Tug-of-War in the Selection of Materials for Battery Technologies
Abstract
:1. Introduction
2. Battery Specifications
2.1. Electrical Parameters
2.2. Battery Types
- Rechargeable or Non-Rechargeable
- Monovalent and Multivalent
- Organic and Inorganic
- Flow Batteries
- Rigid Batteries or Flexible Batteries
2.3. Sustainability Factors
3. Anodes
3.1. Anodes for Lithium-Based Batteries
3.2. Anodes for Sodium-Based Batteries
3.3. Anodes for Potassium-Based Batteries
3.4. Anodes for Zinc-Based Batteries
3.5. Anodes for Calcium-Based Batteries
3.6. Anodes for Magnesium-Based Batteries
3.7. Anodes for Aluminum-Based Batteries
4. Cathodes
- Transition metal compounds, oxides, or complex oxides have olivine (1D), layered (2D), or spinel (3D) crystal structures [8,204]. Olivine crystal structures have 1D tunnels to allow ions to flow, causing lower rate capability. Reducing the size of the active material is a strategy to address this issue. Layered oxides have a general formula ABO, where A represents the ion carrier such as Li, Na, K, Zn, Ca, Al, and Mg, and B represents one or more metal ions such as Ni, Co, Fe, Mn, and Cu. Spinel oxides have a general formula ABO, where A represents the ion carrier such as Li, Na, K, Zn, Ca, Al, and Mg, and B can be Ti, V, and Mn [9,205]. The layered and spinel oxides offer good electronic conductivity and high densities.
- Polyanionic compounds have a general formula ABB’(XO), where A represents one ion carrier, Li, Na, K, Zn, Ca, Al, or Mg; B could be V, Ti, Fe, Tr, Al, or Nb; and X is P or S. Polyanionic compounds offer higher thermal stability and safety than the layered and spinel oxide cathodes due to the covalent bond between the oxygen and the P, S, or Si. Moreover, polyanionic cathodes include abundant transition metals, such as Fe, which contributed to their applications in storage devices for renewable energy sources. The use of polyanionic compounds requires synthesized small particles with coated conductive carbon due to the poor electronic conductivity, increasing the cost, reducing the volumetric energy density, and leading to low performance [9].
- Prussian blue analogues (PBA) have a general formula ABB(CN). A is usually Li, Na, K, Zn, Ca, Mg, or Al, while B and B can be Fe, Mn, Ni, Co, or Cu. The use of PBA as an electrode is due to two structural characteristics: (1) large 3D diffusion channels that facilitate its inward and outward transport by the weak interaction with the diffusing ion, and (2) control of the [B(CN)] vacancies that improve the crystallinity by changing the stoichiometry and the preparation conditions. Moreover, PBA has a high theoretical specific capacity, a simple synthesis, and a low cost [206].
4.1. Cathodes for Lithium-Based Batteries
4.2. Cathodes for Sodium-Based Batteries
4.3. Cathodes for Potassium-Based Batteries
4.4. Cathodes for Zinc-Based Batteries
4.5. Cathodes for Calcium-Based Batteries
4.6. Cathodes for Magnesium-Based Batteries
4.7. Cathodes for Aluminum-Based Batteries
5. Electrolyte and Separator
- Chemical inertness toward inactive and active battery components.
- Thermal stability with low melting and high boiling temperatures.
- Electrochemical stability window.
- High ionic conductivity and no electronic conductivity.
- Environmental friendliness and nontoxicity.
- Sustainable chemistry.
- Simple synthesis, preparation, and scaling processes.
- Tunable interphase property on both electrodes.
| Additive | Boiling Point (K) | Density (g cm) |
|---|---|---|
| TEPa | 488.15 | 1.072 |
| TMPa | 453.15 | 1.197 |
| TFPa | 355.15 | 1.594 |
| DMMPb | 453.15 | 1.145 |
| DMMEMPc | 553.15 | |
| MFEa | 333.15 | 1.529 |
| MFAa | 358.15 | 1.272 |
| Polymer | S cm | Ceramic | S cm |
|---|---|---|---|
| PEO | 10−8 to 10−6 | LISICON | 10−5 to 10−3 |
| PMMA | 10−4 to 10−5 | NASICON | 10−5 to 10−3 |
| PAN | Garnet | 10−5 to 10−3 | |
| PVdF | Perovskite | 10−5 to 10−3 | |
| PVdF-HFP | Sulfide | 10−7 to 10−3 | |
| PVdF-TrFE | LiPON | 10−6 | |
| PPO | |||
| PVA | |||
| PAM | |||
| PNA | |||
| PAA | |||
| PNIPAM |
5.1. Lithium Batteries
5.2. Sodium Batteries
5.3. Potassium Batteries
5.4. Zinc Batteries
5.5. Calcium Batteries
5.6. Magnesium Batteries
5.7. Aluminum Batteries
6. Applications of Batteries
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Zhao, C.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.; Yu, D.; Liu, Y.; Titirici, M.; Chueh, Y.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Nadeem, F.; Hussain, S.M.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative review of energy storage systems, their roles, and impacts on future power systems. IEEE Access 2019, 7, 4555–4585. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Fan, X.; Liu, X.; Hu, W.; Zhong, C.; Lu, J. Advances in the development of power supplies for the Internet of Everything. InfoMat 2019, 1, 130–139. [Google Scholar] [CrossRef]
- Raj, A.; Steingart, D. Review—Power Sources for the Internet of Things. J. Electrochem. Soc. 2018, 165, B3130–B3136. [Google Scholar] [CrossRef]
- Salama, M.; Rosy.; Attias, R.; Yemini, R.; Gofer, Y.; Aurbach, D.; Noked, M. Metal-Sulfur Batteries: Overview and Research Methods. ACS Energy Lett. 2019, 4, 436–446. [Google Scholar] [CrossRef]
- Mao, M.; Gao, T.; Hou, S.; Wang, C. A critical review of cathodes for rechargeable Mg batteries. Chem. Soc. Rev. 2018, 47, 8804–8841. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Mukherjee, S.; Singh, G. Two-Dimensional Anode Materials for Non-lithium Metal-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 932–955. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.B.; Huang, J.Q.; Kaskel, S.; Chou, S.; Park, H.S.; Zhang, Q. Alloy Anodes for Rechargeable Alkali-Metal Batteries: Progress and Challenge. ACS Mater. Lett. 2019, 1, 217–229. [Google Scholar] [CrossRef]
- Puthusseri, D.; Wahid, M.; Ogale, S. Conversion-type Anode Materials for Alkali-Ion Batteries: State of the Art and Possible Research Directions. ACS Omega 2018, 3, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hwang, J.; Kaushik, S.; Chen, C.Y.; Hagiwara, R. Advances in sodium secondary batteries utilizing ionic liquid electrolytes. Energy Environ. Sci. 2019, 12, 3247–3287. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Yin, D.; Du, Y. Electrolytes for Batteries with Earth-Abundant Metal Anodes. Chem. A Eur. J. 2018, 24, 18220–18234. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.J.; Geiger, S.; Falusi, S.; Roth, M. Material Review of Li Ion Battery Separators; American Institute of Physics: College Park, MD, USA, 2014; Volume 1597, pp. 66–81. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.H.; Kim, J.H.; Lee, S.Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- “Engineering and Technology History Wiki”. Milestones: Volta’s Electrical Battery Invention, 1799. 1999. Available online: https://ethw.org/ (accessed on 30 January 2022).
- Cadex Electronics Inc. When Was the Battery Invented? Cadex Electronics Inc.: Richmond, BC, Canada, 2019; Available online: https://batteryuniversity.com/learn/ (accessed on 30 January 2022).
- Viswanathan, B. Energy Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–313. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Bondarev, D.; Singhal, V.; Yushin, G. Ten years left to redesign lithium-ion batteries. Nature 2018, 14, 467–470. [Google Scholar] [CrossRef]
- Bernhart, W. Recycling of Lithium-Ion Batteries in the Context of Technology and Price Developments. ATZelectronics Worldw. 2019, 14, 38–43. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Lei, Y. Recent Research Progress of Anode Materials for Potassium-ion Batteries. Energy Environ. Mater. 2020, 3, 105–120. [Google Scholar] [CrossRef]
- Yao, Z.; Hegde, V.I.; Aspuru-Guzik, A.; Wolverton, C. Discovery of Calcium-Metal Alloy Anodes for Reversible Ca-Ion Batteries. Adv. Energy Mater. 2019, 9, 1802994. [Google Scholar] [CrossRef]
- Zhao, S.; Qin, B.; Chan, K.; Li, C.V.; Li, F. Recent Development of Aprotic Na-O2 Batteries. Batter. Supercaps 2019, 2, 725–742. [Google Scholar] [CrossRef]
- Lewis, D. The COVID pandemic has harmed researcher productivity—And mental health. Nature 2021. [Google Scholar] [CrossRef]
- Holland, A.; He, X. Advanced Li-Ion and beyond Lithium Batteries 2022–2032: Technologies, Players, Trends, Markets; Technical Report; IDTechEx: Cambridge, UK, 2021. [Google Scholar]
- Zhu, J.; Zhu, P.; Yan, C.; Dong, X.; Zhang, X. Recent progress in polymer materials for advanced lithium-sulfur batteries. Prog. Polym. Sci. 2019, 90, 118–163. [Google Scholar] [CrossRef]
- Xu, X.L.; Wang, S.J.; Wang, H.; Xu, B.; Hu, C.; Jin, Y.; Liu, J.B.; Yan, H. The suppression of lithium dendrite growth in lithium sulfur batteries: A review. J. Energy Storage 2017, 13, 387–400. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Jiao, M.; Xie, Z.; Zhou, Z. Understanding Rechargeable Li-O2 Batteries via First-Principles Computations. Batter. Supercaps 2019, 2, 498–508. [Google Scholar] [CrossRef]
- Huang, J.; Peng, Z. Understanding the Reaction Interface in Lithium-Oxygen Batteries. Energy Environ. 2019, 2, 37–48. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, J.; Jung, J.W.; Park, J.; Jang, T.; Kim, H.S.; Nam, J.S.; Lim, H.; Yoon, K.R.; Ryu, W.H.; et al. Lithium—Air Batteries: Air-Breathing Challenges and Perspective. ACS Nano 2020, 14, 14549–14578. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Peters, J.; Peña Cruz, A.; Weil, M. Exploring the Economic Potential of Sodium-Ion Batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef]
- Kumar, D.; Kuhar, S.B.; Kanchan, D. Room temperature sodium-sulfur batteries as emerging energy source. J. Energy Storage 2018, 18, 133–148. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.K.; Myung, S.T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, H.; Fan, W.; Zhong, C.; Hu, W.; Mitlin, D. Review of Emerging Potassium–Sulfur Batteries. Adv. Mater. 2020, 32, 1908007. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Ren, W.; Zhang, D.; Yang, Y.; Yang, J.; Wang, J.; Zeng, X.; NuLi, Y. Challenges and prospects of Mg-air batteries: A review. Energy Mater. 2022, 2, 200024. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium Metal Anodes: Emerging Solutions to Dendrite Growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Xiao, Y.; Abbasi, N.M.; Zhu, Y.; Li, S.; Tan, S.; Ling, W.; Peng, L.; Yang, T.; Wang, L.; Guo, X.; et al. Layered Oxide Cathodes Promoted by Structure Modulation Technology for Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 2001334. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Electric VehicleTeam. A Guide to Understanding Battery Specifications; Electric VehicleTeam: Cambridge, MA, USA, 2008. [Google Scholar]
- Farahani, S. ZigBee Wireless Networks and Transceivers; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 6; pp. 207–224. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M.R. Multivalent rechargeable batteries. Energy Storage Mater. 2019, 20, 253–262. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Das, S.K.; Archer, L.A. The rechargeable aluminum-ion battery. Chem. Commun. 2011, 47, 12610. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a calcium-based rechargeable battery. Nat. Mater. 2016, 15, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Dong, Y.; Li, S.; Zhou, J.; Wang, L.; Wang, B. MOFs and COFs for Batteries and Supercapacitors. Electrochem. Energy Rev. 2020, 3, 81–126. [Google Scholar] [CrossRef]
- Poizot, P.; Dolhem, F.; Gaubicher, J. Progress in all-organic rechargeable batteries using cationic and anionic configurations: Toward low-cost and greener storage solutions? Curr. Opin. Electrochem. 2018, 9, 70–80. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 1804975. [Google Scholar] [CrossRef]
- Lindahl, N.; Bitenc, J.; Dominko, R.; Johansson, P. Aluminum Metal—Organic Batteries with Integrated 3D Thin Film Anodes. Adv. Funct. Mater. 2020, 30, 3–9. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, P.; Song, M. Metal-Organic Frameworks for High-Energy Lithium Batteries with Enhanced Safety: Recent Progress and Future Perspectives. Batter. Supercaps 2019, 2, 591–626. [Google Scholar] [CrossRef]
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Hu, J.K.; Huang, J.Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef] [PubMed]
- Isidor Buchmann. Types of Battery Cells. 2019. Available online: https://batteryuniversity.com/article/bu-301a-types-of-battery-cells (accessed on 30 January 2022).
- Copyright Epec, L. Prismatic and Pouch Battery Packs. 2021. Available online: https://www.epectec.com/batteries/prismatic-pouch-packs.html (accessed on 30 January 2022).
- Xiaoxi, H. Flexible, Printed and Thin Film Batteries 2020–2030: Technologies, Markets and Players; Technical Report; IDTechEx: Cambridge, UK, 2020. [Google Scholar]
- Song, W.; Yoo, S.; Song, G.; Lee, S.; Kong, M.; Rim, J.; Jeong, U.; Park, S. Recent Progress in Stretchable Batteries for Wearable Electronics. Batter. Supercaps 2019, 2, 181–199. [Google Scholar] [CrossRef]
- Praveen, S.; Santhoshkumar, P.; Joe, Y.C.; Senthil, C.; Lee, C.W. 3D-printed architecture of Li-ion batteries and its applications to smart wearable electronic devices. Appl. Mater. Today 2020, 20, 100688. [Google Scholar] [CrossRef]
- Qian, G.; Liao, X.; Zhu, Y.; Pan, F.; Chen, X.; Yang, Y. Designing Flexible Lithium-Ion Batteries by Structural Engineering. ACS Energy Lett. 2019, 4, 690–701. [Google Scholar] [CrossRef]
- Qu, S.; Liu, B.; Wu, J.; Zhao, Z.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Kirigami-Inspired Flexible and Stretchable Zinc-Air Battery Based on Metal-Coated Sponge Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 54833–54841. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Tervoort, E.; Kraus, T.; Niederberger, M. Design and Fabrication of Transparent and Stretchable Zinc Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 6166–6179. [Google Scholar] [CrossRef]
- Guo, Z.H.; Liu, M.; Cong, Z.; Guo, W.; Zhang, P.; Hu, W.; Pu, X. Stretchable Textile Rechargeable Zn Batteries Enabled by a Wax Dyeing Method. Adv. Mater. Technol. 2020, 5, 2000544. [Google Scholar] [CrossRef]
- Fu, W.; Turcheniuk, K.; Naumov, O.; Mysyk, R.; Wang, F.; Liu, M.; Kim, D.; Ren, X.; Magasinski, A.; Yu, M.; et al. Materials and technologies for multifunctional, flexible or integrated supercapacitors and batteries. Mater. Today 2021, 48, 1. [Google Scholar] [CrossRef]
- Peng, J.; Jeffrey Snyder, G. A figure of merit for flexibility. Science 2019, 366, 690–691. [Google Scholar] [CrossRef]
- Kong, L.; Tang, C.; Peng, H.; Huang, J.; Zhang, Q. Advanced energy materials for flexible batteries in energy storage: A review. SmartMat 2020, 1. [Google Scholar] [CrossRef]
- Song, J.; Yan, W.; Cao, H.; Song, Q.; Ding, H.; Lv, Z.; Zhang, Y.; Sun, Z. Material flow analysis on critical raw materials of lithium-ion batteries in China. J. Clean. Prod. 2019, 215, 570–581. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.; Tharumalingam, E.; Dusseault, M.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.B.; Jin, Z.; Zhang, R.; Wang, G.; Chen, L.Q.; Liu, Q.B.; Huang, J.Q.; Zhang, Q. Recent advances in understanding dendrite growth on alkali metal anodes. EnergyChem 2019, 1, 100003. [Google Scholar] [CrossRef]
- Chang, H.; Wu, Y.R.; Han, X.; Yi, T.F. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater. 2021, 1, 24. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, Q.; Zhu, Y.; Chen, Y.; Fu, L.; Liu, L.; Yu, N.; Wu, Y.; van Ree, T. In Pursuit of a Dendrite-Free Electrolyte/Electrode Interface on Lithium Metal Anodes: A Minireview. Energy Fuels 2020, 34, 10503–10512. [Google Scholar] [CrossRef]
- Xiao, X.; Yao, W.; Tang, J.; Liu, C.; Lian, R.; Urbankowski, P.; Anayee, M.; He, S.; Li, J.; Wang, H.; et al. Interconnected Two-dimensional Arrays of Niobium Nitride Nanocrystals as Stable Lithium Host. Batter. Supercaps 2021, 4, 106–111. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.Y.; Li, J.; Zou, Y.L.; Tang, J.J. Study of nano-porous hard carbons as anode materials for lithium ion batteries. Mater. Chem. Phys. 2012, 135, 445–450. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Zhang, Y.; Shen, F.; Yang, G.; Wang, L.; Zhang, X.; He, Y.; Luo, L.; Deng, S. Ultrafine layered graphite as an anode material for lithium ion batteries. Mater. Lett. 2018, 229, 134–137. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous Doped Silicon Nanowires for Lithium Ion Battery Anode with Long Cycle Life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, Q.; Guan, Z.; Zhao, Q.; Sun, N.; Xu, B. A Flexible Si@C Electrode with Excellent Stability Employing an MXene as a Multifunctional Binder for Lithium-Ion Batteries. ChemSusChem 2020, 13, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Liu, X.C.; Ke, F.S.; Zhou, X.D. Porous germanium enabled high areal capacity anode for lithium-ion batteries. Compos. Part B Eng. 2019, 163, 158–164. [Google Scholar] [CrossRef]
- Xiao, X.; Li, X.; Zheng, S.; Shao, J.; Xue, H.; Pang, H. Nanostructured Germanium Anode Materials for Advanced Rechargeable Batteries. Adv. Mater. Interfaces 2017, 4, 1600798. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Grishanov, D.A.; Yu, D.Y.W.; Gun, J.; Sladkevich, S.; Lev, O.; Prikhodchenko, P.V. GeO2 Thin Film Deposition on Graphene Oxide by the Hydrogen Peroxide Route: Evaluation for Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2017, 9, 9152–9160. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, Y.; Ding, F.; Sang, L.; Mai, Y. An artificial Li-Al interphase layer on Li-B alloy for stable lithium-metal anode. Electrochim. Acta 2019, 304, 255–262. [Google Scholar] [CrossRef]
- Kong, L.L.; Wang, L.; Ni, Z.C.; Liu, S.; Li, G.R.; Gao, X.P. Lithium—Magnesium Alloy as a Stable Anode for Lithium–Sulfur Battery. Adv. Funct. Mater. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on Advanced Materials for Li-ion Batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Coelho, J.; Pokle, A.; Park, S.H.; McEvoy, N.; Berner, N.C.; Duesberg, G.S.; Nicolosi, V. Lithium Titanate/Carbon Nanotubes Composites Processed by Ultrasound Irradiation as Anodes for Lithium Ion Batteries. Sci. Rep. 2017, 7, 7614. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.K.; Amine, K. Titanium-Based Anode Materials for Safe Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 959–969. [Google Scholar] [CrossRef]
- Trang, N.T.H.; Ali, Z.; Kang, D.J. Mesoporous TiO2 spheres interconnected by multiwalled carbon nanotubes as an anode for high-performance lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 3676–3683. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Lei, Z.; Sun, K.; Rooney, D. Facile Synthesis of Anatase TiO2 Quantum-Dot/Graphene-Nanosheet Composites with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Adv. Mater. 2014, 26, 2084–2088. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kim, J.C.; Seo, S.D.; Lee, S.; Lee, J.H.; Kim, D.W. A binder-free Ge-nanoparticle anode assembled on multiwalled carbon nanotube networks for Li-ion batteries. Chem. Commun. 2012, 48, 7061–7063. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liu, M.; Naik, D.; Gole, J.L. Electrochemical properties of Li–Mg alloy electrodes for lithium batteries. J. Power Sources 2001, 92, 70–80. [Google Scholar] [CrossRef]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Hallac, P.B.; Jiang, J.; Hurley, P.T.; Chen, J. Multilayered Si Nanoparticle/Reduced Graphene Oxide Hybrid as a High-Performance Lithium-Ion Battery Anode. Adv. Mater. 2014, 26, 758–764. [Google Scholar] [CrossRef]
- Huang, Y.H.; Bao, Q.; Chen, B.H.; Duh, J.G. Nano-to-microdesign of marimo-like carbon nanotubes supported frameworks via in-spaced polymerization for high performance silicon lithium ion battery anodes. Small 2015, 11, 2314–2322. [Google Scholar] [CrossRef]
- Qin, J.; He, C.; Zhao, N.; Wang, Z.; Shi, C.; Liu, E.Z.; Li, J. Graphene networks anchored with Sn@Graphene as lithium ion battery anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.J.; Guo, Y.G. Binding SnO2 Nanocrystals in Nitrogen-Doped Graphene Sheets as Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef]
- Meng, X.; He, K.; Su, D.; Zhang, X.; Sun, C.; Ren, Y.; Wang, H.H.; Weng, W.; Trahey, L.; Canlas, C.P.; et al. Gallium Sulfide-Single-Walled Carbon Nanotube Composites: High-Performance Anodes for Lithium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 5435–5442. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Dong, R.; Wu, F.; Bai, Y.; Wu, C. Sodium Storage Mechanism and Optimization Strategies for Hard Carbon Anode of Sodium Ion Batteries. Acta Chim. Sin. 2021, 79, 1461–1476. [Google Scholar] [CrossRef]
- Arie, A.A.; Tekin, B.; Demir, E.; Demir-Cakan, R. Hard carbons derived from waste tea bag powder as anodes for sodium ion battery. Mater. Technol. 2019, 34, 515–524. [Google Scholar] [CrossRef]
- Ding, C.; Huang, L.; Lan, J.; Yu, Y.; Zhong, W.H.; Yang, X. Superresilient Hard Carbon Nanofabrics for Sodium-Ion Batteries. Small 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Guerfi, A.; Kim, C.; Zaghib, K. Roles of ti in electrode materials for sodium-ion batteries. Front. Energy Res. 2019, 7, 28. [Google Scholar] [CrossRef]
- Bayhan, Z.; Huang, G.; Yin, J.; Xu, X.; Lei, Y.; Liu, Z.; Alshareef, H.N. Two-Dimensional TiO2/TiS2Hybrid Nanosheet Anodes for High-Rate Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 8721–8727. [Google Scholar] [CrossRef]
- Morito, H.; Yamada, T.; Ikeda, T.; Yamane, H. Na-Si binary phase diagram and solution growth of silicon crystals. J. Alloy. Compd. 2009, 480, 723–726. [Google Scholar] [CrossRef]
- Xu, Y.; Swaans, E.; Basak, S.; Zandbergen, H.W.; Borsa, D.M.; Mulder, F.M. Reversible na-ion uptake in Si nanoparticles. Adv. Energy Mater. 2016, 6, 1501436. [Google Scholar] [CrossRef]
- Tseng, K.W.; Huang, S.B.; Chang, W.C.; Tuan, H.Y. Synthesis of Mesoporous Germanium Phosphide Microspheres for High-Performance Lithium-Ion and Sodium-Ion Battery Anodes. Chem. Mater. 2018, 30, 4440–4447. [Google Scholar] [CrossRef]
- Sung, G.K.; Nam, K.H.; Choi, J.H.; Park, C.M. Germanium telluride: Layered high-performance anode for sodium-ion batteries. Electrochim. Acta 2020, 331, 135393. [Google Scholar] [CrossRef]
- Ni, J.; Li, L.; Lu, J. Phosphorus: An Anode of Choice for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1137–1144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Liu, X.; Chen, C.; Fan, L.Z.; Jiao, L. Red phosphorus nanoparticles embedded in porous N-doped carbon nanofibers as high-performance anode for sodium-ion batteries. Energy Storage Mater. 2017, 9, 170–178. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Z.; Li, W.; Gu, X.; Huang, L. Hybrid phosphorene/graphene nanocomposite as an anode material for Na-ion batteries: A first-principles study. J. Phys. D Appl. Phys. 2017, 50, 165501. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Zhong, X.; Yu, Y. Superior sodium storage in phosphorus@porous multichannel flexible freestanding carbon nanofibers. Energy Storage Mater. 2017, 9, 112–118. [Google Scholar] [CrossRef]
- Zhu, J.; He, Q.; Liu, Y.; Key, J.; Nie, S.; Wu, M.; Shen, P.K. Three-dimensional, hetero-structured, Cu3P@C nanosheets with excellent cycling stability as Na-ion battery anode material. J. Mater. Chem. A 2019, 7, 16999–17007. [Google Scholar] [CrossRef]
- Kaushik, S.; Matsumoto, K.; Sato, Y.; Hagiwara, R. Vanadium phosphide–phosphorus composite as a high-capacity negative electrode for sodium secondary batteries using an ionic liquid electrolyte. Electrochem. Commun. 2019, 102, 46–51. [Google Scholar] [CrossRef]
- Liu, D.; Huang, X.; Qu, D.; Zheng, D.; Wang, G.; Harris, J.; Si, J.; Ding, T.; Chen, J.; Qu, D. Confined phosphorus in carbon nanotube-backboned mesoporous carbon as superior anode material for sodium/potassium-ion batteries. Nano Energy 2018, 52, 1–10. [Google Scholar] [CrossRef]
- Ma, M.; Yao, Y.; Wu, Y.; Yu, Y. Progress and Prospects of Transition Metal Sulfides for Sodium Storage. Adv. Fiber Mater. 2020, 2, 314–337. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, W.; Gu, H.; Hong, Z.; Xiao, W.; Wang, F.; Li, W.; Gu, D. Versatile Preparation of Mesoporous Single-Layered Transition-Metal Sulfide/Carbon Composites for Enhanced Sodium Storage. Adv. Mater. 2022, 34. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, Y.U.; Chang, S.J.; Kim, B.H.; Choi, J.; Wang, J.; Zhang, D.; Braun, P.V.; Jin, H.J.; Kang, K. Crumpled graphene paper for high power sodium battery anode. Carbon 2016, 99, 658–664. [Google Scholar] [CrossRef]
- Wang, G.Z.; Feng, J.M.; Dong, L.; Li, X.F.; Li, D.J. Porous graphene anchored with Sb/SbOxas sodium-ion battery anode with enhanced reversible capacity and cycle performance. J. Alloy. Compd. 2017, 693, 141–149. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Li, X.; Liu, X.; Wang, H.; Bai, J. Multi-layer graphene assembled fibers with porous structure as anode materials for highly reversible lithium and sodium storage. Electrochim. Acta 2018, 259, 702–710. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, K.; Liu, S.; Song, H.; Zhou, J. Flexible Co0.85Se nanosheets/graphene composite film as binder-free anode with high Li- and Na-Ion storage performance. J. Alloy. Compd. 2018, 731, 714–722. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, B.; Liu, P.; Li, F.; Zheng, M.; Chen, M.; Shi, Y.; Zhou, H. Fe2O3 nanocrystals anchored onto graphene nanosheets as the anode material for low-cost sodium-ion batteries. Chem. Commun. 2014, 50, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, Z.; Gordin, M.L.; Hu, S.; Yi, R.; Tang, D.; Walter, T.; Regula, M.; Choi, D.; Li, X.; et al. Chemically bonded phosphorus/graphene hybrid as a high performance anode for sodium-ion batteries. Nano Lett. 2014, 14, 6329–6335. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, H.; Jiao, L.; Chen, C.; Cao, K.; Wang, Y.; Yuan, H. Exfoliated-SnS2 restacked on graphene as a high-capacity, high-rate, and long-cycle life anode for sodium ion batteries. Nanoscale 2015, 7, 1325–1332. [Google Scholar] [CrossRef]
- Li, S.; Cao, X.; Schmidt, C.N.; Xu, Q.; Uchaker, E.; Pei, Y.; Cao, G. TiNb2O7/graphene composites as high-rate anode materials for lithium/sodium ion batteries. J. Mater. Chem. A 2016, 4, 4242–4251. [Google Scholar] [CrossRef]
- Wang, H.G.; Wu, Z.; Meng, F.L.; Ma, D.L.; Huang, X.L.; Wang, L.M.; Zhang, X.B. Nitrogen-doped porous carbon nanosheets as low-cost, high-performance anode material for sodium-ion batteries. ChemSusChem 2013, 6, 56–60. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, X.; Guo, P.; Jaeger, F.; Wang, Z. Design of NaTi2(PO4)3 nanocrystals embedded in N-doped graphene sheets for sodium-ion battery anode with superior electrochemical performance. Ceram. Int. 2017, 43, 12338–12342. [Google Scholar] [CrossRef]
- Yue, X.; Huang, N.; Jiang, Z.; Tian, X.; Wang, Z.; Hao, X.; Jiang, Z.J. Nitrogen-rich graphene hollow microspheres as anode materials for sodium-ion batteries with super-high cycling and rate performance. Carbon 2018, 130, 574–583. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Q.; Yang, M.; Wang, Y.; Chen, T.; Chen, Q.; Zhu, X.; Xia, Q.; Li, S.; Xia, H. Highly doped graphene with multi-dopants for high-capacity and ultrastable sodium-ion batteries. Energy Storage Mater. 2018, 13, 134–141. [Google Scholar] [CrossRef]
- Sultana, I.; Rahman, M.M.; Ramireddy, T.; Chen, Y.; Glushenkov, A.M. High capacity potassium-ion battery anodes based on black phosphorus. J. Mater. Chem. A 2017, 5, 23506–23512. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, W.; Wang, H.; Qi, X.; Xing, Z.; Zhuang, Q.; Ju, Z. Enhanced capacity of chemically bonded phosphorus/carbon composite as an anode material for potassium-ion batteries. J. Power Sources 2018, 378, 460–467. [Google Scholar] [CrossRef]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew. Chem. Int. Ed. 2013, 52, 4633–4636. [Google Scholar] [CrossRef] [PubMed]
- Dahbi, M.; Yabuuchi, N.; Fukunishi, M.; Kubota, K.; Chihara, K.; Tokiwa, K.; Yu, X.F.; Ushiyama, H.; Yamashita, K.; Son, J.Y.; et al. Black Phosphorus as a High-Capacity, High-Capability Negative Electrode for Sodium-Ion Batteries: Investigation of the Electrode/Electrolyte Interface. Chem. Mater. 2016, 28, 1625–1635. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.; Liu, K.; Wang, X.; Mulder, F.M. High-Performance and Low-Cost Sodium-Ion Anode Based on a Facile Black Phosphorus-Carbon Nanocomposite. ChemElectroChem 2017, 4, 2140–2144. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Dou, S.; Wang, G. Ultrathin MoS2 Nanosheets as Anode Materials for Sodium-Ion Batteries with Superior Performance. Adv. Energy Mater. 2015, 5, 1401205. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, J.; Bong, S.; Jang, B.; Seong, K.D.; Piao, Y. Scalable Synthesis of Few-Layer MoS2 Incorporated into Hierarchical Porous Carbon Nanosheets for High-Performance Li- and Na-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2016, 8, 19456–19465. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef]
- Wang, H.; Lan, X.; Jiang, D.; Zhang, Y.; Zhong, H.; Zhang, Z.; Jiang, Y. Sodium storage and transport properties in pyrolysis synthesized MoSe2 nanoplates for high performance sodium-ion batteries. J. Power Sources 2015, 283, 187–194. [Google Scholar] [CrossRef]
- Share, K.; Lewis, J.; Oakes, L.; Carter, R.E.; Cohn, A.P.; Pint, C.L. Tungsten diselenide (WSe2) as a high capacity, low overpotential conversion electrode for sodium ion batteries. RSC Adv. 2015, 5, 101262–101267. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Fu, Y. Nanostructured WSe2/C composites as anode materials for sodium-ion batteries. RSC Adv. 2016, 6, 12726–12729. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef]
- Jian, Z.; Luo, W.; Ji, X. Carbon Electrodes for K-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Fei, H.; Zeng, G.; Ci, L.; Xi, B.; Xiong, S.; Feng, J. Commercial expanded graphite as a low–cost, long-cycling life anode for potassium—Ion batteries with conventional carbonate electrolyte. J. Power Sources 2018, 378, 66–72. [Google Scholar] [CrossRef]
- Jian, Z.; Xing, Z.; Bommier, C.; Li, Z.; Ji, X. Hard Carbon Microspheres: Potassium-Ion Anode Versus Sodium-Ion Anode. Adv. Energy Mater. 2016, 6, 1501874. [Google Scholar] [CrossRef]
- Kishore, B.; G, V.; Munichandraiah, N. K2Ti4O9: A Promising Anode Material for Potassium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2551–A2554. [Google Scholar] [CrossRef]
- Han, J.; Xu, M.; Niu, Y.; Li, G.N.; Wang, M.; Zhang, Y.; Jia, M.; Li, C.M. Exploration of K2Ti8O17 as an anode material for potassium-ion batteries. Chem. Commun. 2016, 52, 11274–11276. [Google Scholar] [CrossRef]
- Sha, M.; Liu, L.; Zhao, H.; Lei, Y. Anode materials for potassium-ion batteries: Current status and prospects. Carbon Energy 2020, 2, 350–369. [Google Scholar] [CrossRef]
- Sultana, I.; Rahman, M.M.; Mateti, S.; Ahmadabadi, V.G.; Glushenkov, A.M.; Chen, Y. K-ion and Na-ion storage performances of Co3O4–Fe2O3 nanoparticle-decorated super P carbon black prepared by a ball milling process. Nanoscale 2017, 9, 3646–3654. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, T.; Zheng, Y.; Zhang, Q.; Liu, Y.; Chen, J.; Liu, H.; Guo, Z. CoS Quantum Dot Nanoclusters for High-Energy Potassium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702634. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Niu, Y.; Bao, S.J.; Yu, Y.N.; Lu, S.Y.; Xu, M. Nanocubic KTi2(PO4)3 electrodes for potassium-ion batteries. Chem. Commun. 2016, 52, 11661–11664. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, Q.; McCulloch, W.D.; Wu, Y. MoS2 as a long-life host material for potassium ion intercalation. Nano Res. 2017, 10, 1313–1321. [Google Scholar] [CrossRef]
- Xie, K.; Yuan, K.; Li, X.; Lu, W.; Shen, C.; Liang, C.; Vajtai, R.; Ajayan, P.; Wei, B. Superior Potassium Ion Storage via Vertical MoS2 “Nano-Rose” with Expanded Interlayers on Graphene. Small 2017, 13, 1701471. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Robust self-supported anode by integrating Sb2S3 nanoparticles with S,N-codoped graphene to enhance K-storage performance. Sci. China Chem. 2017, 60, 1533–1539. [Google Scholar] [CrossRef]
- Lei, K.; Wang, C.; Liu, L.; Luo, Y.; Mu, C.; Li, F.; Chen, J. A Porous Network of Bismuth Used as the Anode Material for High-Energy-Density Potassium-Ion Batteries. Angew. Chem. 2018, 130, 4777–4781. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Ding, S.; Chen, C.; Zhang, M. Enhanced conductivity and properties of SnO2-graphene-carbon nanofibers for potassium-ion batteries by graphene modification. Mater. Lett. 2018, 219, 19–22. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, W.K.; Sencadas, V.; Guo, Z. Understanding High-Energy-Density Sn4P3 Anodes for Potassium-Ion Batteries. Joule 2018, 2, 1534–1547. [Google Scholar] [CrossRef]
- Verma, R.; Didwal, P.N.; Ki, H.S.; Cao, G.; Park, C.J. SnP3/Carbon Nanocomposite as an Anode Material for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 26976–26984. [Google Scholar] [CrossRef]
- Lakshmi, V.; Chen, Y.; Mikhaylov, A.A.; Medvedev, A.G.; Sultana, I.; Rahman, M.M.; Lev, O.; Prikhodchenko, P.V.; Glushenkov, A.M. Nanocrystalline SnS2 coated onto reduced graphene oxide: Demonstrating the feasibility of a non-graphitic anode with sulfide chemistry for potassium-ion batteries. Chem. Commun. 2017, 53, 8272–8275. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Dong, Y.; Wu, Z.S.; Zheng, S.; Wang, S.; Sun, C.; Qin, J.; Shi, X.; Bao, X. Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Naguib, M.; Adams, R.A.; Zhao, Y.; Zemlyanov, D.; Varma, A.; Nanda, J.; Pol, V.G. Electrochemical performance of MXenes as K-ion battery anodes. Chem. Commun. 2017, 53, 6883–6886. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, X.; Yu, H.; Zhao, G.; Shu, J.; Xu, X.; Sun, W.; Dou, S.X. Electrochemical potassium/lithium-ion intercalation into TiSe2: Kinetics and mechanism. Energy Storage Mater. 2019, 16, 512–518. [Google Scholar] [CrossRef]
- Yang, C.; Feng, J.; Lv, F.; Zhou, J.; Lin, C.; Wang, K.; Zhang, Y.; Yang, Y.; Wang, W.; Li, J.; et al. Metallic Graphene-Like VSe2 Ultrathin Nanosheets: Superior Potassium-Ion Storage and Their Working Mechanism. Adv. Mater. 2018, 30, 1800036. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, G.; Lim, K.; Kang, K. A comparative study of graphite electrodes using the co-intercalation phenomenon for rechargeable Li, Na and K batteries. Chem. Commun. 2016, 52, 12618–12621. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zou, X.; Zhu, Y.; Xu, Y.; Wang, C. Electrochemical Intercalation of Potassium into Graphite. Adv. Funct. Mater. 2016, 26, 8103–8110. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Xu, Z.; Lv, X.; Chen, J.; Jiang, L.; Lai, Y.; Li, J. Dispersion-corrected DFT investigation on defect chemistry and potassium migration in potassium-graphite intercalation compounds for potassium ion batteries anode materials. Carbon 2016, 107, 885–894. [Google Scholar] [CrossRef]
- Deng, Q.; Pei, J.; Fan, C.; Ma, J.; Cao, B.; Li, C.; Jin, Y.; Wang, L.; Li, J. Potassium salts of para-aromatic dicarboxylates as the highly efficient organic anodes for low-cost K-ion batteries. Nano Energy 2017, 33, 350–355. [Google Scholar] [CrossRef]
- Lei, K.; Li, F.; Mu, C.; Wang, J.; Zhao, Q.; Chen, C.; Chen, J. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes. Energy Environ. Sci. 2017, 10, 552–557. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y. Recent Progress on Zinc-Ion Rechargeable Batteries. Nano-Micro Lett. 2019, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.; Dongmo, S.; Janek, J.; Schröder, D. Benchmarking Anode Concepts: The Future of Electrically Rechargeable Zinc–Air Batteries. ACS Energy Lett. 2019, 4, 1287–1300. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Wu, Y.; Wang, Z.; Mathur, A.; Yang, H.; Chen, P.; Nair, S.; Liu, N. A Lasagna-Inspired Nanoscale ZnO Anode Design for High-Energy Rechargeable Aqueous Batteries. ACS Appl. Energy Mater. 2018, 1, 6345–6351. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Ma, Y.; Howe, J.D.; Yang, H.; Chen, P.; Aluri, S.; Liu, N. Ion-Sieving Carbon Nanoshells for Deeply Rechargeable Zn-Based Aqueous Batteries. Adv. Energy Mater. 2018, 8, 1802470. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Miyazaki, K.; Abe, T.; Janek, J.; Schröder, D. Towards zinc-oxygen batteries with enhanced cycling stability: The benefit of anion-exchange ionomer for zinc sponge anodes. J. Power Sources 2018, 395, 195–204. [Google Scholar] [CrossRef]
- Chamoun, M.; Hertzberg, B.J.; Gupta, T.; Davies, D.; Bhadra, S.; Van Tassell, B.; Erdonmez, C.; Steingart, D.A. Hyper-dendritic nanoporous zinc foam anodes. NPG Asia Mater. 2015, 7, e178. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, E.; Jiang, L.; Sun, G. Superior cycling stability and high rate capability of three-dimensional Zn/Cu foam electrodes for zinc-based alkaline batteries. RSC Adv. 2015, 5, 83781–83787. [Google Scholar] [CrossRef]
- Hwang, H.J.; Chi, W.S.; Kwon, O.; Lee, J.G.; Kim, J.H.; Shul, Y.G. Selective Ion Transporting Polymerized Ionic Liquid Membrane Separator for Enhancing Cycle Stability and Durability in Secondary Zinc-Air Battery Systems. ACS Appl. Mater. Interfaces 2016, 8, 26298–26308. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Feng, Z.; Xie, X.; Wen, X. A novel ZnO@Ag@Polypyrrole hybrid composite evaluated as anode material for zinc-based secondary cell. Sci. Rep. 2016, 6, 24471. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, B.; Park, M.S.; Kim, K. Improved reversibility of Zn anodes for rechargeable Zn-air batteries by using alkoxide and acetate ions. Electrochim. Acta 2016, 199, 164–171. [Google Scholar] [CrossRef]
- Kakeya, T.; Nakata, A.; Arai, H.; Ogumi, Z. Enhanced zinc electrode rechargeability in alkaline electrolytes containing hydrophilic organic materials with positive electrode compatibility. J. Power Sources 2018, 407, 180–184. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Z.; Wang, Y.; Li, H.; Zhang, J. In-situ growth of ZnO nanoplates on graphene for the application of high rate flexible quasi-solid-state Ni-Zn secondary battery. J. Power Sources 2018, 407, 137–146. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Damtew, D.; Stumpp, M.; Konovalova, A.; Henkensmeier, D.; Schlettwein, D.; Schröder, D. Design Strategy for Zinc Anodes with Enhanced Utilization and Retention: Electrodeposited Zinc Oxide on Carbon Mesh Protected by Ionomeric Layers. ACS Appl. Energy Mater. 2018, 1, 5579–5588. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-De Dompablo, M.E.; Ponrouch, A.; Johansson, P.; Palacín, M.R. Achievements, Challenges, and Prospects of Calcium Batteries. Chem. Rev. 2020, 120, 6331–6357. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, F.; Tang, Y. A Novel Calcium-Ion Battery Based on Dual-Carbon Configuration with High Working Voltage and Long Cycling Life. Adv. Sci. 2018, 5, 1701082. [Google Scholar] [CrossRef]
- Ponrouch, A.; Tchitchekova, D.; Frontera, C.; Bardé, F.; Dompablo, M.A.d.; Palacín, M. Assessing Si-based anodes for Ca-ion batteries: Electrochemical decalciation of CaSi2. Electrochem. Commun. 2016, 66, 75–78. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef]
- Adil, M.; Sarkar, A.; Roy, A.; Panda, M.R.; Nagendra, A.; Mitra, S. Practical Aqueous Calcium-Ion Battery Full-Cells for Future Stationary Storage. ACS Appl. Mater. Interfaces 2020, 12, 11489–11503. [Google Scholar] [CrossRef]
- Gheytani, S.; Liang, Y.; Wu, F.; Jing, Y.; Dong, H.; Rao, K.K.; Chi, X.; Fang, F.; Yao, Y. An Aqueous Ca-Ion Battery. Adv. Sci. 2017, 4, 1700465. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Yao, W.; Song, X.; Zhang, G.; Chen, B.; Yang, J.; Tang, Y. A Calcium-Ion Hybrid Energy Storage Device with High Capacity and Long Cycling Life under Room Temperature. Adv. Energy Mater. 2019, 9, 1803865. [Google Scholar] [CrossRef]
- Tsai, P.c.; Chung, S.C.; Lin, S.k.; Yamada, A. Ab initio study of sodium intercalation into disordered carbon. J. Mater. Chem. A 2015, 3, 9763–9768. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, I.A.; Yuan, Y.; Bommier, C.; Wang, X.; Ma, L.; Leonard, D.P.; Lerner, M.M.; Carter, R.G.; Wu, T.; Greaney, P.A.; et al. Mg-Ion Battery Electrode: An Organic Solid’s Herringbone Structure Squeezed upon Mg-Ion Insertion. J. Am. Chem. Soc. 2017, 139, 13031–13037. [Google Scholar] [CrossRef]
- Attias, R.; Salama, M.; Hirsch, B.; Goffer, Y.; Aurbach, D. Anode-Electrolyte Interfaces in Secondary Magnesium Batteries. Joule 2019, 3, 27–52. [Google Scholar] [CrossRef]
- Wu, N.; Lyu, Y.C.; Xiao, R.J.; Yu, X.; Yin, Y.X.; Yang, X.Q.; Li, H.; Gu, L.; Guo, Y.G. A highly reversible, low-strain Mg-ion insertion anode material for rechargeable Mg-ion batteries. NPG Asia Mater. 2014, 6, e120. [Google Scholar] [CrossRef]
- Meng, Z.; Foix, D.; Brun, N.; Dedryvère, R.; Stievano, L.; Morcrette, M.; Berthelot, R. Alloys to Replace Mg Anodes in Efficient and Practical Mg-Ion/Sulfur Batteries. ACS Energy Lett. 2019, 4, 2040–2044. [Google Scholar] [CrossRef]
- Arthur, T.S.; Singh, N.; Matsui, M. Electrodeposited Bi, Sb and Bi1-xSbx alloys as anodes for Mg-ion batteries. Electrochem. Commun. 2012, 16, 103–106. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, M.; Li, X.; Nie, Z.; Zuo, P.; Li, G.; Liu, T.; Xiao, J.; Cheng, Y.; Wang, C.; et al. Highly reversible Mg insertion in nanostructured Bi for Mg ion batteries. Nano Lett. 2014, 14, 255–260. [Google Scholar] [CrossRef]
- Cheng, Y.; Shao, Y.; Parent, L.R.; Sushko, M.L.; Li, G.; Sushko, P.V.; Browning, N.D.; Wang, C.; Liu, J. Interface Promoted Reversible Mg Insertion in Nanostructured Tin-Antimony Alloys. Adv. Mater. 2015, 27, 6598–6605. [Google Scholar] [CrossRef]
- Murgia, F.; Weldekidan, E.T.; Stievano, L.; Monconduit, L.; Berthelot, R. First investigation of indium-based electrode in Mg battery. Electrochem. Commun. 2015, 60, 56–59. [Google Scholar] [CrossRef]
- Faegh, E.; Ng, B.; Hayman, D.; Mustain, W.E. Practical assessment of the performance of aluminium battery technologies. Nat. Energy 2021, 6, 21–29. [Google Scholar] [CrossRef]
- Zhang, K.; Kirlikovali, K.O.; Suh, J.M.; Choi, J.W.; Jang, H.W.; Varma, R.S.; Farha, O.K.; Shokouhimehr, M. Recent Advances in Rechargeable Aluminum-Ion Batteries and Considerations for Their Future Progress. ACS Appl. Energy Mater. 2020, 3, 6019–6035. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Liu, M.; Rong, Z.; Malik, R.; Canepa, P.; Jain, A.; Ceder, G.; Persson, K.A. Spinel compounds as multivalent battery cathodes: A systematic evaluation based on ab initio calculations. Energy Environ. Sci. 2015, 8, 964–974. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian Blue Analogs for Rechargeable Batteries. iScience 2018, 3, 110–133. [Google Scholar] [CrossRef]
- Wang, H.F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Xing, W. High Energy Density Li-Ion Batteries with ALD Multi-Functional Modified LiCoO2 Cathode. ECS Trans. 2017, 80, 55–63. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, G.; Gu, C.; Ni, J. Revisiting polyanionic LiFePO4 battery material for electric vehicles. Funct. Mater. Lett. 2021, 14, 2130006. [Google Scholar] [CrossRef]
- Wang, B.; Liu, T.; Liu, A.; Liu, G.; Wang, L.; Gao, T.; Wang, D.; Zhao, X.S. A Hierarchical Porous C@LiFePO4/Carbon Nanotubes Microsphere Composite for High-Rate Lithium-Ion Batteries: Combined Experimental and Theoretical Study. Adv. Energy Mater. 2016, 6, 1600426. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, J.; Zhang, C. Mechanical Composite of LiNi0.8Co0.15Al0.05O2/Carbon Nanotubes with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 376. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Amin, R.; Jafta, C.; Zhang, J.; Liu, J.; Du, Z.; Meyer, H.M.; Self, E.; et al. Lithium Iron Aluminum Nickelate, LiNixFeyAlzO2 —New Sustainable Cathodes for Next-Generation Cobalt-Free Li-Ion Batteries. Adv. Mater. 2020, 32, 2002960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chang, L.; Ji, R. Controllable preparation of Fe-containing Li-rich cathode material Li[Li1/5Fe1/10Ni3/20Mn11/20]O2 with stable high-rate properties for Li-ion batteries. Funct. Mater. Lett. 2021, 14, 2150004. [Google Scholar] [CrossRef]
- Ould Ely, T.; Kamzabek, D.; Chakraborty, D.; Doherty, M.F. Lithium-Sulfur Batteries: State of the Art and Future Directions. ACS Appl. Energy Mater. 2018, 1, 1783–1814. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium-Ion and Lithium-Sulfur Batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Yuan, H.; Li, H.; Zhang, T.; Li, G.; He, T.; Du, F.; Feng, S. A K2Fe4O7 superionic conductor for all-solid-state potassium metal batteries. J. Mater. Chem. A 2018, 6, 8413–8418. [Google Scholar] [CrossRef]
- Sun, Q.; Lau, K.C.; Geng, D.; Meng, X. Atomic and Molecular Layer Deposition for Superior Lithium-Sulfur Batteries: Strategies, Performance, and Mechanisms. Batter. Supercaps 2018, 1, 41–68. [Google Scholar] [CrossRef]
- Deng, C.; Wang, Z.; Wang, S.; Yu, J. Inhibition of polysulfide diffusion in lithium-sulfur batteries: Mechanism and improvement strategies. J. Mater. Chem. A 2019, 7, 12381–12413. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Song, A.; Sun, G.; Shao, G. 3D interconnected porous carbon nanosheets/carbon nanotubes as a polysulfide reservoir for high performance lithium–sulfur batteries. Nanoscale 2018, 10, 816–824. [Google Scholar] [CrossRef]
- Gueon, D.; Hwang, J.T.; Yang, S.B.; Cho, E.; Sohn, K.; Yang, D.K.; Moon, J.H. Spherical Macroporous Carbon Nanotube Particles with Ultrahigh Sulfur Loading for Lithium—Sulfur Battery Cathodes. ACS Nano 2018, 12, 226–233. [Google Scholar] [CrossRef]
- Ummethala, R.; Fritzsche, M.; Jaumann, T.; Balach, J.; Oswald, S.; Nowak, R.; Sobczak, N.; Kaban, I.; Rümmeli, M.H.; Giebeler, L. Lightweight, free-standing 3D interconnected carbon nanotube foam as a flexible sulfur host for high performance lithium-sulfur battery cathodes. Energy Storage Mater. 2018, 10, 206–215. [Google Scholar] [CrossRef]
- Amin, K.; Meng, Q.; Ahmad, A.; Cheng, M.; Zhang, M.; Mao, L.; Lu, K.; Wei, Z. A Carbonyl Compound-Based Flexible Cathode with Superior Rate Performance and Cyclic Stability for Flexible Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1703868. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qi, J.; Xu, M.; Zhou, D.; Xiao, Z. Selective S/Li2S Conversion via in-Built Crystal Facet Self-Mediation: Toward High Volumetric Energy Density Lithium–Sulfur Batteries. ACS Nano 2020, 14, 15011–15022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Mu, X.; He, P.; Zhou, H. Advances and Challenges for Aprotic Lithium-Oxygen Batteries using Redox Mediators. Batter. Supercaps 2019, 2, 803–819. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, C.P.; Rui, X.H.; Cheng, T.; Chen, C.H. V2O3 modified LiFePO4/C composite with improved electrochemical performance. J. Power Sources 2011, 196, 5623–5630. [Google Scholar] [CrossRef]
- Wang, B.; Al Abdulla, W.; Wang, D.; Zhao, X.S. A three-dimensional porous LiFePO4 cathode material modified with a nitrogen-doped graphene aerogel for high-power lithium ion batteries. Energy Environ. Sci. 2015, 8, 869–875. [Google Scholar] [CrossRef]
- Fang, X.; Shen, C.; Ge, M.; Rong, J.; Liu, Y.; Zhang, A.; Wei, F.; Zhou, C. High-power lithium ion batteries based on flexible and light-weight cathode of LiNi0.5Mn1.5O4/carbon nanotube film. Nano Energy 2015, 12, 43–51. [Google Scholar] [CrossRef]
- Babu, G.; Kalaiselvi, N.; Bhuvaneswari, D. Synthesis and surface modification of LiCo1/3Ni1/3Mn1/3O2 for lithium battery applications. J. Electron. Mater. 2014, 43, 1062–1070. [Google Scholar] [CrossRef]
- Kong, D.; Li, X.; Zhang, Y.; Hai, X.; Wang, B.; Qiu, X.; Song, Q.; Yang, Q.H.; Zhi, L. Encapsulating V2O4 into carbon nanotubes enables the synthesis of flexible high-performance lithium ion batteries. Energy Environ. Sci. 2016, 9, 906–911. [Google Scholar] [CrossRef]
- Kim, J.H.; Fu, K.; Choi, J.; Sun, S.; Kim, J.; Hu, L.; Paik, U. Hydroxylated carbon nanotube enhanced sulfur cathodes for improved electrochemical performance of lithium-sulfur batteries. Chem. Commun. 2015, 51, 13682–13685. [Google Scholar] [CrossRef]
- Carter, R.; Davis, B.; Oakes, L.; Maschmann, M.R.; Pint, C.L. A high areal capacity lithium–sulfur battery cathode prepared by site-selective vapor infiltration of hierarchical carbon nanotube arrays. Nanoscale 2017, 9, 15018–15026. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Cheng, Z.; Xiao, Z.; Li, X.; Wang, R. The Fusion of Imidazolium-Based Ionic Polymer and Carbon Nanotubes: One Type of New Heteroatom-Doped Carbon Precursors for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1703936. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Li, X.; Adams, F.; Luo, W.; Huang, Y.; Hu, L. Electrode Materials of Sodium-Ion Batteries toward Practical Application. ACS Energy Lett. 2018, 3, 1604–1612. [Google Scholar] [CrossRef]
- Martinez De Ilarduya, J.; Otaegui, L.; López del Amo, J.M.; Armand, M.; Singh, G. NaN3 addition, a strategy to overcome the problem of sodium deficiency in P2-Na0.67[Fe0.5Mn0.5]O2 cathode for sodium-ion battery. J. Power Sources 2017, 337, 197–203. [Google Scholar] [CrossRef]
- Wang, P.F.; You, Y.; Yin, Y.X.; Guo, Y.G. An O3-type NaNi0.5Mn0.5O2 cathode for sodium-ion batteries with improved rate performance and cycling stability. J. Mater. Chem. A 2016, 4, 17660–17664. [Google Scholar] [CrossRef]
- Wang, P.F.; Yao, H.R.; Liu, X.Y.; Yin, Y.X.; Zhang, J.N.; Wen, Y.; Yu, X.; Gu, L.; Guo, Y.G. Na+/vacancy disordering promises high-rate Na-ion batteries. Sci. Adv. 2018, 4, eaar6018. [Google Scholar] [CrossRef]
- Manzi, J.; Paolone, A.; Palumbo, O.; Corona, D.; Massaro, A.; Cavaliere, R.; Muñoz-García, A.B.; Trequattrini, F.; Pavone, M.; Brutti, S. Monoclinic and Orthorhombic NaMnO2 for Secondary Batteries: A Comparative Study. Energies 2021, 14, 1230. [Google Scholar] [CrossRef]
- Song, T.; Yao, W.; Kiadkhunthod, P.; Zheng, Y.; Wu, N.; Zhou, X.; Tunmee, S.; Sattayaporn, S.; Tang, Y. A Low-Cost and Environmentally Friendly Mixed Polyanionic Cathode for Sodium-Ion Storage. Angew. Chem. 2020, 132, 750–755. [Google Scholar] [CrossRef]
- Xie, B.; Sun, B.; Gao, T.; Ma, Y.; Yin, G.; Zuo, P. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478. [Google Scholar] [CrossRef]
- Hu, P.; Peng, W.; Wang, B.; Xiao, D.; Ahuja, U.; Réthoré, J.; Aifantis, K.E. Concentration-Gradient Prussian Blue Cathodes for Na-Ion Batteries. ACS Energy Lett. 2020, 5, 100–108. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Huang, Y.; Ou, C.; Yuan, X.; Yan, L.; Li, W.; Zhang, H.; Shen, P.K. General Strategy To Synthesize Highly Dense Metal Oxide Quantum Dots-Anchored Nitrogen-Rich Graphene Compact Monoliths To Enable Fast and High-Stability Volumetric Lithium/Sodium Storage. ACS Appl. Energy Mater. 2019, 2, 3500–3512. [Google Scholar] [CrossRef]
- Mao, Q.; Gao, R.; Li, Q.; Ning, D.; Zhou, D.; Schuck, G.; Schumacher, G.; Hao, Y.; Liu, X. O3-type NaNi0.5Mn0.5O2 hollow microbars with exposed {0 1 0} facets as high performance cathode materials for sodium-ion batteries. Chem. Eng. J. 2020, 382, 122978. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, F.; Lu, Y.; Chen, L.; Hu, Y. High-Entropy Layered Oxide Cathodes for Sodium-Ion Batteries. Angew. Chem. 2020, 132, 270–275. [Google Scholar] [CrossRef]
- Wang, P.F.; Yao, H.R.; Liu, X.Y.; Zhang, J.N.; Gu, L.; Yu, X.Q.; Yin, Y.X.; Guo, Y.G. Ti-Substituted NaNi0.5Mn0.5−xTixO2 Cathodes with Reversible O3-P3 Phase Transition for High-Performance Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700210. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Guo, Y.J.; Duan, H.; Zuo, T.T.; Hu, E.; Attenkofer, K.; Li, H.; Zhao, X.S.; Yin, Y.X.; Yu, X.; et al. Honeycomb-Ordered Na3Ni1.5M0.5BiO6 (M = Ni, Cu, Mg, Zn) as High-Voltage Layered Cathodes for Sodium-Ion Batteries. ACS Energy Lett. 2017, 2, 2715–2722. [Google Scholar] [CrossRef]
- Talaie, E.; Duffort, V.; Smith, H.L.; Fultz, B.; Nazar, L.F. Structure of the high voltage phase of layered P2-Na2/3-z [Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its stability. Energy Environ. Sci. 2015, 8, 2512–2523. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. A Practical High-Energy Cathode for Sodium-Ion Batteries Based on Uniform P2-Na0.7CoO2 Microspheres. Angew. Chem. 2017, 129, 5895–5899. [Google Scholar] [CrossRef]
- Mu, L.; Xu, S.; Li, Y.; Hu, Y.S.; Li, H.; Chen, L.; Huang, X. Prototype Sodium-Ion Batteries Using an Air-Stable and Co/Ni-Free O3-Layered Metal Oxide Cathode. Adv. Mater. 2015, 27, 6928–6933. [Google Scholar] [CrossRef]
- Wang, H.; Liao, X.Z.; Yang, Y.; Yan, X.; He, Y.S.; Ma, Z.F. Large-Scale Synthesis of NaNi1/3Fe1/3Mn1/3O2 as High Performance Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2016, 163, A565–A570. [Google Scholar] [CrossRef]
- Wang, P.F.; You, Y.; Yin, Y.X.; Wang, Y.S.; Wan, L.J.; Gu, L.; Guo, Y.G. Suppressing the P2-O2 Phase Transition of Na0.67Mn0.67Ni0.33O2 by Magnesium Substitution for Improved Sodium-Ion Batteries. Angew. Chem. 2016, 128, 7571–7575. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Qiao, R.; Wray, L.A.; Hossain, M.A.; Chuang, Y.D.; Yang, W.; Lu, Y.; Evans, D.; Lee, J.J.; et al. Rhombohedral Prussian White as Cathode for Rechargeable Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, M.; Xiao, P.; Wang, Y.; Ceder, G. Additional Sodium Insertion into Polyanionic Cathodes for Higher-Energy Na-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700514. [Google Scholar] [CrossRef]
- Carter, R.; Oakes, L.; Douglas, A.; Muralidharan, N.; Cohn, A.P.; Pint, C.L. A Sugar-Derived Room-Temperature Sodium Sulfur Battery with Long Term Cycling Stability. Nano Lett. 2017, 17, 1863–1869. [Google Scholar] [CrossRef]
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Chou, S.; Davey, K.; Dou, S.X.; Qiao, S.Z. Long-Life Room-Temperature Sodium–Sulfur Batteries by Virtue of Transition-Metal-Nanocluster—Sulfur Interactions. Angew. Chem. Int. Ed. 2019, 58, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.C.; Bo, S.; Shi, T.; Kwon, D.; Ceder, G. K-Ion Batteries Based on a P2-Type K0.6CoO2 Cathode. Adv. Energy Mater. 2017, 7, 1700098. [Google Scholar] [CrossRef]
- Vaalma, C.; Giffin, G.A.; Buchholz, D.; Passerini, S. Non-Aqueous K-Ion Battery Based on Layered K0.3MnO2 and Hard Carbon/Carbon Black. J. Electrochem. Soc. 2016, 163, A1295–A1299. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.; Kim, J.C.; Bo, S.; Liu, L.; Shi, T.; Ceder, G. Investigation of Potassium Storage in Layered P3-Type K0.5MnO2 Cathode. Adv. Mater. 2017, 29, 1702480. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Meng, J.; Xia, F.; Wu, J.; Liu, F.; Zhang, X.; Xu, L.; Lin, X.; Mai, L. K+modulated K+/vacancy disordered layered oxide for high-rate and high-capacity potassium-ion batteries. Energy Environ. Sci. 2020, 13, 3129–3137. [Google Scholar] [CrossRef]
- Chihara, K.; Katogi, A.; Kubota, K.; Komaba, S. KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries. Chem. Commun. 2017, 53, 5208–5211. [Google Scholar] [CrossRef]
- Park, W.B.; Han, S.C.; Park, C.; Hong, S.U.; Han, U.; Singh, S.P.; Jung, Y.H.; Ahn, D.; Sohn, K.S.; Pyo, M. KVP2O7 as a Robust High-Energy Cathode for Potassium-Ion Batteries: Pinpointed by a Full Screening of the Inorganic Registry under Specific Search Conditions. Adv. Energy Mater. 2018, 8, 1703099. [Google Scholar] [CrossRef]
- Lin, X.; Huang, J.; Tan, H.; Huang, J.; Zhang, B. K3V2(PO4)2F3 as a robust cathode for potassium-ion batteries. Energy Storage Mater. 2019, 16, 97–101. [Google Scholar] [CrossRef]
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. A novel K-ion battery: Hexacyanoferrate(ii)/graphite cell. J. Mater. Chem. A 2017, 5, 4325–4330. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Gao, H.; Zhou, W.; Lü, X.; Kaveevivitchai, W.; Manthiram, A.; Goodenough, J.B. Low-Cost High-Energy Potassium Cathode. J. Am. Chem. Soc. 2017, 139, 2164–2167. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Sun, P.; Xu, Y. Sodium sulfonate groups substituted anthraquinone as an organic cathode for potassium batteries. Electrochem. Commun. 2018, 86, 34–37. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Lu, Y.; Li, Y.; Liang, G.; Chen, J. Oxocarbon Salts for Fast Rechargeable Batteries. Angew. Chem. Int. Ed. 2016, 55, 12528–12532. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Zhang, S.; Zhou, T.; Mao, J.; Guo, Z. Cathode Materials for Potassium-Ion Batteries: Current Status and Perspective. Electrochem. Energy Rev. 2018, 1, 625–658. [Google Scholar] [CrossRef]
- Jian, Z.; Liang, Y.; Rodríguez-Pérez, I.A.; Yao, Y.; Ji, X. Poly(anthraquinonyl sulfide) cathode for potassium-ion batteries. Electrochem. Commun. 2016, 71, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, W.; Wang, J.; Zhang, Y.; Zhu, Y.; Chen, Y.; Fu, L.; Wu, Y. Sulfur nanocomposite as a positive electrode material for rechargeable potassium—Sulfur batteries. Chem. Commun. 2018, 54, 2288–2291. [Google Scholar] [CrossRef]
- Ma, R.; Fan, L.; Wang, J.; Lu, B. Confined and covalent sulfur for stable room temperature potassium-sulfur battery. Electrochim. Acta 2019, 293, 191–198. [Google Scholar] [CrossRef]
- Xiong, P.; Han, X.; Zhao, X.; Bai, P.; Liu, Y.; Sun, J.; Xu, Y. Room-Temperature Potassium–Sulfur Batteries Enabled by Microporous Carbon Stabilized Small-Molecule Sulfur Cathodes. ACS Nano 2019, 13, 2536–2543. [Google Scholar] [CrossRef]
- Sada, K.; Senthilkumar, B.; Barpanda, P. Electrochemical potassium-ion intercalation in Na:XCoO2: A novel cathode material for potassium-ion batteries. Chem. Commun. 2017, 53, 8588–8591. [Google Scholar] [CrossRef] [PubMed]
- Naveen, N.; Park, W.B.; Han, S.C.; Singh, S.P.; Jung, Y.H.; Ahn, D.; Sohn, K.S.; Pyo, M. Reversible K+-Insertion/Deinsertion and Concomitant Na+-Redistribution in P’3-Na0.52CrO2 for High-Performance Potassium-Ion Battery Cathodes. Chem. Mater. 2018, 30, 2049–2057. [Google Scholar] [CrossRef]
- Deng, T.; Fan, X.; Luo, C.; Chen, J.; Chen, L.; Hou, S.; Eidson, N.; Zhou, X.; Wang, C. Self-Templated Formation of P2-type K0.6CoO
2 Microspheres for High Reversible Potassium-Ion Batteries. Nano Lett. 2018, 18, 1522–1529. [Google Scholar] [CrossRef] [PubMed] - Wang, X.; Xu, X.; Niu, C.; Meng, J.; Huang, M.; Liu, X.; Liu, Z.; Mai, L. Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries. Nano Lett. 2017, 17, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Fan, X.; Chen, J.; Chen, L.; Luo, C.; Zhou, X.; Yang, J.; Zheng, S.; Wang, C. Layered P2-Type K0.65Fe0.5Mn0.5O2 Microspheres as Superior Cathode for High-Energy Potassium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1800219. [Google Scholar] [CrossRef]
- Liu, C.; Luo, S.; Huang, H.; Wang, Z.; Hao, A.; Zhai, Y.; Wang, Z. K0.67Ni0.17Co0.17Mn0.66O2: A cathode material for potassium-ion battery. Electrochem. Commun. 2017, 82, 150–154. [Google Scholar] [CrossRef]
- Deng, L.; Niu, X.; Ma, G.; Yang, Z.; Zeng, L.; Zhu, Y.; Guo, L. Layered Potassium Vanadate K0.5V2O5 as a Cathode Material for Nonaqueous Potassium Ion Batteries. Adv. Funct. Mater. 2018, 28, 1800670. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Pei, Y.; Mu, C.; Li, H.; Li, F.; Chen, J. Low-Cost K4Fe(CN)6 as a High-Voltage Cathode for Potassium-Ion Batteries. ChemSusChem 2018, 11, 1285–1289. [Google Scholar] [CrossRef]
- Eftekhari, A. Potassium secondary cell based on Prussian blue cathode. J. Power Sources 2004, 126, 221–228. [Google Scholar] [CrossRef]
- Chong, S.; Chen, Y.; Zheng, Y.; Tan, Q.; Shu, C.; Liu, Y.; Guo, Z. Potassium ferrous ferricyanide nanoparticles as a high capacity and ultralong life cathode material for nonaqueous potassium-ion batteries. J. Mater. Chem. A 2017, 5, 22465–22471. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, T.; Yang, L.; Li, G.; Lee, J.Y. A Fe/Mn-Based Prussian Blue Analogue as a K-Rich Cathode Material for Potassium-Ion Batteries. ChemElectroChem 2017, 4, 2237–2242. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; Huggins, R.A.; Cui, Y. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett. 2011, 11, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Nossol, E.; Souza, V.H.; Zarbin, A.J. Carbon nanotube/Prussian blue thin films as cathodes for flexible, transparent and ITO-free potassium secondary battery. J. Colloid Interface Sci. 2016, 478, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.h.; Yin, Y.b.; Yang, X.; Sun, T.; Wang, S.; Jiang, Y.s.; Yan, J.m.; Zhang, X.b. Transformation of Rusty Stainless-Steel Meshes into Stable, Low-Cost, and Binder-Free Cathodes for High-Performance Potassium-Ion Batteries. Angew. Chem. 2017, 129, 7989–7993. [Google Scholar] [CrossRef]
- Han, J.; Li, G.N.; Liu, F.; Wang, M.; Zhang, Y.; Hu, L.; Dai, C.; Xu, M. Investigation of K3V2(PO4)3/C nanocomposites as high-potential cathode materials for potassium-ion batteries. Chem. Commun. 2017, 53, 1805–1808. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Carter, M.; Zhou, L.; Dai, J.; Fu, K.; Lacey, S.; Li, T.; Wan, J.; Han, X.; et al. Organic electrode for non-aqueous potassium-ion batteries. Nano Energy 2015, 18, 205–211. [Google Scholar] [CrossRef]
- Lu, X.; Bowden, M.E.; Sprenkle, V.L.; Liu, J. A Low Cost, High Energy Density, and Long Cycle Life Potassium-Sulfur Battery for Grid-Scale Energy Storage. Adv. Mater. 2015, 27, 5915–5922. [Google Scholar] [CrossRef]
- Wang, L.; Bao, J.; Liu, Q.; Sun, C.F. Concentrated electrolytes unlock the full energy potential of potassium-sulfur battery chemistry. Energy Storage Mater. 2019, 18, 470–475. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Performance Enhancement and Mechanistic Studies of Room-Temperature Sodium-Sulfur Batteries with a Carbon-Coated Functional Nafion Separator and a Na2S/Activated Carbon Nanofiber Cathode. Chem. Mater. 2016, 28, 896–905. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, H.M.; Sun, Y.K. High performance potassium-sulfur batteries based on a sulfurized polyacrylonitrile cathode and polyacrylic acid binder. J. Mater. Chem. A 2018, 6, 14587–14593. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically Induced Structural Transformation in a γ-MnO2 Cathode of a High Capacity Zinc-Ion Battery System. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5 · nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Wang, F.; Hu, E.; Sun, W.; Gao, T.; Ji, X.; Fan, X.; Han, F.; Yang, X.Q.; Xu, K.; Wang, C. A rechargeable aqueous Zn2+-battery with high power density and a long cycle-life. Energy Environ. Sci. 2018, 11, 3168–3175. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. An electrochemically induced bilayered structure facilitates long-life zinc storage of vanadium dioxide. J. Mater. Chem. A 2018, 6, 8006–8012. [Google Scholar] [CrossRef]
- Ding, J.; Du, Z.; Gu, L.; Li, B.; Wang, L.; Wang, S.; Gong, Y.; Yang, S. Ultrafast Zn2+ Intercalation and Deintercalation in Vanadium Dioxide. Adv. Mater. 2018, 30, 1800762. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 Nanowire/Graphene Electrodes for Aqueous Rechargeable Zinc Ion Batteries with High Rate Capability and Large Capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Deng, W.; Zhou, Z.; Li, Y.; Zhang, M.; Yuan, X.; Hu, J.; Li, Z.; Li, C.; Li, R. High-Capacity Layered Magnesium Vanadate with Concentrated Gel Electrolyte toward High-Performance and Wide-Temperature Zinc-Ion Battery. ACS Nano 2020, 14, 15776–15785. [Google Scholar] [CrossRef]
- Trócoli, R.; La Mantia, F. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries Beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Zhao, H.B.; Hu, C.J.; Cheng, H.W.; Fang, J.H.; Xie, Y.P.; Fang, W.Y.; Doan, T.N.L.; Hoang, T.K.A.; Xu, J.Q.; Chen, P. Novel Rechargeable M3V2(PO4)3//Zinc (M = Li, Na) Hybrid Aqueous Batteries with Excellent Cycling Performance. Sci. Rep. 2016, 6, 25809. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, Z.; Jiang, Y.; Jin, C.; Huang, W.; Ding, X.; Huang, Y. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Cheng, S.; Jiang, K. A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Storage Mater. 2018, 15, 14–21. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, K.; Huo, W.; Wang, Y.; Yao, G.; Gu, X.; Cheng, H.; Mai, L.; Hu, C.; Wang, X. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019, 62, 275–281. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene Scroll-Coated α-MnO2 Nanowires as High-Performance Cathode Materials for Aqueous Zn-Ion Battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry with H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- He, P.; Quan, Y.; Xu, X.; Yan, M.; Yang, W.; An, Q.; He, L.; Mai, L. High-Performance Aqueous Zinc-Ion Battery Based on Layered H2V3O8 Nanowire Cathode. Small 2017, 13, 1702551. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li + intercalated V2O5 · n H2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Islam, S.; Pham, D.T.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Electrochemical Zinc Intercalation in Lithium Vanadium Oxide: A High-Capacity Zinc-Ion Battery Cathode. Chem. Mater. 2017, 29, 1684–1694. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Rechargeable Aqueous Zn–V2O5 Battery with High Energy Density and Long Cycle Life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Shin, J.; Choi, D.S.; Lee, H.J.; Jung, Y.; Choi, J.W. Hydrated Intercalation for High-Performance Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2019, 9, 1900083. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Li, N.; Xie, K.; Wang, J.G.; Liu, X.; Wei, B. Graphene-Boosted, High-Performance Aqueous Zn-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 25446–25453. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Han, L.; Wang, Z.; Wang, H.; Zhao, Q.; Liu, J.; Pan, F. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 2019, 56, 92–99. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Shan, L.; Yang, Y.; Zhang, W.; Chen, H.; Fang, G.; Zhou, J.; Liang, S. Observation of combination displacement/intercalation reaction in aqueous zinc-ion battery. Energy Storage Mater. 2019, 18, 10–14. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. 2018, 130, 4007–4012. [Google Scholar] [CrossRef]
- Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.K.; Kim, J. K
2V6O16·2.7H2O nanorod cathode: An advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 15530–15539. [Google Scholar] [CrossRef] - He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium Ion Stabilized Vanadium Oxide Nanowire Cathode for High-Performance Zinc-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhu, T.; Wang, X.; Wei, X.; Yan, M.; Li, J.; Luo, W.; Yang, W.; Zhang, W.; Zhou, L.; et al. Highly Durable Na2V6O16·1.63H2O Nanowire Cathode for Aqueous Zinc-Ion Battery. Nano Lett. 2018, 18, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, F.; Luo, Z.; Fang, G.; Zhou, J.; Pan, A.; Liang, S. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Fang, G.; Liu, F.; Zhu, C.; Wang, C.; Pan, A.; Liang, S. Engineering the interplanar spacing of ammonium vanadates as a high-performance aqueous zinc-ion battery cathode. J. Mater. Chem. A 2019, 7, 940–945. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, K.; Wang, Y. Electrochemical activated MoO2/Mo2N heterostructured nanobelts as superior zinc rechargeable battery cathode. Energy Storage Mater. 2018, 15, 374–379. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Kasiri, G.; Trócoli, R.; Bani Hashemi, A.; La Mantia, F. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries. Electrochim. Acta 2016, 222, 74–83. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Morphology-Dependent Electrochemical Performance of Zinc Hexacyanoferrate Cathode for Zinc-Ion Battery. Sci. Rep. 2015, 5, 18263. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Jiang, Y.; Zhang, W.; Huang, Y. Hybrid aqueous battery based on Na3V2(PO4)3/C cathode and zinc anode for potential large-scale energy storage. J. Power Sources 2016, 308, 52–57. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, W.; Luo, Z.; Liu, L.; Lu, Y.; Li, Y.; Li, L.; Hu, J.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, eaao1761. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Payal, R. Rechargeable Batteries: History, Progress, and Applications. In Wiley; Boddula, R., Pothu, R., Asiri, A.M., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2020; Chapter 10; pp. 195–216. [Google Scholar]
- Cabello, M.; Nacimiento, F.; González, J.R.; Ortiz, G.; Alcántara, R.; Lavela, P.; Pérez-Vicente, C.; Tirado, J.L. Advancing towards a veritable calcium-ion battery: CaCo2O4 positive electrode material. Electrochem. Commun. 2016, 67, 59–64. [Google Scholar] [CrossRef]
- Tchitchekova, D.S.; Frontera, C.; Ponrouch, A.; Krich, C.; Bardé, F.; Palacín, M.R. Electrochemical calcium extraction from 1D-Ca3Co2O6. Dalton Trans. 2018, 47, 11298–11302. [Google Scholar] [CrossRef] [PubMed]
- Tchitchekova, D.S.; Ponrouch, A.; Verrelli, R.; Broux, T.; Frontera, C.; Sorrentino, A.; Bardé, F.; Biskup, N.; Arroyo-de Dompablo, M.E.; Palacín, M.R. Electrochemical Intercalation of Calcium and Magnesium in TiS2: Fundamental Studies Related to Multivalent Battery Applications. Chem. Mater. 2018, 30, 847–856. [Google Scholar] [CrossRef]
- Lipson, A.L.; Pan, B.; Lapidus, S.H.; Liao, C.; Vaughey, J.T.; Ingram, B.J. Rechargeable Ca-Ion Batteries: A New Energy Storage System. Chem. Mater. 2015, 27, 8442–8447. [Google Scholar] [CrossRef]
- Cabello, M.; Nacimiento, F.; Alcántara, R.; Lavela, P.; Pérez Vicente, C.; Tirado, J.L. Applicability of Molybdite as an Electrode Material in Calcium Batteries: A Structural Study of Layer-type CaxMoO3. Chem. Mater. 2018, 30, 5853–5861. [Google Scholar] [CrossRef]
- Hayashi, M.; Arai, H.; Ohtsuka, H.; Sakurai, Y. Electrochemical characteristics of calcium in organic electrolyte solutions and vanadium oxides as calcium hosts. J. Power Sources 2003, 119–121, 617–620. [Google Scholar] [CrossRef]
- Xu, X.; Duan, M.; Yue, Y.; Li, Q.; Zhang, X.; Wu, L.; Wu, P.; Song, B.; Mai, L. Bilayered Mg0.25V2O5 ·H2O as a Stable Cathode for Rechargeable Ca-Ion Batteries. ACS Energy Lett. 2019, 4, 1328–1335. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Xiong, F.; Yu, R.; Wu, P.; Cui, L.; An, Q. VOPO4·2H2O as a new cathode material for rechargeable Ca-ion batteries. Chem. Commun. 2020, 56, 3805–3808. [Google Scholar] [CrossRef]
- See, K.A.; Gerbec, J.A.; Jun, Y.S.; Wudl, F.; Stucky, G.D.; Seshadri, R. A High Capacity Calcium Primary Cell Based on the Ca-S System. Adv. Energy Mater. 2013, 3, 1056–1061. [Google Scholar] [CrossRef]
- Cheng, Y.; Shao, Y.; Zhang, J.G.; Sprenkle, V.L.; Liu, J.; Li, G. High performance batteries based on hybrid magnesium and lithium chemistry. Chem. Commun. 2014, 50, 9644–9646. [Google Scholar] [CrossRef]
- Ma, Z.; MacFarlane, D.R.; Kar, M. Mg Cathode Materials and Electrolytes for Rechargeable Mg Batteries: A Review. Batter. Supercaps 2019, 2, 115–127. [Google Scholar] [CrossRef]
- Wan, L.F.; Incorvati, J.T.; Poeppelmeier, K.R.; Prendergast, D. Building a fast lane for Mg diffusion in α-MoO3 by fluorine doping. Chem. Mater. 2016, 28, 6900–6908. [Google Scholar] [CrossRef]
- Incorvati, J.T.; Wan, L.F.; Key, B.; Zhou, D.; Liao, C.; Fuoco, L.; Holland, M.; Wang, H.; Prendergast, D.; Poeppelmeier, K.R.; et al. Reversible Magnesium Intercalation into a Layered Oxyfluoride Cathode. Chem. Mater. 2016, 28, 17–20. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Jacobson, A.J. High capacity microporous molybdenum-vanadium oxide electrodes for rechargeable lithium batteries. Chem. Mater. 2013, 25, 2708–2715. [Google Scholar] [CrossRef]
- Sun, X.; Bonnick, P.; Duffort, V.; Liu, M.; Rong, Z.; Persson, K.A.; Ceder, G.; Nazar, L.F. A high capacity thiospinel cathode for Mg batteries. Energy Environ. Sci. 2016, 9, 2273–2277. [Google Scholar] [CrossRef]
- Gao, T.; Han, F.; Zhu, Y.; Suo, L.; Luo, C.; Xu, K.; Wang, C. Hybrid Mg2+/Li+ Battery with Long Cycle Life and High Rate Capability. Adv. Energy Mater. 2015, 5, 1401507. [Google Scholar] [CrossRef]
- Taniguchi, K.; Gu, Y.; Katsura, Y.; Yoshino, T.; Takagi, H. Rechargeable Mg battery cathode TiS3 with d–p orbital hybridized electronic structures. Appl. Phys. Express 2016, 9, 011801. [Google Scholar] [CrossRef]
- Arsentev, M.; Missyul, A.; Petrov, A.V.; Hammouri, M. TiS3 Magnesium Battery Material: Atomic-Scale Study of Maximum Capacity and Structural Behavior. J. Phys. Chem. C 2017, 121, 15509–15515. [Google Scholar] [CrossRef]
- Gu, Y.; Katsura, Y.; Yoshino, T.; Takagi, H.; Taniguchi, K. Rechargeable magnesium-ion battery based on a TiSe2-cathode with d-p orbital hybridized electronic structure. Sci. Rep. 2015, 5, 12486. [Google Scholar] [CrossRef]
- Gershinsky, G.; Yoo, H.D.; Gofer, Y.; Aurbach, D. Electrochemical and spectroscopic analysis of Mg2+ intercalation into thin film electrodes of layered oxides: V2O5 and MoO3. Langmuir 2013, 29, 10964–10972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ling, C.; Mizuno, F. A Conceptual Magnesium Battery with Ultrahigh Rate Capability. Chem. Commun. 2015, 51, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Fan, Y.; Tan, S.; Zhou, L.; Xu, Y.; Pei, C.; An, Q.; Mai, L. Magnesium storage performance and mechanism of CuS cathode. Nano Energy 2018, 47, 210–216. [Google Scholar] [CrossRef]
- Duffort, V.; Sun, X.; Nazar, L.F. Screening for positive electrodes for magnesium batteries: A protocol for studies at elevated temperatures. Chem. Commun. 2016, 52, 12458–12461. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Taniguchi, K.; Miyasaka, H. Copper Selenide as a New Cathode Material based on Displacement Reaction for Rechargeable Magnesium Batteries. Electrochim. Acta 2016, 210, 655–661. [Google Scholar] [CrossRef]
- Zhang, R.; Ling, C. Unveil the Chemistry of Olivine FePO4 as Magnesium Battery Cathode. ACS Appl. Mater. Interfaces 2016, 8, 18018–18026. [Google Scholar] [CrossRef]
- Zhang, R.; Arthur, T.S.; Ling, C.; Mizuno, F. Manganese dioxides as rechargeable magnesium battery cathode; Synthetic approach to understand magnesiation process. J. Power Sources 2015, 282, 630–638. [Google Scholar] [CrossRef]
- Chen, X.; Bleken, F.L.; Løvvik, O.M.; Vullum-Bruer, F. Comparing electrochemical performance of transition metal silicate cathodes and chevrel phase Mo6S8 in the analogous rechargeable Mg-ion battery system. J. Power Sources 2016, 321, 76–86. [Google Scholar] [CrossRef]
- Pan, B.; Zhou, D.; Huang, J.; Zhang, L.; Burrell, A.K.; Vaughey, J.T.; Zhang, Z.; Liao, C. 2,5-Dimethoxy-1,4-Benzoquinone (DMBQ) as Organic Cathode for Rechargeable Magnesium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A580–A583. [Google Scholar] [CrossRef]
- Pan, B.; Huang, J.; Feng, Z.; Zeng, L.; He, M.; Zhang, L.; Vaughey, J.T.; Bedzyk, M.J.; Fenter, P.; Zhang, Z.; et al. Polyanthraquinone-Based Organic Cathode for High-Performance Rechargeable Magnesium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600140. [Google Scholar] [CrossRef]
- Gao, T.; Hou, S.; Wang, F.; Ma, Z.; Li, X.; Xu, K.; Wang, C. Reversible S0/MgSx Redox Chemistry in a MgTFSI2/MgCl2/DME Electrolyte for Rechargeable Mg/S Batteries. Angew. Chem. Int. Ed. 2017, 56, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
- Zhao-Karger, Z.; Liu, R.; Dai, W.; Li, Z.; Diemant, T.; Vinayan, B.P.; Bonatto Minella, C.; Yu, X.; Manthiram, A.; Behm, R.J.; et al. Toward Highly Reversible Magnesium–Sulfur Batteries with Efficient and Practical Mg[B(hfip)4]2 Electrolyte. ACS Energy Lett. 2018, 3, 2005–2013. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Z.; Qiao, L.; Guan, J.; Xu, H.; Wang, X.; Hu, P.; Du, H.; Li, S.; Zhou, X.; et al. Novel Design Concepts of Efficient Mg-Ion Electrolytes toward High-Performance Magnesium-Selenium and Magnesium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602055. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Zhao, X.; Wang, D.; Diemant, T.; Behm, R.J.; Fichtner, M. Performance Improvement of Magnesium Sulfur Batteries with Modified Non-Nucleophilic Electrolytes. Adv. Energy Mater. 2015, 5, 1401155. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Gil Bardaji, M.E.; Fuhr, O.; Fichtner, M. A new class of non-corrosive, highly efficient electrolytes for rechargeable magnesium batteries. J. Mater. Chem. A 2017, 5, 10815–10820. [Google Scholar] [CrossRef]
- Du, A.; Zhang, Z.; Qu, H.; Cui, Z.; Qiao, L.; Wang, L.; Chai, J.; Lu, T.; Dong, S.; Dong, T.; et al. An efficient organic magnesium borate-based electrolyte with non-nucleophilic characteristics for magnesium–sulfur battery. Energy Environ. Sci. 2017, 10, 2616–2625. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Zhao-Karger, Z.; Diemant, T.; Chakravadhanula, V.S.K.; Schwarzburger, N.I.; Cambaz, M.A.; Behm, R.J.; Kübel, C.; Fichtner, M. Performance study of magnesium-sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 2016, 8, 3296–3306. [Google Scholar] [CrossRef] [Green Version]
- Vardar, G.; Nelson, E.G.; Smith, J.G.; Naruse, J.; Hiramatsu, H.; Bartlett, B.M.; Sleightholme, A.E.; Siegel, D.J.; Monroe, C.W. Identifying the Discharge Product and Reaction Pathway for a Secondary Mg/O2 Battery. Chem. Mater. 2015, 27, 7564–7568. [Google Scholar] [CrossRef]
- Smith, J.G.; Naruse, J.; Hiramatsu, H.; Siegel, D.J. Theoretical Limiting Potentials in Mg/O2 Batteries. Chem. Mater. 2016, 28, 1390–1401. [Google Scholar] [CrossRef]
- Tian, H.; Gao, T.; Li, X.; Wang, X.; Luo, C.; Fan, X.; Yang, C.; Suo, L.; Ma, Z.; Han, W.; et al. High power rechargeable magnesium/iodine battery chemistry. Nat. Commun. 2017, 8, 14083. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Yu, Z.; Yuan, Y.; Wang, S.; Jiao, S. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 2015, 51, 11892–11895. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gong, M.; Lin, M.C.; Yuan, C.; Angell, M.; Huang, L.; Wang, D.Y.; Zhang, X.; Yang, J.; Hwang, B.J.; et al. 3D Graphitic Foams Derived from Chloroaluminate Anion Intercalation for Ultrafast Aluminum-Ion Battery. Adv. Mater. 2016, 28, 9218–9222. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, F.; Liu, Y.; Huang, T.; Zheng, B.; Ananth, N.; Xu, Z.; Gao, W.; Gao, C. A Defect-Free Principle for Advanced Graphene Cathode of Aluminum-Ion Battery. Adv. Mater. 2017, 29, 1605958. [Google Scholar] [CrossRef]
- Hu, Y.; Debnath, S.; Hu, H.; Luo, B.; Zhu, X.; Wang, S.; Hankel, M.; Searles, D.J.; Wang, L. Unlocking the potential of commercial carbon nanofibers as free-standing positive electrodes for flexible aluminum ion batteries. J. Mater. Chem. A 2019, 7, 15123–15130. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Huang, J.; Li, K.; Zeng, J.; Yang, Y.; Li, J.; Wang, Y.; Wang, J.; Zhao, J. Investigation of the Reversible Intercalation/Deintercalation of Al into the Novel Li3VO4@C Microsphere Composite Cathode Material for Aluminum-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 28486–28494. [Google Scholar] [CrossRef]
- Wei, J.; Chen, W.; Chen, D.; Yang, K. Molybdenum Oxide as Cathode for High Voltage Rechargeable Aluminum Ion Battery. J. Electrochem. Soc. 2017, 164, A2304–A2309. [Google Scholar] [CrossRef]
- Wang, S.; Jiao, S.; Wang, J.; Chen, H.S.; Tian, D.; Lei, H.; Fang, D.N. High-Performance Aluminum-Ion Battery with CuS@C Microsphere Composite Cathode. ACS Nano 2017, 11, 469–477. [Google Scholar] [CrossRef]
- Geng, L.; Scheifers, J.P.; Fu, C.; Zhang, J.; Fokwa, B.P.T.; Guo, J. Titanium Sulfides as Intercalation-Type Cathode Materials for Rechargeable Aluminum Batteries. ACS Appl. Mater. Interfaces 2017, 9, 21251–21257. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Wang, S.; Li, S.; Jiao, S. Porous CuO microsphere architectures as high-performance cathode materials for aluminum-ion batteries. J. Mater. Chem. A 2018, 6, 3084–3090. [Google Scholar] [CrossRef]
- Yu, X.; Boyer, M.J.; Hwang, G.S.; Manthiram, A. Room-Temperature Aluminum-Sulfur Batteries with a Lithium-Ion-Mediated Ionic Liquid Electrolyte. Chem 2018, 4, 586–598. [Google Scholar] [CrossRef]
- Yang, H.; Yin, L.; Liang, J.; Sun, Z.; Wang, Y.; Li, H.; He, K.; Ma, L.; Peng, Z.; Qiu, S.; et al. An Aluminum-Sulfur Battery with a Fast Kinetic Response. Angew. Chem. Int. Ed. 2018, 57, 1898–1902. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, H.; Wang, S.; Huang, T.; Xi, J.; Cai, S.; Guo, F.; Xu, Z.; Gao, W.; Gao, C. Ultrafast all-climate aluminum-graphene battery with quarter-million cycle life. Sci. Adv. 2017, 3, eaao7233. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, F.; Shi, X.; Qin, J.; Zhang, Z.; Bao, X.; Wu, Z.S. Graphene aerogel derived compact films for ultrafast and high-capacity aluminum ion batteries. Energy Storage Mater. 2019, 23, 664–669. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, W.; Gao, W.; Chen, H.; Gao, C. Wet-Spun Superelastic Graphene Aerogel Millispheres with Group Effect. Adv. Mater. 2017, 29, 1701482. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, B.; Gong, D.; Xu, Z.; Lu, B. Graphene Nanoribbons on Highly Porous 3D Graphene for High-Capacity and Ultrastable Al-Ion Batteries. Adv. Mater. 2017, 29, 1604118. [Google Scholar] [CrossRef]
- Wang, S.; Kravchyk, K.V.; Krumeich, F.; Kovalenko, M.V. Kish Graphite Flakes as a Cathode Material for an Aluminum Chloride-Graphite Battery. ACS Appl. Mater. Interfaces 2017, 9, 28478–28485. [Google Scholar] [CrossRef] [Green Version]
- Smajic, J.; Alazmi, A.; Batra, N.; Palanisamy, T.; Anjum, D.H.; Costa, P.M.F.J. Mesoporous Reduced Graphene Oxide as a High Capacity Cathode for Aluminum Batteries. Small 2018, 14, 1803584. [Google Scholar] [CrossRef]
- Stadie, N.P.; Wang, S.; Kravchyk, K.V.; Kovalenko, M.V. Zeolite-Templated Carbon as an Ordered Microporous Electrode for Aluminum Batteries. ACS Nano 2017, 11, 1911–1919. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Huq, A.; Wang, S.; Park, M.J.; Manthiram, A. Rechargeable Aluminum-Ion Batteries Based on an Open-Tunnel Framework. Small 2017, 13, 1701296. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, B.; Xiong, W.; Sun, H.; Lin, Z.; Hu, L.; Tu, J.; Hou, J.; Zhu, H.; Jiao, S. A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci. Rep. 2013, 3, 3383. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bai, Y.; Chen, S.; Luo, X.; Wu, C.; Wu, F.; Lu, J.; Amine, K. Binder-free V2O5 cathode for greener rechargeable aluminum battery. ACS Appl. Mater. Interfaces 2015, 7, 80–84. [Google Scholar] [CrossRef]
- Chiku, M.; Takeda, H.; Matsumura, S.; Higuchi, E.; Inoue, H. Amorphous Vanadium Oxide/Carbon Composite Positive Electrode for Rechargeable Aluminum Battery. ACS Appl. Mater. Interfaces 2015, 7, 24385–24389. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, Z.; Hu, Z.; Lu, J.; Zhou, Z.; Jiao, S. Hexagonal NiS nanobelts as advanced cathode materials for rechargeable Al-ion batteries. Chem. Commun. 2016, 52, 10427–10430. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, Z.; Tu, J.; Wang, J.; Tian, D.; Liu, Y.; Jiao, S. A Novel Aluminum-Ion Battery: Al/AlCl3 -[EMIm]Cl/Ni3S2@Graphene. Adv. Energy Mater. 2016, 6, 1600137. [Google Scholar] [CrossRef]
- Geng, L.; Lv, G.; Xing, X.; Guo, J. Reversible Electrochemical Intercalation of Aluminum in Mo6S8. Chem. Mater. 2015, 27, 4926–4929. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, B.; Ye, D.; Zhu, X.; Lyu, M.; Wang, L. An Innovative Freeze-Dried Reduced Graphene Oxide Supported SnS2 Cathode Active Material for Aluminum-Ion Batteries. Adv. Mater. 2017, 29, 1606132. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Tu, J.; Zhang, G.; Li, S.; Tian, D.; Jiao, S. Flower-like Vanadium Suflide/Reduced Graphene Oxide Composite: An Energy Storage Material for Aluminum-Ion Batteries. ChemSusChem 2018, 11, 709–715. [Google Scholar] [CrossRef]
- Gao, T.; Li, X.; Wang, X.; Hu, J.; Han, F.; Fan, X.; Suo, L.; Pearse, A.J.; Lee, S.B.; Rubloff, G.W.; et al. A Rechargeable Al/S Battery with an Ionic-Liquid Electrolyte. Angew. Chem. 2016, 128, 10052–10055. [Google Scholar] [CrossRef]
- Cohn, G.; Ma, L.; Archer, L.A. A novel non-aqueous aluminum sulfur battery. J. Power Sources 2015, 283, 416–422. [Google Scholar] [CrossRef]
- Yuan, F.; Chi, S.; Dong, S.; Zou, X.; Lv, S.; Bao, L.; Wang, J. Ionic liquid crystal with fast ion-conductive tunnels for potential application in solvent-free Li-ion batteries. Electrochim. Acta 2019, 294, 249–259. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.; Gao, T.; Sun, W.; Ma, Z.; Yang, C.; Han, F.; Xu, K.; Wang, C. High-Voltage Aqueous Magnesium Ion Batteries. ACS Cent. Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, L.; Zou, X.; Dong, S.; Chi, S.; Xie, J.; Xing, H.; Bian, L.; Bao, L.; Wang, J. Flexible all-solid-state electrolytes with ordered fast Li-ion-conductive nano-pathways for rechargeable lithium batteries. J. Power Sources 2019, 444, 227305. [Google Scholar] [CrossRef]
- Gao, H.; Xue, L.; Xin, S.; Goodenough, J.B. A High-Energy-Density Potassium Battery with a Polymer-Gel Electrolyte and a Polyaniline Cathode. Angew. Chem. Int. Ed. 2018, 57, 5449–5453. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-solid-state Al—Air batteries with polymer alkaline gel electrolyte. J. Power Sources 2014, 251, 470–475. [Google Scholar] [CrossRef]
- Jiang, G.; Li, F.; Wang, H.; Wu, M.; Qi, S.; Liu, X.; Yang, S.; Ma, J. Perspective on High-Concentration Electrolytes for Lithium Metal Batteries. Small Struct. 2021, 2, 2000122. [Google Scholar] [CrossRef]
- Cao, X.; Jia, H.; Xu, W.; Zhang, J.G. Review—Localized High-Concentration Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2021, 168, 010522. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093. [Google Scholar] [CrossRef]
- Wu, X.; Leonard, D.P.; Ji, X. Emerging Non-Aqueous Potassium-Ion Batteries: Challenges and Opportunities. Chem. Mater. 2017, 29, 5031–5042. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Sun, J.; Liu, Y.; Hou, L. Recent Progress in “Water-in-Salt” Electrolytes Toward Non-lithium Based Rechargeable Batteries. Front. Chem. 2020, 8, 595. [Google Scholar] [CrossRef]
- Montanino, M.; Passerini, S.; Appetecchi, G. Rechargeable Lithium Batteries; Elsevier: Amsterdam, The Netherlands, 2015; Chapter Four; pp. 73–116. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Wang, X.; Xia, S.; Zhang, Y.; Han, Y.; Yu, J.; Ding, B. Polymer Template Synthesis of Soft, Light, and Robust Oxide Ceramic Films. iScience 2019, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Le Ruyet, R.; Berthelot, R.; Salager, E.; Florian, P.; Fleutot, B.; Janot, R. Investigation of Mg(BH4)(NH2)-Based Composite Materials with Enhanced Mg2+ Ionic Conductivity. J. Phys. Chem. C 2019, 123, 10756–10763. [Google Scholar] [CrossRef]
- Kotobuki, M. Recent progress of ceramic electrolytes for post Li and Na batteries. Funct. Mater. Lett. 2021, 14, 2130003. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, B.; Wang, J.; Zuo, C.; He, D.; Xie, X.; Xue, Z. PEO-based electrolytes blended with star polymers with precisely imprinted polymeric pseudo-crown ether cavities for alkali metal ion batteries. J. Membr. Sci. 2019, 576, 182–189. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Septiana, A.R.; Honggowiranto, W.; Sudaryanto; Kartini, E.; Hidayat, R. Comparative study on the ionic conductivities and redox properties of LiPF6 and LiTFSI electrolytes and the characteristics of their rechargeable lithium ion batteries. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012061. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, D.H.; Shin, J.H.; Jang, J.E.; Ahn, K.H.; Lee, C.; Lee, H. Ionic Conduction and Solution Structure in LiPF6 and LiBF4 Propylene Carbonate Electrolytes. J. Phys. Chem. C 2018, 122, 19438–19446. [Google Scholar] [CrossRef]
- Wu, F.; Kim, G.; Diemant, T.; Kuenzel, M.; Schür, A.R.; Gao, X.; Qin, B.; Alwast, D.; Jusys, Z.; Behm, R.J.; et al. Reducing Capacity and Voltage Decay of Co-Free Li1.2Ni0.2Mn0.6O2 as Positive Electrode Material for Lithium Batteries Employing an Ionic Liquid-Based Electrolyte. Adv. Energy Mater. 2020, 10, 2001830. [Google Scholar] [CrossRef]
- Bommier, C.; Ji, X. Electrolytes, SEI Formation, and Binders: A Review of Nonelectrode Factors for Sodium-Ion Battery Anodes. Small 2018, 14, 1703576. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, D.; Qin, X.; Lin, K.; Kang, F.; Li, B.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. A room-temperature sodium–sulfur battery with high capacity and stable cycling performance. Nat. Commun. 2018, 9, 3870. [Google Scholar] [CrossRef]
- Amara, S.; Toulc’Hoat, J.; Timperman, L.; Biller, A.; Galiano, H.; Marcel, C.; Ledigabel, M.; Anouti, M. Comparative Study of Alkali-Cation-Based (Li+, Na+, K+) Electrolytes in Acetonitrile and Alkylcarbonates. ChemPhysChem 2019, 20, 581. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Hoang, T.K.; Yang, D.; Liu, Y.; Ahmed, M.; Xu, J.; Qiu, X.; Chen, P. Electrolyte engineering for a highly stable, rechargeable hybrid aqueous battery. J. Energy Storage 2019, 26, 100920. [Google Scholar] [CrossRef]
- Lyu, L.; Gao, Y.; Wang, Y.; Xiao, L.; Lu, J.; Zhuang, L. Improving the cycling performance of silver-zinc battery by introducing PEG-200 as electrolyte additive. Chem. Phys. Lett. 2019, 723, 102–110. [Google Scholar] [CrossRef]
- Abdulla, J.; Cao, J.; Zhang, D.; Zhang, X.; Sriprachuabwong, C.; Kheawhom, S.; Wangyao, P.; Qin, J. Elimination of Zinc Dendrites by Graphene Oxide Electrolyte Additive for Zinc-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4602–4609. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Tian, Q.; Chen, J. Developing improved electrolytes for aqueous zinc-ion batteries to achieve excellent cyclability and antifreezing ability. J. Colloid Interface Sci. 2021, 586, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.S.; Heo, J.W.; Kwak, H.H.; Lee, H.; Hong, S.T. Organic electrolyte-based rechargeable zinc-ion batteries using potassium nickel hexacyanoferrate as a cathode material. J. Power Sources 2017, 337, 204–211. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Wang, Y.; Wang, Y.; Li, J.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Ultrafast Rechargeable Zinc Battery Based on High-Voltage Graphite Cathode and Stable Nonaqueous Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 32978–32986. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, M.; Huang, S.; Tian, J.; Niu, Z. Thermal-Gated Polymer Electrolytes for Smart Zinc-Ion Batteries. Angew. Chem. 2020, 132, 16622–16626. [Google Scholar] [CrossRef]
- Lee, B.S.; Cui, S.; Xing, X.; Liu, H.; Yue, X.; Petrova, V.; Lim, H.D.; Chen, R.; Liu, P. Dendrite Suppression Membranes for Rechargeable Zinc Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38928–38935. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Qin, B.; Passerini, S. High-Voltage Operation of a V2O5 Cathode in a Concentrated Gel Polymer Electrolyte for High-Energy Aqueous Zinc Batteries. ACS Appl. Mater. Interfaces 2020, 12, 15305–15312. [Google Scholar] [CrossRef]
- Melemed, A.M.; Khurram, A.; Gallant, B.M. Current Understanding of Nonaqueous Electrolytes for Calcium-Based Batteries. Batter. Supercaps 2020, 3, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Nielson, K.V.; Luo, J.; Liu, T.L. Optimizing Calcium Electrolytes by Solvent Manipulation for Calcium Batteries. Batter. Supercaps 2020, 3, 766–772. [Google Scholar] [CrossRef]
- Biria, S.; Pathreeker, S.; Li, H.; Hosein, I.D. Plating and stripping of calcium in an alkyl carbonate electrolyte at room temperature. ACS Appl. Energy Mater. 2019, 2, 7738–7743. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Taimoory, S.M.; Trant, J.F. Calcium-Ion Batteries: Identifying Ideal Electrolytes for Next-Generation Energy Storage Using Computational Analysis. J. Phys. Chem. C 2019, 123, 15885–15896. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, C.; Liu, Q.; Li, X.; Zhao, X.; Guo, Z. Progress and Perspective on Rechargeable Magnesium–Sulfur Batteries. Small Methods 2021, 5, 2001303. [Google Scholar] [CrossRef]
- Sheha, E.; Liu, F.; Wang, T.; Farrag, M.; Liu, J.; Yacout, N.; Kebede, M.A.; Sharma, N.; Fan, L.Z. Dual Polymer/Liquid Electrolyte with BaTiO3 Electrode for Magnesium Batteries. ACS Appl. Energy Mater. 2020, 3, 5882–5892. [Google Scholar] [CrossRef]
- Angell, M.; Pan, C.J.; Rong, Y.; Yuan, C.; Lin, M.C.; Hwang, B.J.; Dai, H. High Coulombic efficiency aluminum-ion battery using an AlCl3 -urea ionic liquid analog electrolyte. Proc. Natl. Acad. Sci. USA 2017, 114, 834–839. [Google Scholar] [CrossRef]
- Wu, C.; Gu, S.; Zhang, Q.; Bai, Y.; Li, M.; Yuan, Y.; Wang, H.; Liu, X.; Yuan, Y.; Zhu, N.; et al. Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery. Nat. Commun. 2019, 10, 73. [Google Scholar] [CrossRef]
- Counterpoint. Global Smartphone Market Share: By Quarter. Available online: https://www.counterpointresearch.com/global-smartphone-share/ (accessed on 30 January 2022).
- Statista. Number of Smartphones Sold to End Users Worldwide from 2007 to 2021; Statista: Hamburg, Germany, 2021. [Google Scholar]
- IEA. Global EV Outlook 2021; IEA: Paris, France, 2021. [Google Scholar]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Tesla. Model S. 2021. Available online: https://www.tesla.com/models (accessed on 30 January 2022).
- Fundacio Institut De Recerca De L’Energia De Catalunya. CObalt-Free Batteries for FutuRe Automotive Applications; Fundacio Institut De Recerca De L’Energia De Catalunya: Barcelona, Spain, 2021. [Google Scholar]
- Osterreichische Forschungsforderungsgesellschaft MBH. ERA-NET for Research and Innovation on Materials and Battery Technologies, Supporting the European Green Deal; Osterreichische Forschungsforderungsgesellschaft MBH: Vienna, Austria, 2021. [Google Scholar]
- Epec, L. Battery Standards. 2021. Available online: https://www.epectec.com/batteries/battery-standards.html (accessed on 30 January 2022).
- Woodbank Communications Ltd. International Standards and Testing Applicable to Batteries; Woodbank Communications Ltd.: Chester, UK, 2005. [Google Scholar]
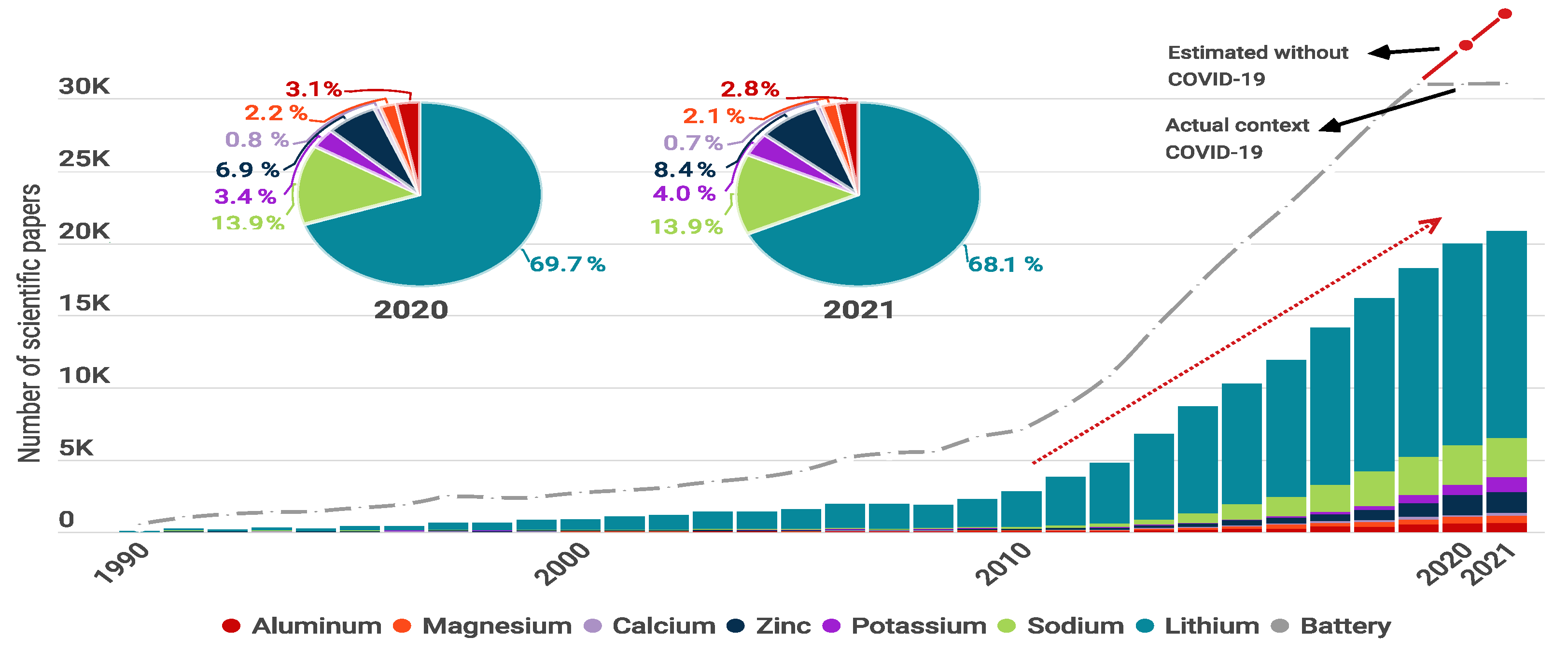













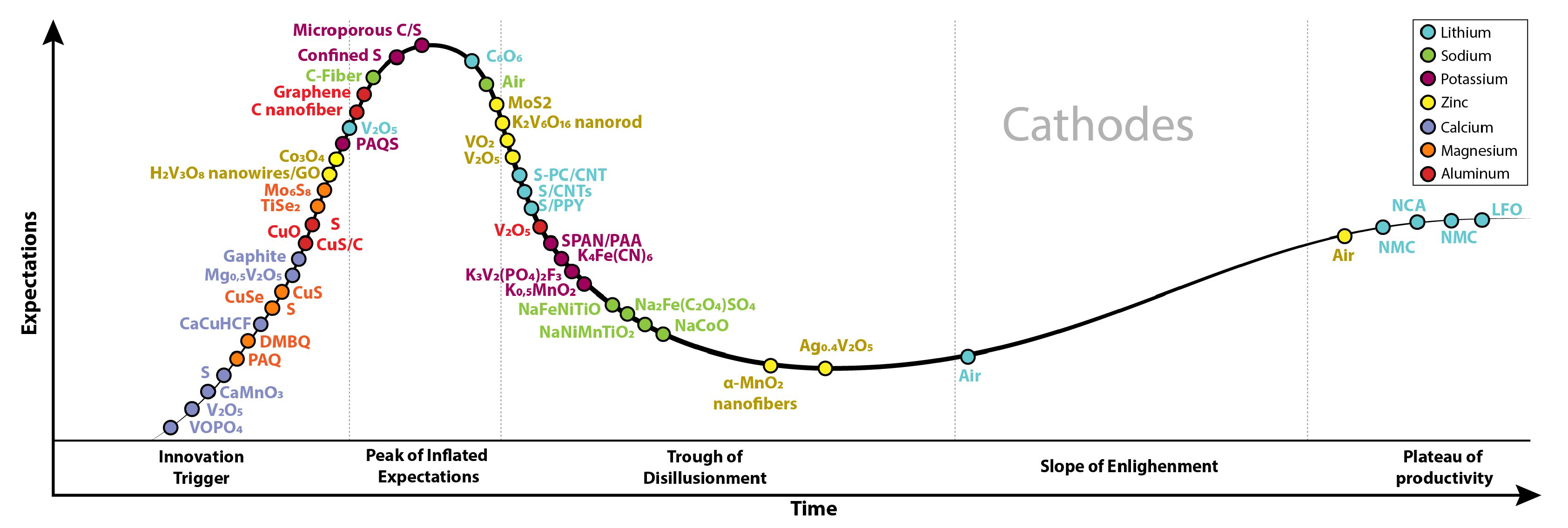
| Parameter | Symbol | Unit | Description |
|---|---|---|---|
| Voltage | V | Cell voltage is cathode potential minus anode potential. | |
| Capacity | Ct | The maximum electrical charge stored in the cell. | |
| Specific Capacity | Cs | kg−1 | The capacity of electrodes is usually provided per mass of active material. |
| Energy | E | Maximum energy delivered by a given system with a theoretical voltage and a theoretical capacity: . | |
| Specific Energy | Es | /kg−1 | Maximum energy of a cell permass of the whole battery:. |
| Energy Density | Ed | W h L−1 | Nominal battery energy per unit volumeof the whole battery:. |
| Coulombic Efficiency | % | Ratio of discharging and charging capacity. . | |
| C-rate | C | - | Measure for the charging/discharging current of an electrochemical cell. A C-rate of 1 corresponds to a full charge/discharge within 1 h. . |
| Current Density | J | m−2 | Electric current per cross-sectional area. |
| Cycle Life | - | - | Cycle numbers. |
| Anode Material | Voltage Range (V) | Specific Capacity (mA h g) | Current (mA g) | Cycles | |
|---|---|---|---|---|---|
| Intercalation | |||||
| Graphite [88] | 449 | 0.1 a | 100 | ||
| Porous Hard Carbon [79] | 503 | 0.2 a | |||
| UF layerd Graphite [80] | 0.1–2.0 | 393 | 40 | 800 | |
| LiTiO NP/CNTs [89] | 1.2–2.0 | 173 | 0.1 a | 1000 | 98.5 |
| TiO [90] | 0.8 | 330 | |||
| TiO/CNT [91] | 316 | 66 | 100 | ||
| TiO/G [92] | 272 | 168 | 100 | ||
| Alloy | |||||
| Ge [93] | 0.02–1.2 | 1300 | 250 | 340 | 60 |
| GeO/GO [85] | 0–2.5 | 1000 | 250 | 50 | 69 |
| Li–B [86] | 213.2 | 200 | |||
| Li–B–Al [86] | 211.9 | 100 | 200 | 83.9 | |
| Li–Mg [94] | 607 | 50 | 200 | ||
| Si MW [81] | 3038 | 400 | 200 | 90 | |
| Si/C film [82] | 0.1–2.5 | 2276 | 8400 | 150 | 73 |
| Si/C [95] | 3500 | 12,600 | 300 | ||
| Si NP/CNTs [96] | 1629 | 200 | 200 | ||
| Sn NP/G [97] | 1022 | 2000 | 1000 | ||
| SnO NC/G [98] | 1865 | 500 | 500 | ||
| Sn/SnO/G | 0.01–3.0 | 2970 | 100 | 75 | 44 |
| ZnCoO/rGO | 1613 | 500 | 400 | ||
| Conversion | |||||
| CoO/rGO | 0.01–3.0 | 2313 | 100 | 500 | 74 |
| CoSe NS/G | 680 | 50 | 300 | ||
| CuMnO/G | 0.01–3.0 | 1491 | 50 | 150 | 75 |
| GaS NF/CNTs [99] | 2118 | 120 | 100 |
| Anode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | |
|---|---|---|---|---|---|
| Alloy | |||||
| Si | 279 | 10 | 100 | ||
| Intercalation | |||||
| Hard carbon [103] | ∼0.0–3.0 | 250 | 1000 | 1200 | |
| crumpled G [119] | ∼0.0–2.5 | 125 | 1000 | 500 | |
| porous G/SbO [120] | ∼0.0–3.0 | 350 | 50 | 100 | |
| porous multilayered G [121] | ∼0.0–3.0 | 392 | 100 | 100 | |
| G/CoSe nanosheets [122] | ∼0.0–2.5 | 180.7 | 500 | 100 | |
| G nanosheets/FeO [123] | ∼0.0–3.0 | 400 | 100 | 200 | |
| G/P stacks [124] | 0.0–2.0 | 1706 | 260 | 60 | |
| G/SnS stacks [125] | ∼0.0–2.5 | 618.9 | 200 | 100 | |
| G/TiNbO [126] | ∼0.0–3.0 | 200 | 200 | 70 | |
| N-doped G sheets [127] | ∼0.0–3.0 | 115.5 | 50 | 260 | |
| N-doped G/NaTi(PO) [128] | ∼1.5–3.0 | 75 | 20 a | 200 | |
| N-rich G [129] | ∼0.0–3.0 | 250 | 50 | 250 | |
| N-/S-doped G sheets [130] | ∼0.0–3.0 | 289 | 100 | 100 | |
| 2D TiO/TiS [105] | 0.1–3.0 | 329.63 | 1000 | 140 | |
| Phosphorene | |||||
| Black P/C [131] | ∼0.0–3.0 [132] | 958 [132] | 2000 | 500 | 58.5 |
| CuP–C [114] | ∼0.0–3.0 | 378.9 | 1000 | 1000 | 89 |
| P/C-amorphous [133] | ∼0.0–2.0 | 1764 | 250 | 140 | 87 |
| P-layered black [134] | 0.0–2.0 | 1500 | 125 | 25 | |
| P/C composite layers [135] | ∼0.0–1.5 | 1500 | 100 | 100 | |
| P/G hybrid [136] | 0.0–1.5 | 2400 | 0.02 C | 100 | |
| P-PMCNFs [113] | ∼0.0–2.0 | 2260 | 100 | 90 | 70 |
| P-C [111] | 1308 | 200 | |||
| P-TBMC [116] | ∼0.0–2.0 | 1544 | 8000 | 800 | 69.8 |
| VP/P [115] | ∼0.0–2.0 | 738 | 8000 | 100 | 85.9 |
| TMS | |||||
| MoS nanosheets [137] | ∼0.0–3.0 | 386 | 40 | 100 | |
| MoS/C nanosheets [138] | ∼0.0–2.9 | 280 | 1 C | 300 | |
| MoS/G sheets [139] | 0.1–2.3 | 218 | 25 | 20 | |
| MoSe nanoplates [140] | 0.1–3.0 | 369 | 0.1 C | 50 | |
| WSe [141] | 0.1–2.5 | 117 | 0.1 C | 30 | |
| WSe/C [142] | ∼0.0–3.0 | 270 | 0.2 C | 50 | |
| MoS/G Carbon [118] | 0.5–3.2 | 310 | 5000 | 2500 |
| Anode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | |
|---|---|---|---|---|---|
| Alloy | |||||
| CoO–FeO/C [150] | 0.01–3.0 | 770 | 1000 | 50 | 54 |
| CoS–G [151] | 0.01–2.9 | 434.5 | 4 a | 64.4 | |
| KTiO [147,152] | 0.01–3.0 | 151 | 500 | 900 | 65.4 [148] |
| KTi(PO)/C [153] | 1.2–2.8 | 75.6 | 5 a | 75 | |
| MoS [154] | 0.5–2.0 | 98 | 2.86 a | 74.4 | |
| MoS–RGO [155] | 0.01–3.0 | 679 | 500 | 30 | |
| SbS–S [156] | 0.1–3.0 | 548 | 1000 | 69.7 | |
| Sb NP/3D C [157] | 0.01–2.0 | 478 | 1000 | 68.2 | |
| SnO–G–C [158] | 0–2.5 | 519 | 1000 | 100 | 44 |
| SnP/C fiber [159] | 0.1–2.0 | 514 | 2000 | 200 | 64 |
| SnP/C [160] | 0.01–2.0 | 697 | 1200 | 50 | 58 |
| SnS/RGO [161] | 0.01–2.0 | 355 | 2000 | 56 | |
| TiC [162] | 0.01–3.0 | 136 | 300 | 27 | |
| TiCNT [163] | 0.01–3.0 | 710 | 28.40 | ||
| TiSe [164] | 1.0–3.0 | 92.7 | 1000 | 67.1 | |
| VSe [165] | 0.01–2.6 | 366 | 2000 | 69.10 | |
| Carbon | |||||
| Graphite [144] | 0.01–1.5 | 273 | 200 [166] | 200 [167] | 87 [168] |
| expanded Graphite [145] | 0.01–3.0 | 267 | 200 | 500 | 81 |
| hard Carbon [169] | 0.01–1.5 [146] | 300 | 1395 | 100 | 87 |
| soft Carbon [144] | 273 | 50 | 50 | ||
| Organic | |||||
| KPC [170] | 0.1–2.0 | 245 | 2 a | 44 | |
| KTP [171] | 0.1–2.0 | 305.8 | 1000 | 76.1 |
| Anode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | |
|---|---|---|---|---|---|
| Carbon-coated ZnO [175] | 1.5–2.0 | 155.5 | 1 | 42 | 48.8 |
| hyper dendritic Zn [177] | 232.6 | 0.2 | 100 | 77 | |
| Zn sponge [176] | 0.9–2.2 | 164 | 0.03 | 36 | |
| Zn on Cu foam [178] | 690 | 25.4 | 9000 | 31 | |
| Zn foil with IL membrane [179] | 0.8–2.3 | 107 | |||
| ZnO–Ag–polypyrrole [180] | 1.2–1.9 | 437 | 1 | 300 | 87.5 |
| Zn foil [181] | 0.4 | 0.06 | 147 | 100 | |
| ZnO [182] | 269.8 | 0.5 | 1000 | 100 | |
| ZnO/C [183] | 1.2–2.0 | 266.7 | 0.17 | 400 | |
| ZnO with ionomer layer [184] | 1.0–1.9 | 124.5 | 0.78 | 67 | 75 |
| ZnO in Carbon matrix [174] | 1.4–2.0 | 241.9 | 4.96 | 150 | 82.2 |
| Zn sponge advanced [185] | 1.4–1.9 | 310 | 3.1 | 141 | 96.6 |
| Anode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | |
|---|---|---|---|---|---|
| Alloy | |||||
| Ca [50] | 30 | 85 | |||
| Ca-Si [188] | 0.37 | 240 | 1 | ||
| Ca-Sn [192] | 0.8 | 526 | 350 [189] | 80 | |
| Carbon | |||||
| Graphene [193] | 225 | ||||
| MCMB [187] | 4.6 | 62 | 1 a | 300 | 82 |
| Organic | |||||
| PANI [190] | 0.4 | 114 | 150 | 200 | 99 |
| PNDIE [191] | −0.45 | 160 | 915 | 4000 | 99 |
| PTCDA [194] | 80 |
| Anode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | |
|---|---|---|---|---|---|
| MgBi [198] | 0.23 | 257 | 385 | 100 | 86 |
| MgBi NT [199] | 350 | 19 | 200 | 95 | |
| BiSb [198] | 298 | 1 a | 100 | 75 | |
| In [201] | 0.09 | 425 | 0.01 a | ||
| LTO [196] | 175 | 15 | 500 | ||
| Sb [198] | 16 | 1 a | 50 | ||
| SnSb [200] | 420 | 50 | 200 |
| Cathode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| LiCoO [208] | 3–4.6 | 220 | C/4 * | 100 | |
| LiFePO4/C/V2O3 [225] | 3.4 | 140 | 750 | 30 | 100 |
| LiFePO4/CNTs [210] | 115 | 1700 | 1000 | 98 | |
| LiFePO4/G [226] | 123 | 1700 | 1000 | 89 | |
| CNT/LiNi0.5Mn1.5O4 [227] | 140 | 70 | 100 | 96 | |
| LiMn2O4 | 80 | ||||
| CNT/LiNi0.8Co0.15Al0.05O2 [211] | 189 | 50 | 60 | 96 | |
| LiCo1/3 Ni1/3Mn1/3O2 [228] | 4.6 | 158 | 30 | 99 | |
| LiNixFeyAlzO2 [212] | 3–4.5 | 200 | 1 * | 100 | 80 |
| V2O5/CNTs [229] | 298 | 150 | 200 | 71 | |
| PDHBQS-SWCNTs [222] | 1.5–3.5 | 182 | 5000 | 500 | 89 |
| Sulfur | |||||
| S coating on | |||||
| hydroxylated CNTs [230] | 1274 | 100 | 57 | ||
| S encapsulated in spherical CNTs particles [220] | 1.5–2.8 | 1343 | 3344 | 100 | 70 |
| S encapsulated in CNTs [219] | 1.5–3 | 1485 | 3344 | 400 | 60 |
| S embedded in CNT foam [221] | 1379 | 1672 | 200 | 76 | |
| S wrapped on CNT array [231] | 1092 | 50 | 64 | ||
| S wrapped on CNTs [232] | 1065 | 300 | 77 | ||
| ZrB2−S [223] | 1243 | 5 * | 600 | 89 |
| Cathode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| Layer metal oxides | |||||
| O3-NaNi0.5Mn0.5O2 [242] | 2.0–4.0 | 133 | 468 | 500 | 70 |
| O3-NaNi0.12Cu0.12Mg0.12Fe0.15 Co0.15Mn0.1Ti0.1Sn0.1Sb0.04O2 [243] | 2.0–3.9 | 110 | 360 | 500 | 83 |
| −NaMnO2 [237] | 2.0–3.8 | 175 | 10 | 50 | |
| −NaMnO2 [237] | 2.0–3.8 | 130 | 10 | 50 | |
| P2−Na2/3Ni1/3Mn1/3Ti1/3O2 [236] | 2.5–4.1 | 88 | 1 a | 500 | 83.9 |
| NaNi0.5Mn0.2Ti0.3O2 [244] | 2.8 | 135 | 240 | 200 | 85 |
| Na3Ni1.5Cu0.5BiO6 [245] | 3.2 | 94 | 10.8 | 200 | 62 |
| NaNi0.5Mn0.5O2 CNT [235] | 2.5 | 141 | 12 | 100 | 90 |
| Na0.67[Mn0.6Ni0.1Fe0.3]O [246] | 4.3 | 200 | 13 | 25 | 75 |
| Na0.7CoO2 Microspheres [247] | 2.9 | 125 | 5 | 300 | 86 |
| Na0.67[Fe0.5Mn0.5]O2 [234] | 2.7 | 183 | 15 | 10 | 90 |
| Na0.9Cu0.22Fe0.3Mn0.48O2 [248] | 3.2 | 100 | 10 | 100 | |
| NaNi1/3Fe1/3Mn1/3O2 [249] | 123 | 130 | 100 | 80 | |
| Na0.67Mn0.67Ni0.28Mg0.05O2 [250] | 123 | 0.1 a | |||
| Na2/3Ni1/3Mn1/3Ti1/3O2 [236] | 88 | 17.3 | 500 | 83 | |
| HD−TiO2−N−RGO [241] | 203.4 | 100 | 100 | 91 | |
| Prussian blue | |||||
| NaxNi0.1Mn0.9Fe− (CN)6·nH2O [240] | 110 | 1000 | 95 | ||
| Prussian white | |||||
| Na1.92FeFe(CN)6 [251] | 3 | 150 | 600 | 1000 | 75 |
| Polyanionic Compoound | |||||
| Na2Fe(C2 O4)SO4 ·H2O [238] | 1.7–4.2 | ~75 | 44 | 500 | 85 |
| Na3V2(PO4)2F3 [252] | 3.7 | 120 | 0.05 a | ||
| Na2Fe(C2O4)SO4 [238] | 3.1 | 170 | 44 | 500 | 85 |
| Sulfur | |||||
| S sugar derived [253] | 0.8–2.6 | 700 | 1675 | 1500 | 81 |
| S/Fe–HC [254] | 0.8–2.7 | 1023 | 100 | 1000 | 38 |
| Cathode Material | Voltage (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| Transition metal Oxide | |||||
| NaCoO2 [271] | 2.9 | 80 | 0.05 a | 50 | 80 |
| NaCrO [272] | 2.95 | 88 | 0.05 a | 200 | 71 |
| K0.6CoO2 [273] | 3 | 82 | 11.8 | 300 | 87 |
| K0−3CrF6 | 5.43 | 284 | |||
| KFMO [274] | 2.45 [275] | 178 | 1000 | 300 | 87 |
| K0.3MnO2 [256] | 1.5–3.5 | 136 | 27.9 | 685 | 91 |
| K0.5MnO2 [257] | 3.6 | 140 | 20 | 50 | 76 |
| KNiCoMnO [276] | 3.1 | 76.5 | 20 | 100 | 87 |
| K0.5V2O5 [277] | 2.5 | 90 | 10 | 250 | 81 |
| Prussian blue analogue | |||||
| KCuFe(CN)6 [278] | 60 | 83 a | 83 | ||
| K4Fe(CN)6 [279] | 3.6 | 65.9 | 20 | 400 | 68 |
| KFeFe(CN)6 | 3.75 [280] | 122 [262] | 100 [281] | 1000 [281] | 90 [280] |
| KMnFe(CN)6 [263] | 3.9 [282] | 142 | 10 a [262] | 100 | 96 |
| KNiFe(CN)6 [283] | 59 | 41 a | 95 | ||
| SWCNT−PB [284] | 1000 | 80 | |||
| MWCNT−PB [284] | 1000 | 60 | |||
| RGO−PB−SSM [285] | 90 | 10 a | 87 | ||
| Polyanionic compounds | |||||
| FePO4 | 2.1 | 160 | 4 | 50 | |
| FeSO4F | 3.5 | 0.05 a | |||
| KVPO4F [259] | 4 | 92 | 665 | 50 | 97 |
| KVP2O7 [260] | 4.4 | 60 | 20 a | 100 | 83 |
| K3V2(PO4)2F3 [261] | 3.7 | 104 | 250 | 100 | 97 |
| K3V2(PO4)3/C [286] | 3.6 | 54 | 20 | 100 | |
| Organic | |||||
| Anthraquinone [264] | 1.7 | 114 | 13 | 100 | |
| K2C6O6 [265] | 1.7 | 213 | 1 a | 100 | |
| PAQS [267] | 2.4 | 200 | 50 | 300 | 80 |
| PTCDA [287] | 2.1 | 130 | 13 | 50 | |
| Sulfur | |||||
| Catholyte: S + K2Sx [288] | 402 | 1 | 1000 | 100 | |
| Confined and covalent S [269] | 873.9 | 100 | 300 | 86.3 | |
| CMK-3/S [289] | 606 | 10 | 10 | 40 | |
| S-CNT [290] | 1140 | 167.5 | 50 | 52.6 | |
| Microporous C/S [270] | 1198.3 | 20 | 150 | 72.5 | |
| Pyrolyzed poly acrylonitrile/S [268] | 0.8–2.9 | 710 | 125 | 100 | 54 |
| Sulfurized Carbonized polyacrylonitrile [291] | 1050 | 837.5 | 100 | 95 | |
| Cathode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| Layered Oxides | |||||
| Manganese | |||||
| −MnO2 nanorods [304] | 115.9 | 5000 | 4000 | 97.7 | |
| −MnO2 nanofibers [305] | 285 | 1520 | 5000 | 92 | |
| −MnO2/rGO [306] | 382 | 300 | 3000 | 94 | |
| MnO2 [307] | 70 | 1885 | 10,000 | ||
| MnO2−PANI [308] | 125 | 2000 | 5000 | ||
| Vanadium | |||||
| H2V3O8 nanowires [309] | 0.2–1.6 | 173.6 | 5000 | 1000 | 94.3 |
| H2V3O8 nanowires/ GO [297] | 0.2–1.6 | 394 | 300 | 2000 | 87 |
| Li−V2O5·nH2O [310] | 0.4–1.4 | 192 | 10,000 | 1000 | |
| LiV3O8 [311] | 0.6–1.2 | 140 | 133 | 65 | |
| Mg0.1V2O5 · H2O (MgVO) | 0.1-1.6 | 470 | 5000 | 3000 | 95 |
| Bilayer V2O5 · nH2O [293] | 0.2–1.6 | 200 | 6000 | 900 | 71 |
| VO [295] | 0.7–1.7 | 133 | 10,000 | 10,000 | 79 |
| VO (B) [296] | 0.3–1.5 | 357 | 100 | 50 | |
| VO [312] | 0.2–1.6 | 372 | 5000 | 4000 | 91.1 |
| V6O13 [313] | 0.2–1.5 | 240 | 4000 | 2000 | 92 |
| V3O7 · H2O/rGO [314] | 0.3–1.5 | 245 | 1500 | 1000 | 79 |
| VO2/rGO [315] | 0.3–1.3 | 240 | 4000 | 1000 | 99 |
| VS2 flake [316] | 0.4–1.0 | 125 | 200 | 250 | 99.7 |
| Zn0.25V2O5 · nH2O nanobelts [317] | 0.5–1.4 | 260 | 2400 | 1000 | 80 |
| others | |||||
| Ag0.4V2O5 [318] | 0.4–1.4 | 144 | 20 | 4000 | |
| Ca0.25V2O5 · nH2O [319] | 0.6–1.6 | 70 | 20 | 3000 | 96 |
| K2V6O16·2·7H2O nanorod [320] | 0.4–1.4 | 188 | 6 | 500 | 82 |
| Na0.33V2O5 [321] | 0.2–1.6 | 218.4 | 1 | 1000 | 93 |
| NaV3O8 [322] | 0.3–1.25 | 165 | 4 | 1000 | 82 |
| Na2V6O16·1·63H2O [323] | 0.2–1.6 | 158 | 5 | 6000 | 90 |
| Na1.1V3O7.9/rGO [324] | 0.4–1.4 | 171 | 300 | 100 | |
| NH4V4O10 [325] | 0.4–1.4 | 255.5 | 10 | 1000 | |
| MoO2/Mo2N nanobelts [326] | 0.25–1.35 | 113 | 1 | 1000 | 78.8 |
| MoS [327] | 0.3–1.5 | 161.7 | 1 | 1000 | 97.7 |
| Prussian blue analogues | |||||
| CuHCF [299] | 0.45–1.4 | 50 | 10,000 | 1000 [328] | 80 |
| ZnHCF [329] | 0.8–1.9 | 68 | 300 | 200 | 85 |
| Polyanionic compound | |||||
| Li3V2(PO4)3 [301] | 0.7–2.1 | 113.5 | 1500 [294] | ||
| Na3V2(PO4)3/C [302] | 0.8–1.7 | 97 | 50 | 200 [330] | 74 |
| Na3V2(PO4)2F3 [303] | 0.8–1.9 | 50 | 1000 | ||
| Organic | |||||
| Polyaniline [53] | 0.5–1.5 | 82 | 5000 | ||
| Quinones [331] | 0.2–1.8 | 120 | 500 | 3000 | 92 |
| Cathode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| Carbon | |||||
| Graphite [189] | 4.45 | 70 | 350 | 95 | |
| Layered Oxide | |||||
| CaCoO [334] | 3 | 150 | 30 | ||
| MoO [337] | 1.3 | 180 | 2 | 12 | |
| VO [338] | 3.2 | 465 | 200 [340] | 200 [340] | |
| Layered sulfide | |||||
| TiS [335] | 1.5 | 520 | 1/50 a | 1 | |
| S/meso -C [341] | 0.75 | 600 | |||
| Polyanionic compound | |||||
| VOPO4·2H2O [340] | 2.8 | 100 | 200 | 200 | |
| Prussian blue analogue | |||||
| K2BaFe(CN)6 | 0–0.8 | 55.8 | 100 | ||
| Na0.2MnFe(CN)6 [336] | 0–3.5 | 70 | 35 | ||
| Organic | |||||
| CaCuHCF [190] | 50 | 300 | 1000 | 95 | |
| Cathode Material | Voltage Range (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| Intercalation | |||||
| Mo6S8 [342] | 1.3 | 122 | 15 a | 3000 | 93 |
| MoO [344] | 1.8 | 210 | |||
| MoOF [345] | 70 | ||||
| MoVO [346] | 2.1 | 235 | |||
| TiS [347] | 1.2 | 200 | 0.2 a | 40 | |
| TiS [348] | 1.5 | 158 | 24 | 400 | 95 |
| TiS [349,350] | 1.2 | 83.7 | 10 | 50 | |
| TiSe [351] | 1 | 110 | 5 | 50 | |
| VO [352] | 2.56 | 150 | |||
| VSe [351] | 1 | 110 | |||
| Polianionic compound | |||||
| AgCl [353] | 2 | 178 | 930 | 100 | |
| CuS [354,355] | 1.6 | 200 | 50 | 30 | |
| Cu2Se [356] | 1.2 | 230 | 5 | 35 | |
| FePO4 [357] | 2 | 15 | 20 | ||
| MnO2 [358] | 2 | 150 | |||
| Silicate [359] | 4 | ||||
| Organic | |||||
| DMBQ [360] | 2 | 100 | 0.2 a | 30 | |
| PAQ [361] | 1.7 | 150 | 130 | 100 | |
| Sulphur | |||||
| S [362] | 1.77 | 600 | 200 | 100 | |
| S-ACCS [363] | 1.5 | 950 | 100 | 48 | |
| S-C [364] | 1.1 | 1081 | 30 | 76 | |
| S-CMK [365] | 1.6 | 800 | 100 [366] | 50 [366] | |
| S-CNT [367] | 1.3 | 1200 | 100 | 83 | |
| S-rGO [368] | 1.5 | 1028 | 50 | 21 | |
| Oxygen | |||||
| O [369,370] | 2.9 | 1300 | |||
| I [371] | 2 | 200 | |||
| Cathode Material | Voltage (V) | Capacity (mA h g) | Current (mA g) | Cycles | Retention (%) |
|---|---|---|---|---|---|
| Carbon | |||||
| 3D graphitic foams [374] | 1.8 | 60 | 12,000 | 7500 [373] | 100 |
| C paper [372] | 1.8 | 85 | 50 | ||
| C NF [376] | 126 | 1000 | 20,000 | 100 | |
| C nanoscrolls [376] | 104 | 1000 | 55,000 | 100 | |
| Defect-free G [375] | 1.94 | 100 | 5000 | 25,000 | 97 |
| G film [384] | 2.3 | 240 [385] | 6000 | 250,000 | 91.7 |
| G microflower [386] | 1.85 | 92 | 100 | 5000 | 100 |
| G nanoribbons [387] | 2 | 148 | 2000 | 10,000 | 98 |
| kish Graphite flakes [388] | 1.79 | 142 | 50 | 200 | 100 |
| mesoporous rGO [389] | 120 | 20 | 100 | 85 | |
| Zeolite-templated C [390] | 1.05 | 382 | 50 | 1000 | 86 |
| Metal Oxides | |||||
| Li3VO4/C [377] | 0.5 | 137 | 20 | 100 | 35 |
| MoO [378] | 1.95 | 90 | 100 | 100 | 28 |
| Mo2,5+yVO9+x [391] | 0.75 | 340 | 2 | 25 | 70 |
| VO [392] | 0.5 | 116 | 50 | 100 | 70 |
| VO [393] | 0.6 | 239 | 44.2 | ||
| VO/C [394] | 1 | 200 | 10 | 15 | 66 |
| VO nanowires [49] | 0.55 | 305 | 20 | 78 | |
| Metal sulfide | |||||
| CuS/C MS [379] | 1 | 90 | 20 | 100 | 32 |
| Cu0,31Ti2S4 [380] | 95 | 5 | 50 | 16 | |
| Hexagonal NiS NB [395] | 0.9 | 105 | 20 | 100 | 100 |
| Ni3S2/G [396] | 1 | 350 | 100 | 100 | 17 |
| Porous CuO MS [381] | 0.6 | 250 | 50 | 100 | 45 |
| Mo6S8 [397] | 80 | 6 | 50 | 47 | |
| SnS2/rGO [398] | 0.68 | 392 | 100 | 100 | 25 |
| Sn porous film [399] | 1.1 | 406 | 20 | 100 | 91 |
| TiS [380] | 50 | 5 | 50 | 72 | |
| VS4/rGO [399] | 407 | 100 | 100 | 20 | |
| Sulfur | |||||
| S/ACC [400] | 0.75 | 1320 | 500 | 20 | |
| S/CNF [382] | 0.76 | 600 | 21 | 20 | |
| S/mesoporous C [383] | 0.5 | 400 | 251 | ||
| S powder [401] | 1.2 | 1300 | 50 | 20 |
| Cyclic Carbonates | Organic Fluorinated Carbonates | Sulfones | Ionic Liquid |
|---|---|---|---|
| ACN | MFAa | EMSa | EMI |
| −BL | FPCa | TMS | DMPI |
| DEC | EMSa | FS | DEDMI |
| DMC | TFPMSa | BS | TMHE |
| DME | GLNb | EVS | PYR |
| DMF | ADNc | ADN | PIP |
| DMSO | SENa | DMMP | MORP |
| EC | TMMP | TFSI | |
| EMC | BC | BETI | |
| MF | FSI | ||
| NM | TSAC | ||
| PC | FSA− | ||
| THF | TFSA− | ||
| VC | BF4− | ||
| diglyme | PF6− | ||
| tetraglyme | N(CN)2− | ||
| DGM | [BH4]− | ||
| UREA | PEGylated |
| Li | Na | K | Zn | Ca | Mg | Al |
|---|---|---|---|---|---|---|
| LiTFSI | NaTFSI | KFTFSI | Zn(TFSI)2 | Ca(TFSI)2 | MgTSFI2 | AlCl4 |
| LiClO4 | NaClO4 | KClO4 | Zn(ClO4)2 | CA(ClO4)2 | MgCl | |
| LiBOB | NaBOB | KPF6 | Zn(CF3SO3)2 | Ca(PF6)2 | Mg(CB11H12)2 | |
| LiPF6 | NaPF6 | KBF4 | ZnSO4 | Ca(BF4)2 | Mg(BBu2Ph2)2 | |
| LiBF4 | NaBF4 | KFSI | Ca(NO3)2 | Mg[B(HFIP)4]2 | ||
| LiAsF6 | NaFSI | KCF3SO3 | Ca(BH4)2 | Mg(BH) | ||
| LiOSO2CF3 | NaFTFSI | |||||
| NaOTf | ||||||
| NaDFOB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantoja, W.; Perez-Taborda, J.A.; Avila, A. Tug-of-War in the Selection of Materials for Battery Technologies. Batteries 2022, 8, 105. https://doi.org/10.3390/batteries8090105
Pantoja W, Perez-Taborda JA, Avila A. Tug-of-War in the Selection of Materials for Battery Technologies. Batteries. 2022; 8(9):105. https://doi.org/10.3390/batteries8090105
Chicago/Turabian StylePantoja, Wendy, Jaime Andres Perez-Taborda, and Alba Avila. 2022. "Tug-of-War in the Selection of Materials for Battery Technologies" Batteries 8, no. 9: 105. https://doi.org/10.3390/batteries8090105








