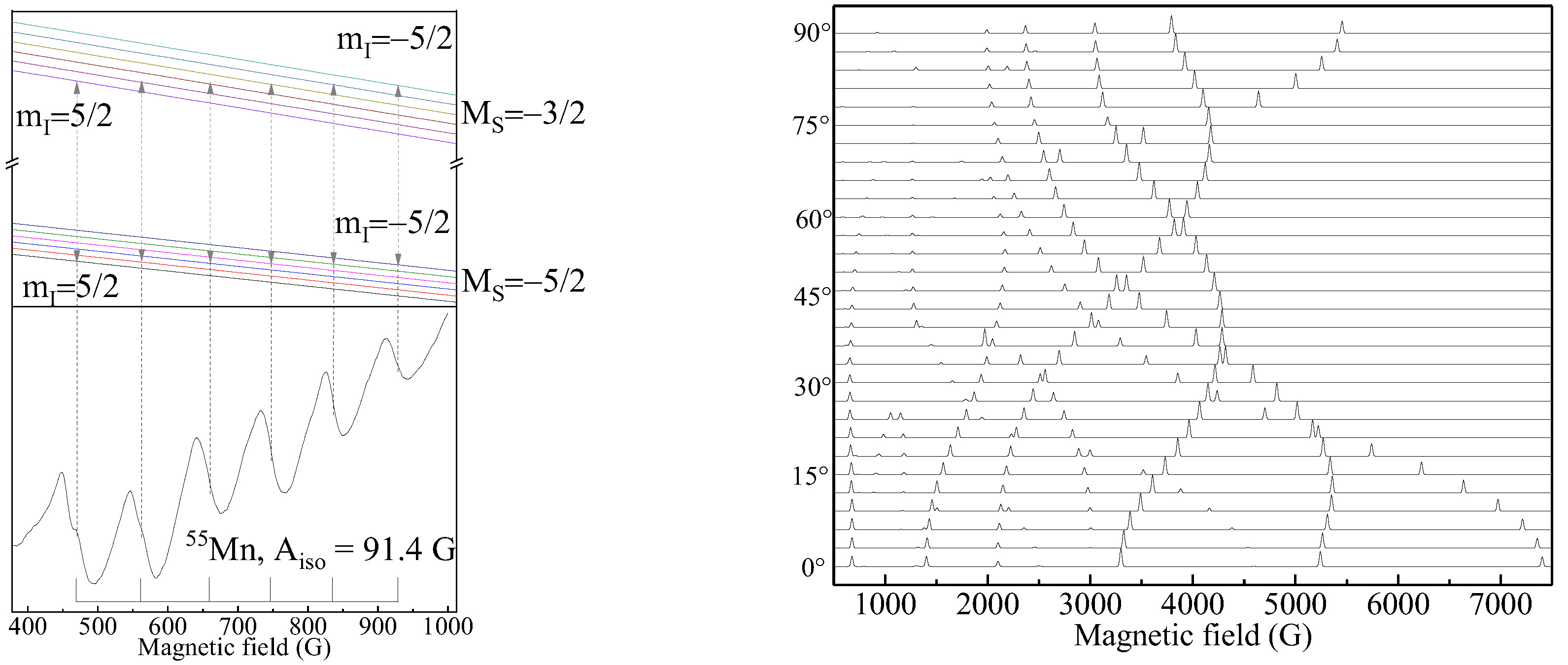
3.1. X-Band Measurements
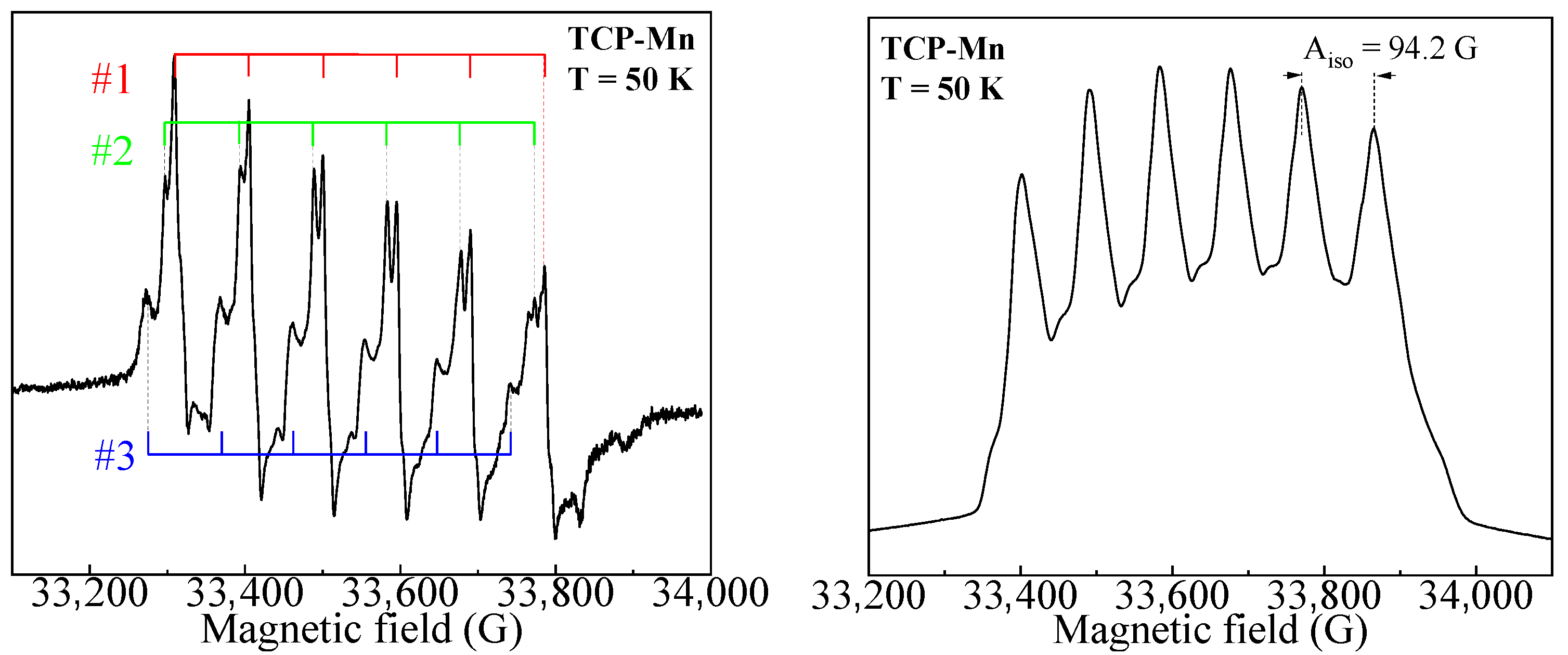
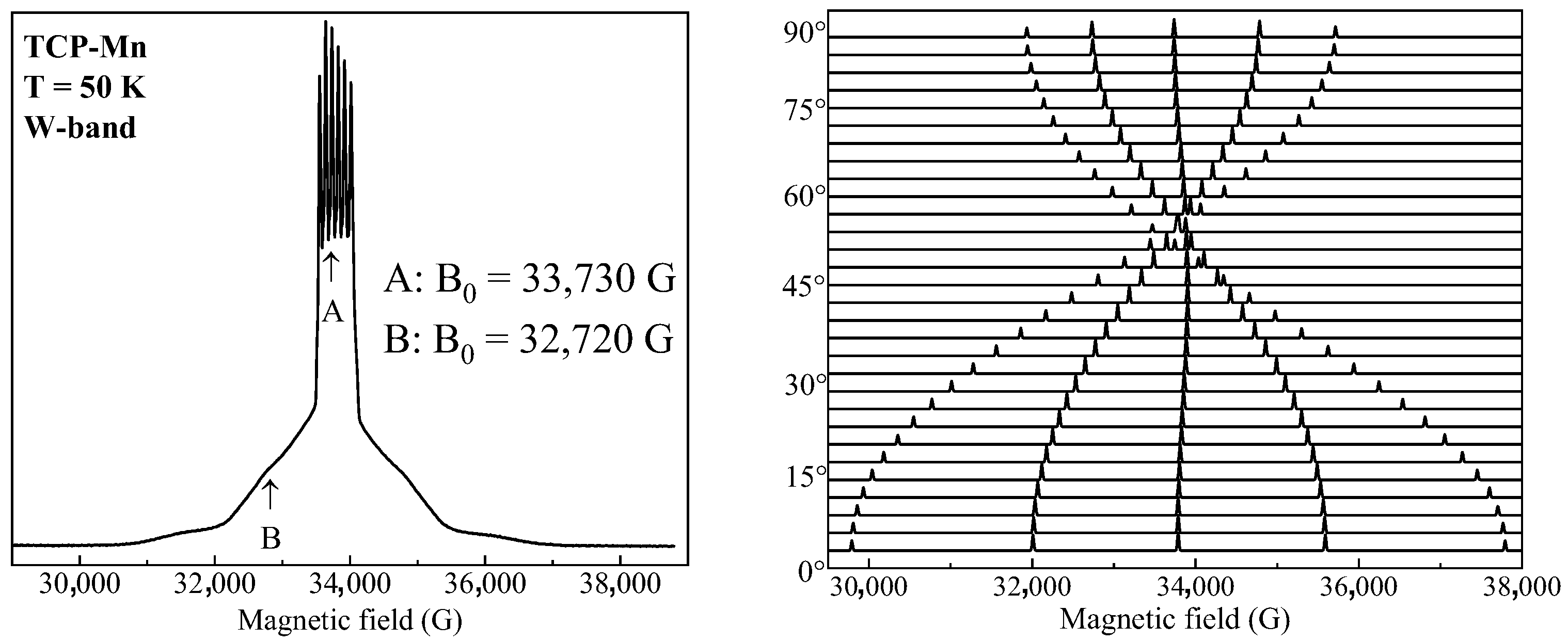
3.2. High Frequency EPR Spectroscopy
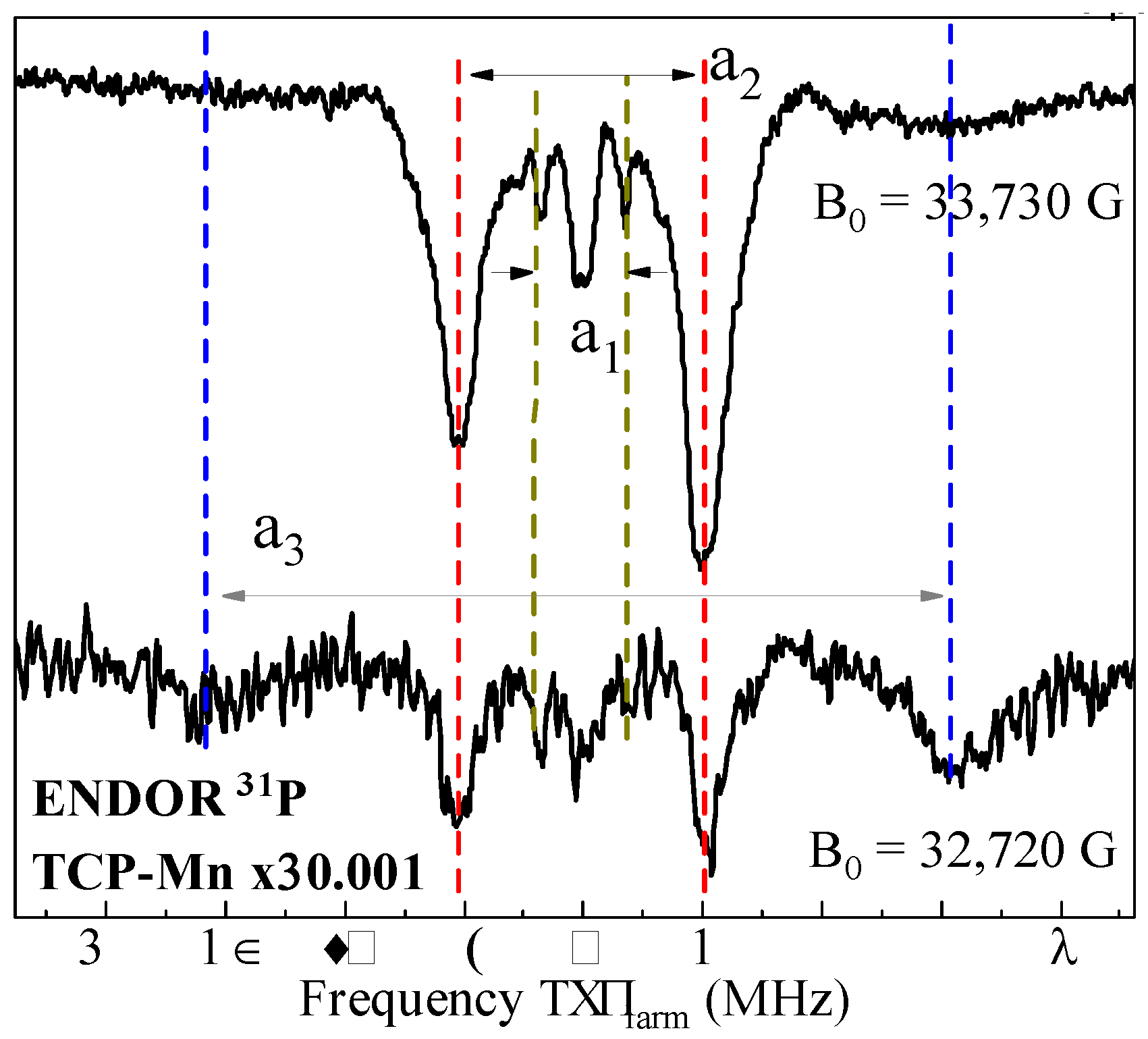
3.3. ENDOR Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makshakova, O.N.; Shurtakova, D.V.; Vakhin, A.V.; Grishin, P.O.; Gafurov, M.R. Incorporation of iron(II) and (III) in hydroxyapatite—A theoretical study. Crystals 2021, 11, 1219. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent advances in catalytic transfer hydrogenation with formic acid over heterogeneous transition metal catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Kosinov, N.; Liu, C.; Hensen, E.J.M.; Pidko, E.A. Engineering of transition metal catalysts confined in zeolites. Chem. Mater. 2018, 30, 3177–3198. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y.; et al. The role of nanodispersed catalysts in microwave application during the development of unconventional hydrocarbon reserves: A review of potential applications. Processes 2021, 9, 420. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Cuenya, B.R. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef] [PubMed]
- van Vreeswijk, S.H.; Weckhuysen, B.M. Emerging analytical methods to characterize zeolite-based materials. Natl. Sci. Rev. 2022, nwac047. [Google Scholar] [CrossRef]
- Saraev, V.V.; Shmidt, F.K. EPR for catalysts based on nickel and cobalt complexes. J. Mol. Catal. A Chem. 2000, 158, 149–154. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, L.; Yu, H.; Khan, A.; Haq, F.; Zhu, L. Recent progress in preparation of branched polyethylene with nickel, titanium, vanadium and chromium catalytic systems and EPR study of related catalytic systems. Eur. Polym. J. 2019, 121, 109339. [Google Scholar] [CrossRef]
- Van Doorslaer, S.; Murphy, D.M. EPR spectroscopy in catalysis. Top. Curr. Chem. 2011, 321, 1–39. [Google Scholar] [CrossRef]
- Morra, E.; Giamello, E.; Chiesa, M. EPR approaches to heterogeneous catalysis. The chemistry of titanium in heterogeneous catalysts and photocatalysts. J. Magn. Reson. 2017, 280, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Dyrek, K.; Che, M. EPR as a tool to investigate the transition metal chemistry on oxide surfaces. Chem. Rev. 1997, 97, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Brustolon, M.; Giamello, E. Electron Paramagnetic Resonance: A Practitioners Toolkit; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Mukhambetov, I.N.; Lamberov, A.A.; Yavkin, B.V.; Gafurov, M.R.; Mamin, G.V.; Orlinskii, S.B. Electron paramagnetic resonance and electron nuclear double resonance study of the paramagnetic complexes of anthraquinone on the surface of γ-Al2O3. J. Phys. Chem. C 2014, 118, 14998–15003. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Synthetic Amorphous Calcium Phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef] [PubMed]
- Kalinnikova, E.; Sadovnikova, M.; Rodionov, A.; Murzakhanov, F.; Grishin, P. Analysis of the osseointegration process of dental implants by electron paramagnetic resonance: An in vivo study. Dent. J. 2022, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.M.C.; Marote, A.; Cerqueira, A.R.; Calado, A.J.; Abrantes, J.C.C.; Olhero, S.; Da Cruz e Silva, O.A.B.; Vieira, S.I.; Ferreira, J.M.F. Injectable MnSr-doped brushite bone cements with improved biological performance. J. Mater. Chem. B 2017, 5, 2775–2787. [Google Scholar] [CrossRef]
- Monma, H. Catalytic behavior of calcium phosphates for decompositions of 2-propanol and ethanol. J. Catal. 1982, 75, 200–203. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Akopyan, A.V.; Gafurov, M.R.; Makshakova, O.N.; Donskaya, N.O.; Fomin, A.S.; Polikarpova, P.P.; Anisimov, A.V.; Murzakhanov, F.F.; Leonov, A.V.; et al. Iron-doped mesoporous powders of hydroxyapatite as molybdenum-impregnated catalysts for deep oxidative desulfurization of model fuel: Synthesis and experimental and theoretical studies. J. Phys. Chem. C 2021, 125, 11604–11619. [Google Scholar] [CrossRef]
- Mota, G.; do Carmo, J.V.C.; Paz, C.B.; Saraiva, G.D.; Campos, A.; Duarte, G.; da Silva Filho, E.C.; Oliveira, A.C.; Soares, J.M.; Rodríguez-Castellón, E.; et al. Influence of the metal incorporation into hydroxyapatites on the deactivation behavior of the solids in the esterification of glycerol. Catalysts 2022, 12, 10. [Google Scholar] [CrossRef]
- Kozlowski, J.T.; Davis, R.J. Heterogeneous catalysts for the guerbet coupling of alcohols. ACS Catal. 2013, 3, 1588–1600. [Google Scholar] [CrossRef]
- Benarafa, A.; Kacimi, M.; Coudurier, G.; Ziyad, M. Characterisation of the active sites in butan-2-ol dehydrogenation over calcium–copper and calcium–sodium–copper phosphates. Appl. Catal. A Gen. 2000, 196, 25–35. [Google Scholar] [CrossRef]
- Dikhtyar, Y.Y.; Spassky, D.A.; Morozov, V.A.; Deyneko, D.V.; Belik, A.A.; Baryshnikova, O.V.; Nikiforov, I.V.; Lazoryak, B.I. Site occupancy, luminescence and dielectric properties of β-Ca3(PO4)2-type Ca8ZnLn(PO4)7 host materials. J. Alloys Compd. 2022, 908, 164521. [Google Scholar] [CrossRef]
- Bessière, A.; Benhamou, R.A.; Wallez, G.; Lecointre, A.; Viana, B. Site occupancy and mechanisms of thermally stimulated luminescence in Ca9Ln(PO4)7 (Ln = lanthanide). Acta Mater. 2012, 60, 6641–6649. [Google Scholar] [CrossRef]
- Mayer, I.; Cohen, S.; Gdalya, S.; Burghaus, O.; Reinen, D. Crystal structure and EPR study of Mn-doped β-tricalcium phosphate. Mater. Res. Bull. 2008, 43, 447–452. [Google Scholar] [CrossRef]
- Sinusaite, L.; Renner, A.M.; Schütz, M.B.; Antuzevics, A.; Rogulis, U.; Grigoraviciute-Puroniene, I.; Mathur, S.; Zarkov, A. Effect of Mn doping on the low-temperature synthesis of Tricalcium Phosphate (TCP) polymorphs. J. Eur. Ceram. Soc. 2019, 39, 3257–3263. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Sharifullin, A.V.; Vakhin, A.V. Differential scanning calorimetric study of heavy oil catalytic oxidation in the presence of manganese tallates. Pet. Sci. Technol. 2019, 37, 1194–1200. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Nuns, N.; Giraudon, J.-M.; Lamonier, J.-F. Cu−Mn hydroxyapatite materials for toluene total oxidation. ChemCatChem 2020, 12, 550–560. [Google Scholar] [CrossRef]
- Chlala, D.; Griboval-Constant, A.; Nuns, N.; Giraudon, J.-M.; Labaki, M.; Lamonier, J.-F. Effect of Mn loading onto hydroxyapatite supported Mn catalysts for toluene removal: Contribution of PCA assisted ToF-SIMS. Catal. Today 2018, 307, 41–47. [Google Scholar] [CrossRef]
- Galukhin, A.; Khelkhal, M.A.; Gerasimov, A.; Biktagirov, T.; Gafurov, M.; Rodionov, A.; Orlinskii, S. Mn-catalyzed oxidation of heavy oil in porous media: Kinetics and some aspects of the mechanism. Energy Fuels 2016, 30, 7731–7737. [Google Scholar] [CrossRef]
- Sinusaite, L.; Antuzevics, A.; Popov, A.I.; Rogulis, U.; Misevicius, M.; Katelnikovas, A.; Kareiva, A.; Zarkov, A. Synthesis and luminescent properties of Mn-doped alpha-tricalcium phosphate. Ceram. Int. 2021, 47, 5335–5340. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Kalita, V.I.; Komlev, D.I.; Radiuk, A.A.; Fomin, A.S.; Davidova, G.A.; Fursova, N.K.; Murzakhanov, F.F.; Gafurov, M.R.; Fosca, M.; et al. In vitro properties of manganese-substituted tricalcium phosphate coatings for titanium biomedical implants deposited by arc plasma. Materials 2020, 13, 4411. [Google Scholar] [CrossRef]
- Shurtakova, D.V.; Grishin, P.O.; Gafurov, M.R.; Mamin, G.V. Using DFT to calculate the parameters of the crystal field in Mn2+ doped hydroxyapatite crystals. Crystals 2021, 11, 1050. [Google Scholar] [CrossRef]
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Klimashina, E.; Putlayev, V.; Kuznetsova, L.; Orlinskii, S. The interplay of manganese and nitrate in hydroxyapatite nanoparticles as revealed by pulsed EPR and DFT. Phys. Chem. Chem. Phys. 2015, 17, 20331–20337. [Google Scholar] [CrossRef] [PubMed]
- Murzakhanov, F.; Mamin, G.V.; Orlinskii, S.; Goldberg, M.; Petrakova, N.V.; Fedotov, A.Y.; Grishin, P.; Gafurov, M.R.; Komlev, V.S. Study of electron–nuclear interactions in doped calcium phosphates by various pulsed EPR spectroscopy techniques. ACS Omega 2021, 6, 25338–25349. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Mamin, G.V.; Goldberg, M.A.; Knotko, A.V.; Gafurov, M.R.; Orlinskii, S.B. EPR of radiation-induced nitrogen centers in hydroxyapatite: New approaches to the study of electron-nuclear interactions. Russ. J. Coord. Chem. 2020, 46, 729–737. [Google Scholar] [CrossRef]
- Goldfarb, D.; Stoll, S. EPR Spectroscopy: Fundamentals and Methods; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yamane, T.; Sugisaki, K.; Matsuoka, H.; Sato, K.; Toyota, K.; Shiomi, D.; Takui, T. ESR analyses of picket fence MnII and 6th ligand coordinated FeIII porphyrins (S = 5/2) and a CoII(Hfac) complex (S = 3/2) with sizable ZFS parameters revisited: A full spin Hamiltonian approach and quantum chemical calculations. Dalton Trans. 2018, 47, 16429–16444. [Google Scholar] [CrossRef] [Green Version]
- Rau, J.V.; Fadeeva, I.V.; Fomin, A.S.; Barbaro, K.; Galvano, E.; Ryzhov, A.P.; Murzakhanov, F.; Gafurov, M.; Orlinskii, S.; Antoniac, I.; et al. Sic Parvis Magna: Manganese-substituted tricalcium phosphate and its biophysical properties. ACS Biomater. Sci. Eng. 2019, 5, 6632–6644. [Google Scholar] [CrossRef]
- Gabbasov, B.; Gafurov, M.; Starshova, A.; Shurtakova, D.; Murzakhanov, F.; Mamin, G.; Orlinskii, S. Conventional, pulsed and high-field electron paramagnetic resonance for studying metal impurities in calcium phosphates of biogenic and synthetic origins. J. Magn. Magn. Mater. 2018, 470, 109–117. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Deyneko, D.V.; Barbaro, K.; Davydova, G.A.; Sadovnikova, M.A.; Murzakhanov, F.F.; Fomin, A.S.; Yankova, V.G.; Antoniac, I.V.; Barinov, S.M.; et al. Influence of synthesis conditions on gadolinium-substituted tricalcium phosphate ceramics and its physicochemical, biological, and antibacterial properties. Nanomaterials 2022, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]


| −904 MHz | −1.41 MHz | 195.2 MHz |
| g-Factor | Aiso (G) | Linewidth (G) | T1 (μs) | T2 (μs) |
|---|---|---|---|---|
| 2.0040(5) | 95.4(2) | 9(1) | 97(1) | 1.51(3) |
| 2.0055(5) | 95.2(2) | 12(1) | 101(1) | 1.49(3) |
| 2.0080(5) | 93.7(3) | 18(2) | 109(2) | 1.5(3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzakhanov, F.F.; Forysenkova, A.A.; Fadeeva, I.V.; Mamin, G.V.; Gafurov, M.R. Incorporation of Manganese (II) in Beta-Tricalcium Phosphate from EPR and ENDOR Measurements for Powders. Ceramics 2022, 5, 318-329. https://doi.org/10.3390/ceramics5030025
Murzakhanov FF, Forysenkova AA, Fadeeva IV, Mamin GV, Gafurov MR. Incorporation of Manganese (II) in Beta-Tricalcium Phosphate from EPR and ENDOR Measurements for Powders. Ceramics. 2022; 5(3):318-329. https://doi.org/10.3390/ceramics5030025
Chicago/Turabian StyleMurzakhanov, Fadis F., Anna A. Forysenkova, Inna V. Fadeeva, Georgy V. Mamin, and Marat R. Gafurov. 2022. "Incorporation of Manganese (II) in Beta-Tricalcium Phosphate from EPR and ENDOR Measurements for Powders" Ceramics 5, no. 3: 318-329. https://doi.org/10.3390/ceramics5030025








