Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Development of UBE-Loaded Bioadhesive Oral Films
2.3. Physico-Chemical Characterization of the UBE-Loaded Bioadhesive Oral Films
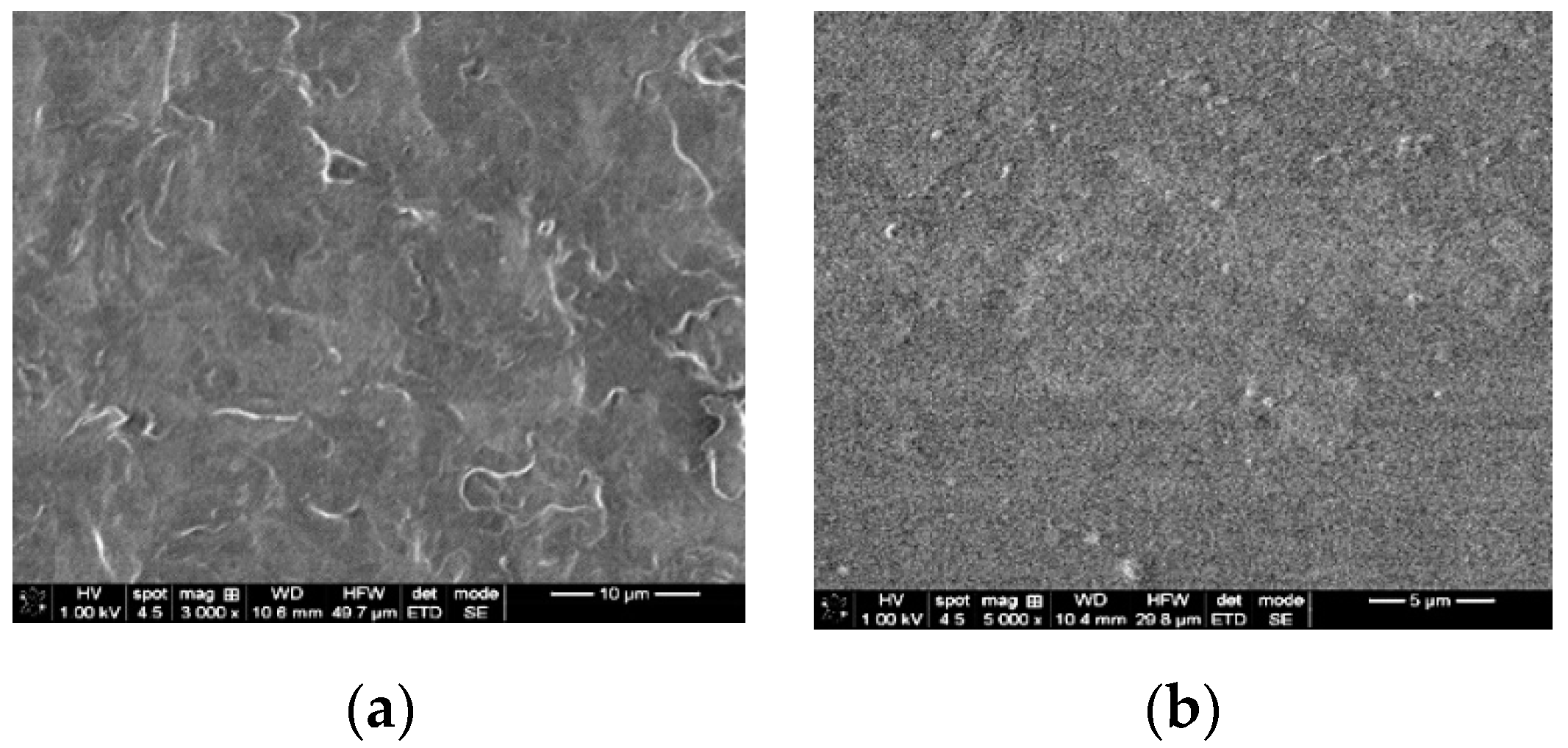
2.3.1. SEM Analysis
2.3.2. Atomic Force Microscopy (AFM)
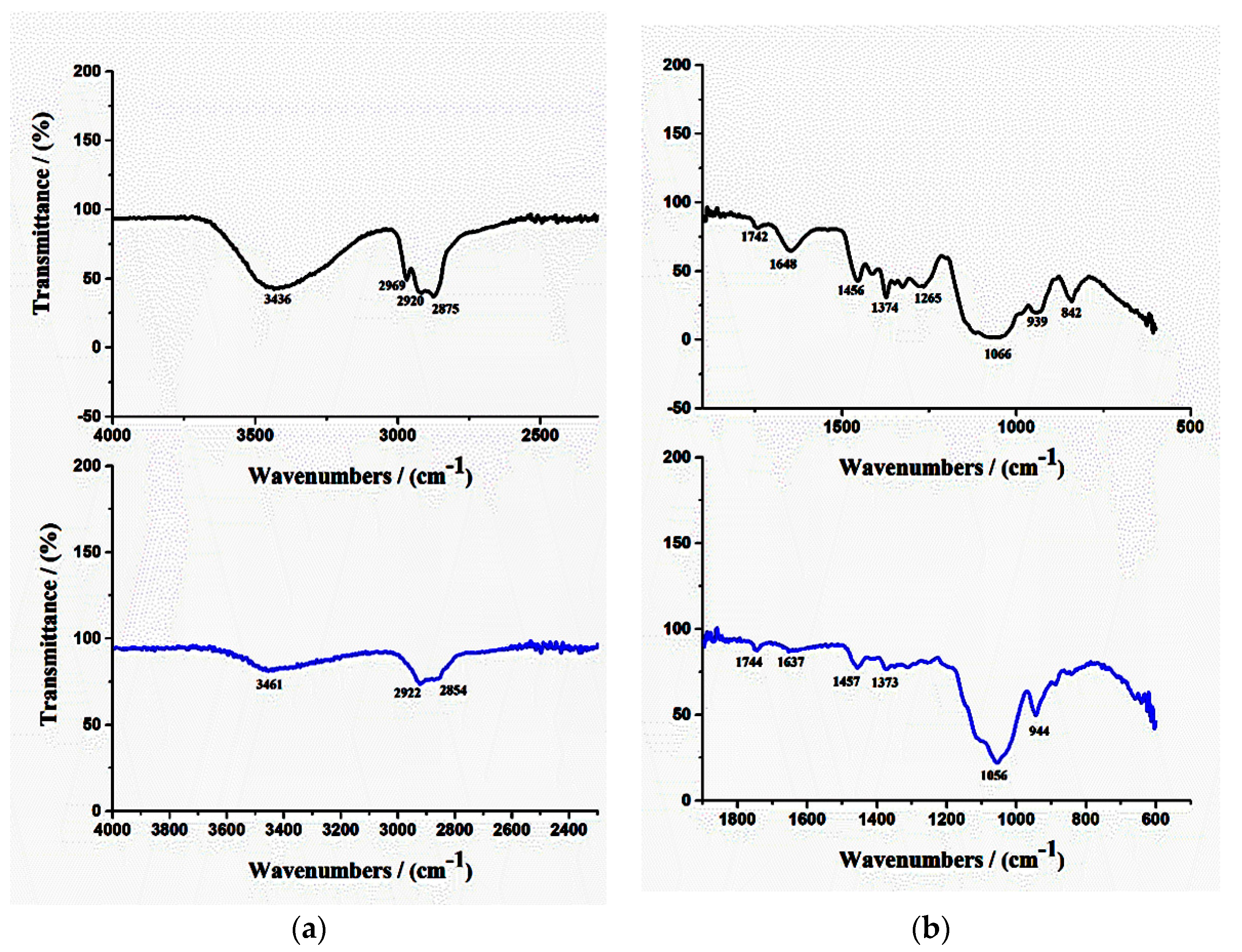
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Powder X-ray Diffractometry
2.3.5. Thermogravimetric Analysis
2.4. Pharmacotechnical Properties of the UBE-Loaded Bioadhesive Oral Films
2.4.1. Weight Uniformity
2.4.2. Thickness
2.4.3. Folding Endurance
2.4.4. Tensile Strength and Elongation Ability
2.4.5. Moisture Content
2.4.6. Surface pH
2.4.7. In Vitro Disintegration Time
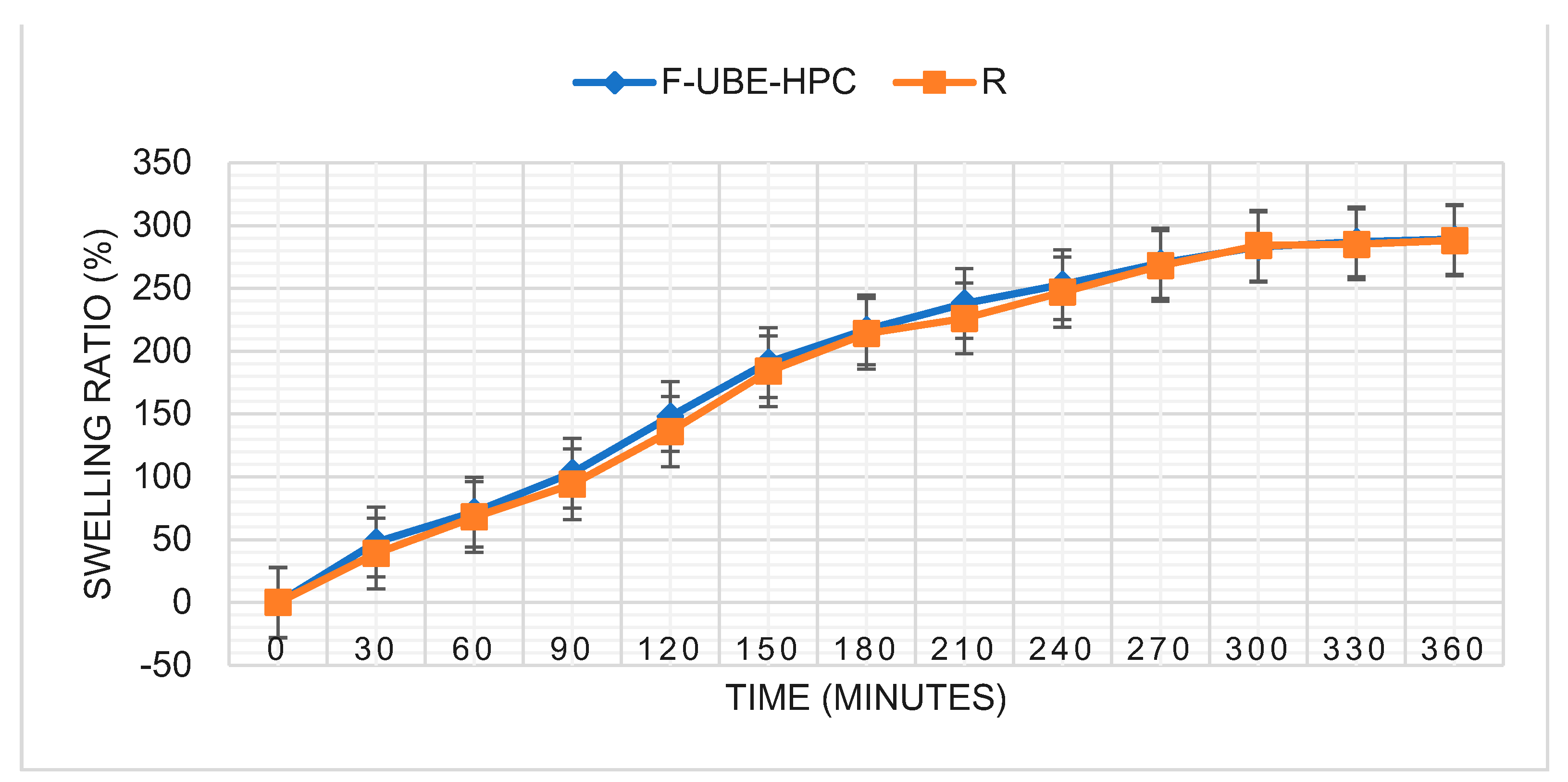
2.4.8. Swelling Ratio
2.4.9. Ex Vivo Bioadhesion Time
2.5. Antimicrobial Activity
2.5.1. Inoculum Preparation
2.5.2. Samples and Standards
2.5.3. Microdilution Method
2.5.4. Reading and Interpreting
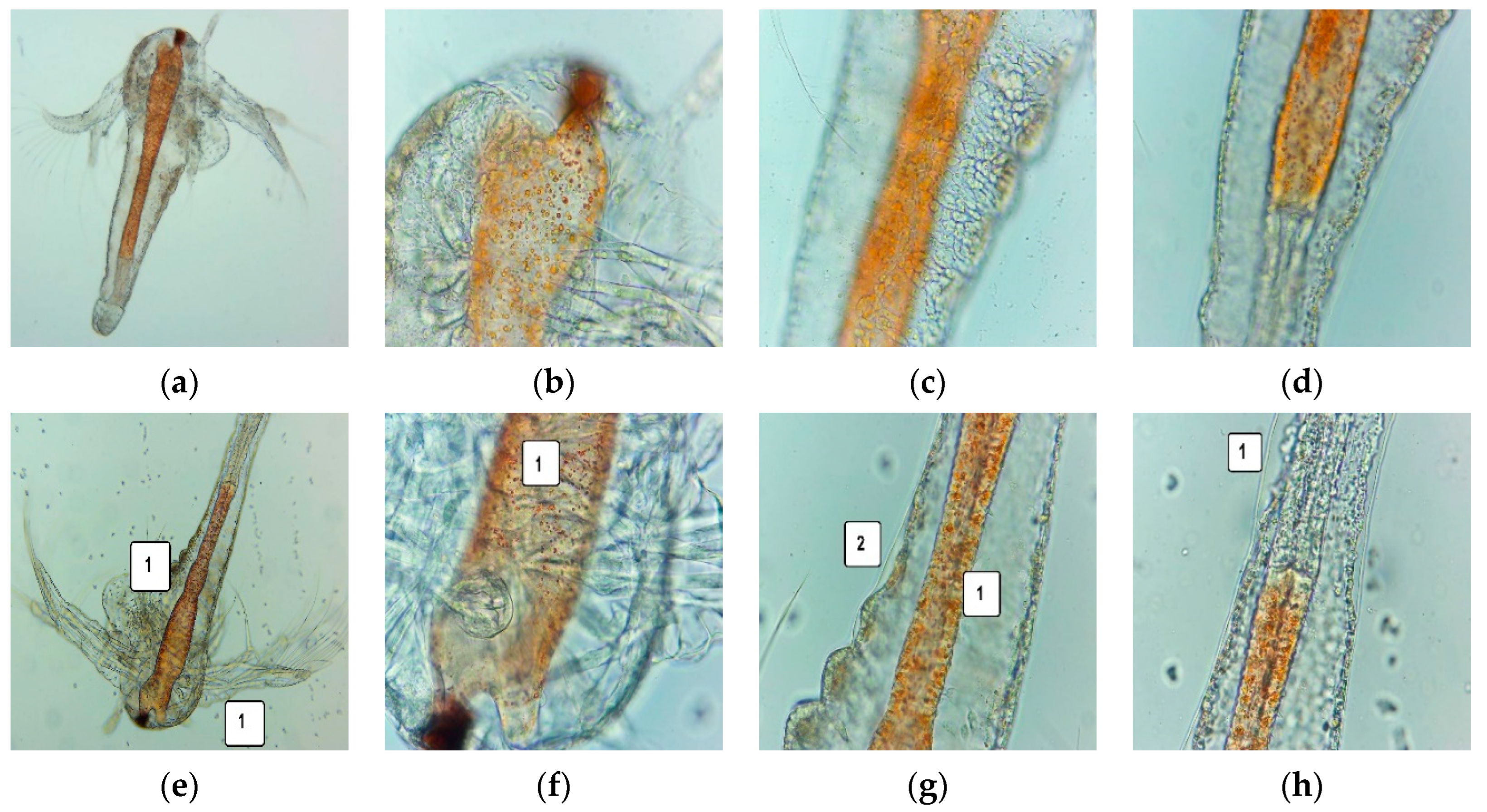
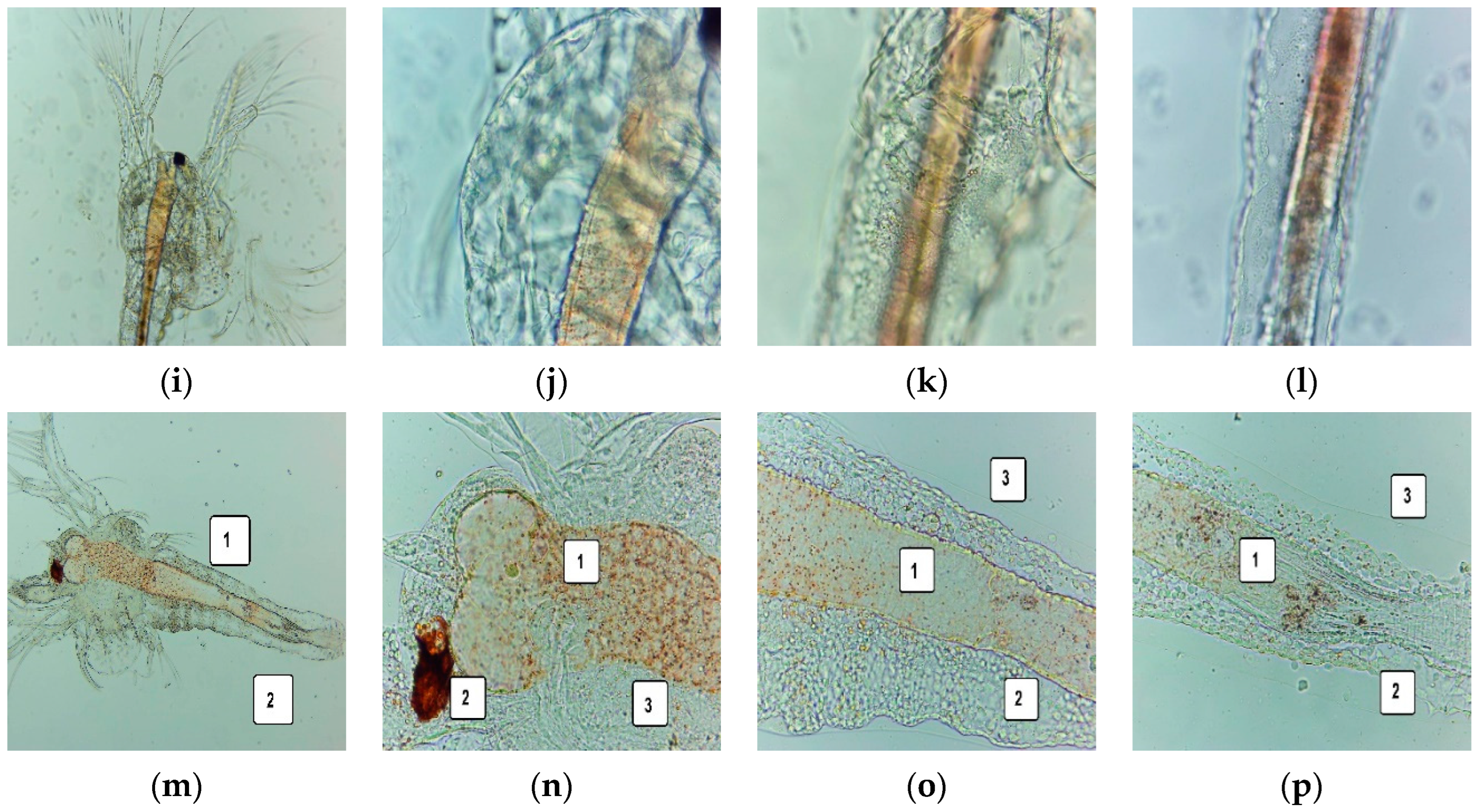
2.6. Cytotoxic Activity of UBE-Loaded Bioadhesive Oral Films on A. salina Larvae
2.6.1. Fluorescent Microscopy
2.6.2. Data Processing
2.7. In Vitro Analysis of the Biological Effects of UBE-Loaded Bioadhesive Oral Films on Human Blood Cell Cultures and CLS-354 Tumor Cell Line
2.7.1. Equipment
2.7.2. Data Processing
2.7.3. Human Blood Cells Cultures
2.7.4. CLS-354 Cell Line, Cell Culture
2.7.5. Samples and Control Solutions
2.7.6. Annexin V-FITC Apoptosis Assay
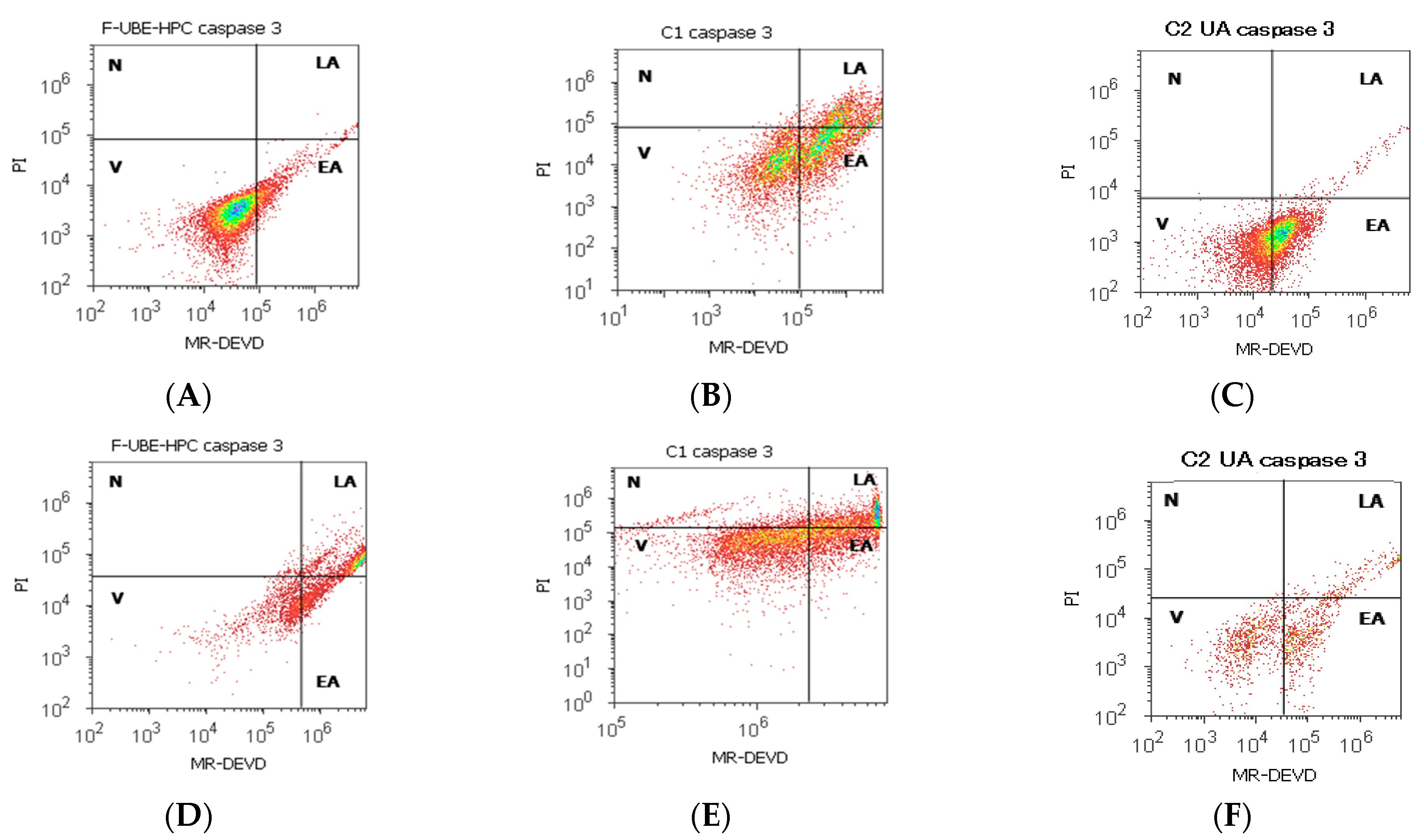
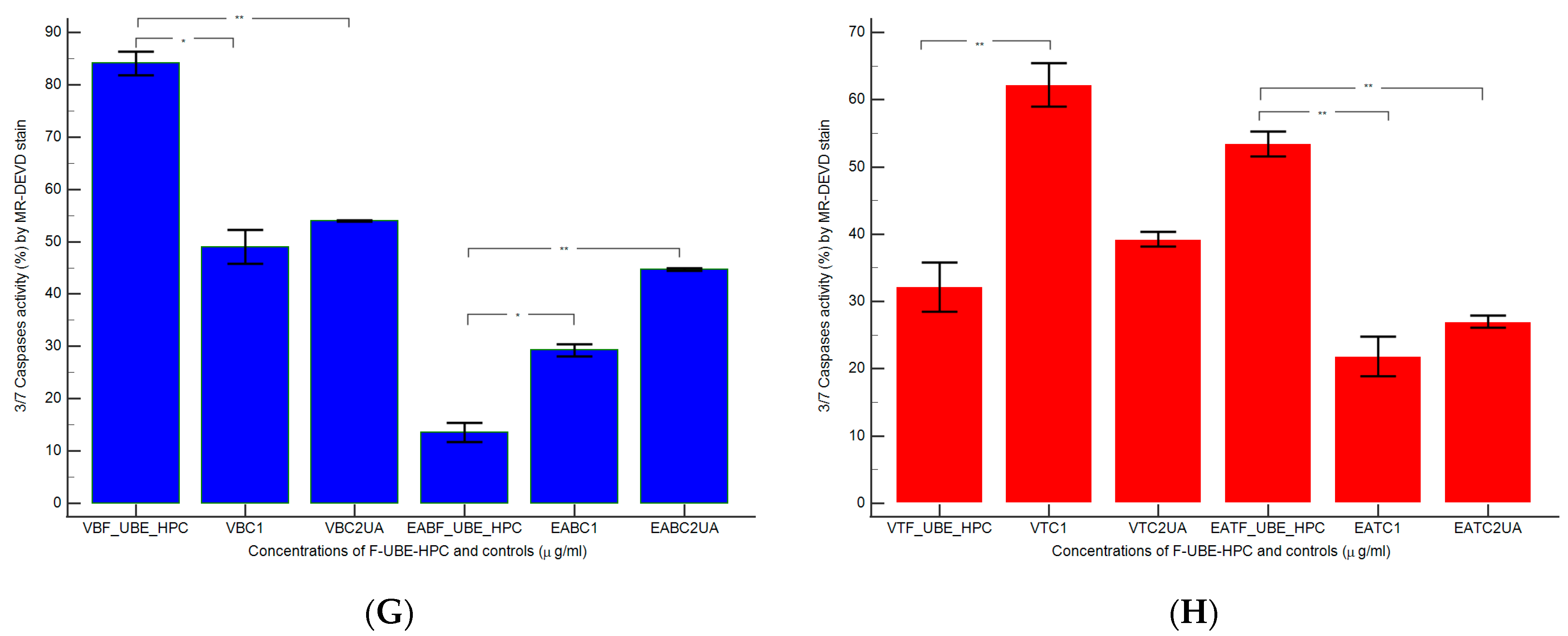
2.7.7. Evaluation of Caspase 3/7 Activity
2.7.8. Evaluation of Nuclear Condensation and Lysosomal Activity
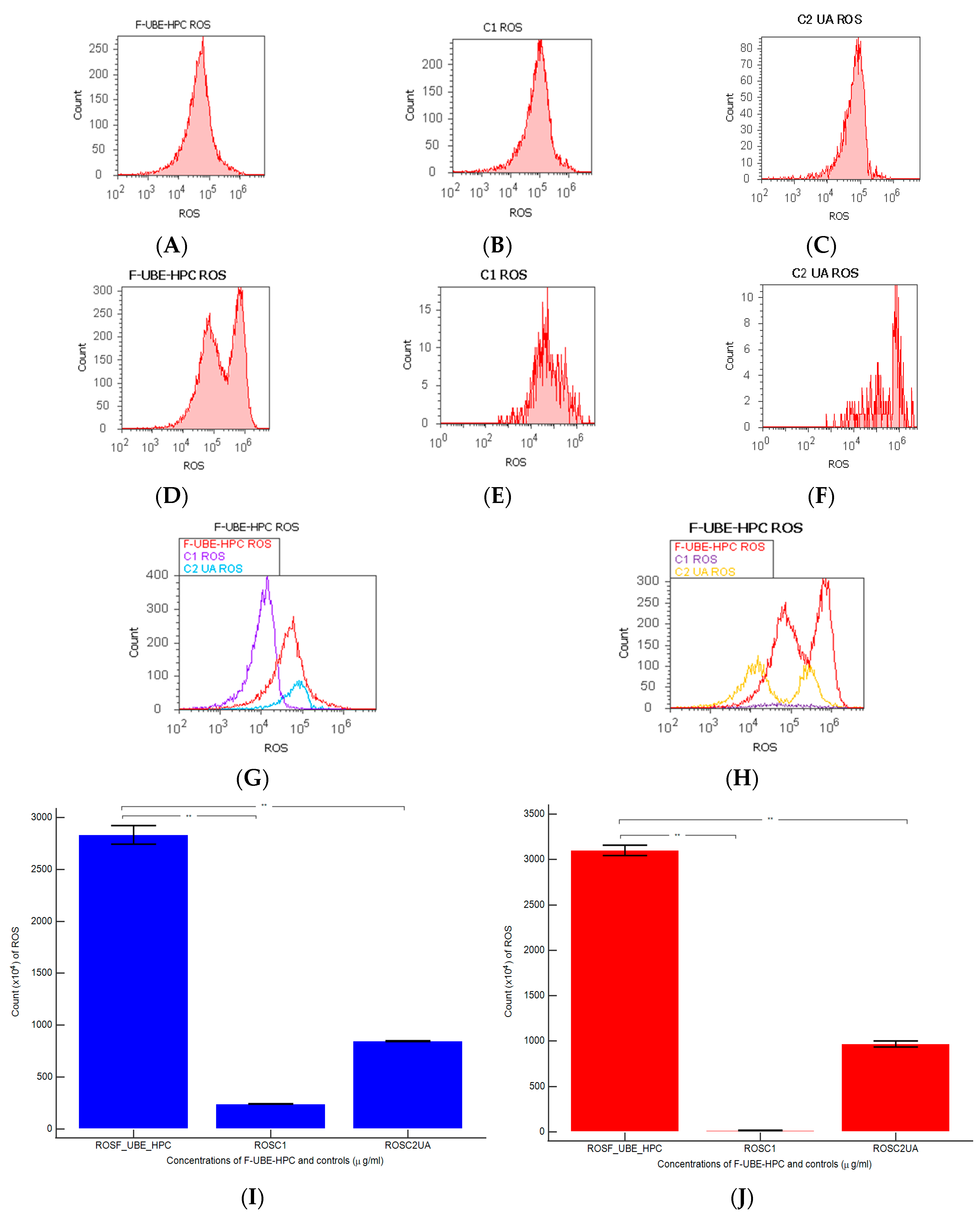
2.7.9. Evaluation of Total ROS Activity
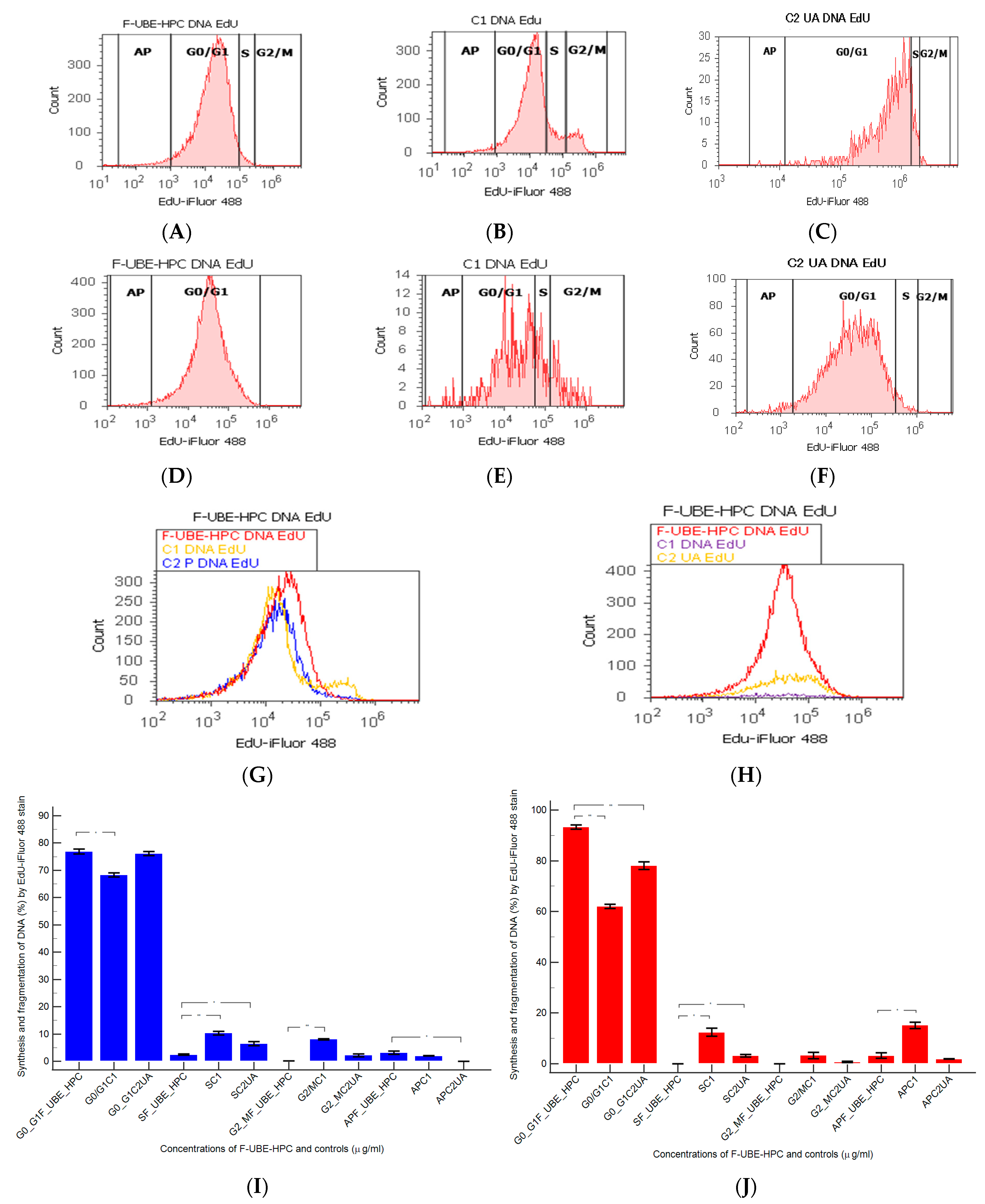
2.7.10. Cell Cycle Analysis
2.7.11. Evaluation of Cell Proliferation
2.8. Data Analysis
3. Results
3.1. Development of the Bioadhesive Oral Films
3.2. Physico-Chemical Characterization of the Bioadhesive Films
3.2.1. SEM Analysis
3.2.2. AFM Analysis
3.2.3. FTIR Analysis
3.2.4. XRD Analysis
3.2.5. TG Analysis
3.3. Pharmacotechnical Properties of the Bioadhesive Oral Films
3.4. Antimicrobial Activity
3.5. Cytotoxic Activity on A. salina Larvae
3.6. In Vitro Analysis of the Biological Effects of UBE-Loaded Mucoadhesive Oral Films on Human Blood Cell Cultures and CLS-354 Cancer Cell Line
3.6.1. Caspase 3/7 Activity
3.6.2. Nuclear Condensation and Lysosomal Activity
3.6.3. ROS Levels
3.6.4. Cell Cycle Analysis
3.6.5. Apoptosis
3.6.6. Cell Proliferation
3.6.7. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PDQ® Screening and Prevention Editorial Board. PDQ Oral Cavity, Oropharyngeal, Hypopharyngeal, and Laryngeal Cancers Prevention; National Cancer Institute: Bethesda, MD, USA, 2022. Available online: https://www.cancer.gov/types/head-and-neck/hp/oral-prevention-pdq (accessed on 30 July 2022).
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in oral cancer—A global scenario. Nepal J. Epidemiol. 2017, 6, 613–619. [Google Scholar] [CrossRef]
- PDQ® Adult Treatment Editorial Board. PDQ Lip and Oral Cavity Cancer Treatment (Adult); National Cancer Institute: Bethesda, MD, USA, 2022. Available online: https://www.cancer.gov/types/head-and-neck/hp/adult/lip-mouth-treatment-pdq (accessed on 30 July 2022).
- Mäkinen, A.; Nawaz, A.; Mäkitie, A.; Meurman, J.H. Role of Non-Albicans Candida and Candida albicans in Oral Squamous Cell Cancer Patients. J. Oral Maxillofac. Surg. 2018, 76, 2564–2571. [Google Scholar] [CrossRef]
- Soussan Irani New Insights into Oral Cancer—Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [CrossRef]
- PDQ® Supportive and Palliative Care Editorial Board. PDQ Oral Complications of Chemotherapy and Head/Neck Radiation; National Cancer Institute: Bethesda, MD, USA, 2022. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq (accessed on 30 July 2022).
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Huebner, J.; Follmann, M. Complementary medicine in guidelines of the German Guideline Program in Oncology: Comparison of the evidence base between complementary and conventional therapy. J. Cancer Res. Clin. Oncol. 2013, 139, 1481–1488. [Google Scholar] [CrossRef]
- Chong, W.Q.; Mogro, M.J.; Arsad, A.; Tai, B.C.; Lee, S.C. Use of decision aid to improve informed decision-making and communication with physicians on the use of oral complementary and alternative medicine (CAM) among cancer patients on chemotherapy treatment: A randomised controlled trial. Support. Care Cancer 2021, 29, 3689–3696. [Google Scholar] [CrossRef]
- Eita, A.A.B. Milk thistle (Silybum marianum (L.)Gaertn.): An overview about its pharmacology and medicinal uses with an emphasis on oral diseases. J. Oral Biosci. 2022, 64, 71–76. [Google Scholar] [CrossRef]
- da Silva, A.C.A.; Ramos, A.I.; Schirmer, E.M.; Massaroli, A.; Araújo, J.S.; da Conceição, V.M. Effect of Chamomilla Recutita in the oncology patient with oral mucositis: A systematic review. Enferm. Glob. 2021, 20, 640–652. [Google Scholar] [CrossRef]
- Santos, H.T.C.; Coimbra, M.C.; Meri Junior, A.E.; Gomes, A.J.P.S. Effectiveness of topically applied chamomile in the treatment of oral mucositis: A literature review. Res. Soc. Dev. 2021, 10, e433101422081. [Google Scholar] [CrossRef]
- Pawar, D.; Neve, R.S.; Kalgane, S.; Riva, A.; Bombardelli, E.; Ronchi, M.; Petrangolini, G.; Morazzoni, P. SAMITAL® improves chemo/radiotherapy-induced oral mucositis in patients with head and neck cancer: Results of a randomized, placebo-controlled, single-blind Phase II study. Support. Care Cancer 2013, 21, 827–834. [Google Scholar] [CrossRef]
- Choi, M.J.; Yoo, H.S.; Park, S.J. The Effects of the Supercritical Extracts of Momordica charantia Linn., Pistacia lentiscus, and Commiphora myrrha on Oral Inflammation and Oral Cancer. Sustainability 2022, 14, 2458. [Google Scholar] [CrossRef]
- de Souza, J.L.S.; Alves, T.; Camerini, L.; Nedel, F.; Campos, A.D.; Lund, R.G. Antimicrobial and cytotoxic capacity of pyroligneous extracts films of Eucalyptus grandis and chitosan for oral applications. Sci. Rep. 2021, 11, 21531. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS Pharm. Sci. Tech. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive buccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int. J. Pharm. 2003, 264, 1–14. [Google Scholar] [CrossRef]
- Balaci, T.; Velescu, B.; Karampelas, O.; Musuc, A.M.; Nițulescu, G.M.; Ozon, E.A.; Nițulescu, G.; Gîrd, C.E.; Fița, C.; Lupuliasa, D. Physico-chemical and pharmaco-technical characterization of inclusion complexes formed by rutoside with β-cyclodextrin and hydroxypropyl-β-cyclodextrin used to develop solid dosage forms. Processes 2021, 9, 26. [Google Scholar] [CrossRef]
- Musuc, A.M.; Anuta, V.; Atkinson, I.; Sarbu, I.; Popa, V.T.; Munteanu, C.; Mircioiu, C.; Ozon, E.A.; Nitulescu, G.M.; Mitu, M.A. Formulation of chewable tablets containing carbamazepine-β-cyclodextrin inclusion complex and f-melt disintegration excipient. The mathematical modeling of the release kinetics of carbamazepine. Pharmaceutics 2021, 13, 915. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Angelici, F.; Ricci, M.; Giovagnoli, S.; Capuccella, M.; Rossi, C. Development of mucoadhesive patches for buccal administration of ibuprofen. J. Control. Release 2004, 99, 73–82. [Google Scholar] [CrossRef]
- Don, T.M.; Huang, M.L.; Chiu, A.C.; Kuo, K.H.; Chiu, W.Y.; Chiu, L.H. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater. Chem. Phys. 2008, 107, 266–273. [Google Scholar] [CrossRef]
- Derle, D.; Joshi, O.; Pawar, A.; Patel, J.; Perdeshi, V. Effect of tablet excipients on mucoadhesive properties of polyoxyethylene and Carbopol 971P. Int. J. Pharm. Pharm. Sci. 2009, 1, 198–205. [Google Scholar]
- Gupta, A.; Garg, S.; Khar, R.K. Measurement of bioadhesive strength of mucoadhesive buccal tablets: Design of an in-vitro assembly. Indian Drugs 1993, 30, 1–6. [Google Scholar]
- Fathi, F.; Ghobeh, M.; Tabarzad, M. Anti-Microbial Peptides: Strategies of Design and Development and Their Promising Wound-Healing Activities. Mol. Biol. Rep. 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Madushan, R.; Vidanarachchi, J.K.; Prasanna, P.H.P.; Werellagama, S.; Priyashantha, H. Use of natural plant extracts as a novel microbiological quality indicator in raw milk: An alternative for resazurin dye reduction method. LWT 2021, 144, 111221. [Google Scholar] [CrossRef]
- Cox, K.D.; Quello, K.; Deford, R.J.; Beckerman, J.L. A rapid method to quantify fungicide sensitivity in the brown rot pathogen Monilinia fructicola. Plant Dis. 2009, 93, 328–331. [Google Scholar] [CrossRef]
- Bitacura, J.G. The Use of Baker’s Yeast in the Resazurin Reduction Test: A Simple, Low-Cost Method for Determining Cell Viability in Proliferation and Cytotoxicity Assays. J. Microbiol. Biol. Educ. 2018, 19, jmbe-19–87. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Rambu, D.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Ungureanu-Iuga, M.; Oroian, M.; Mironeasa, S.; et al. Antioxidant, Cytotoxic, and Rheological Properties of Canola Oil Extract of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 854. [Google Scholar] [CrossRef]
- Schröder, V.; Arcus, M.; Anghel, A.H.; Busuricu, F.; Lepadatu, A.C. Cell differentiation process of Artemia sp. larvae tools for natural products testing. Anim. Sci. 2019, 62, 149–153. [Google Scholar]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Oroian, M.; Mironeasa, S.; Schröder, V.; et al. Advances in the Characterization of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Appl. Sci. 2022, 12, 4234. [Google Scholar] [CrossRef]
- Ionescu, C.; Aschie, M.; Matei, E.; Cozaru, G.-C.; Deacu, M.; Mitroi, A.F.; Baltatescu, G.I.; Nicolau, A.; Mazilu, L.; Tuta, L.A.; et al. Characterization of the Tumor Microenvironment and the Biological Processes with a Role in Prostatic Tumorigenesis. Biomedicines 2022, 10, 1672. [Google Scholar] [CrossRef]
- Eguchi, N.; Kawabata, K.; Goto, H. Electrochemical Polymerization of 4,4-Dimethyl-2,2′-Bithiophene in Concentrated Polymer Liquid Crystal Solution. J. Mater. Sci. Chem. Eng. 2017, 05, 64–70. [Google Scholar] [CrossRef]
- Askari, F.; Zandi, M.; Shokrolahi, P.; Tabatabaei, M.H.; Hajirasoliha, E. Reduction in protein absorption on ophthalmic lenses by PEGDA bulk modification of silicone acrylate-based formulation. Prog. Biomater. 2019, 8, 169–183. [Google Scholar] [CrossRef]
- Ishii, N.; Mizobuchi, S.; Kawano, Y.; Hanawa, T. Preparation and evaluation of a powdered rebamipide mouthwash as in-hospital formulation: Considering dispersion before use in patients. Pharmaceutics 2021, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Matei, E.; Cozaru, G.-C.; Aschie, M.; Bucur, L.; Rambu, D.; Costache, T.; Cucolea, I.E.; Vochita, G.; Gherghel, D.; et al. Usnic acid and Usnea barbata (L.) F.H. wigg. dry extracts promote apoptosis and DNA damage in human blood cells through enhancing ROS levels. Antioxidants 2021, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Vochita, G.; Gherghel, D.; Mihai, C.T.; Rambu, D.; Calcan, S.I.; Costache, T.; Cucolea, I.E.; Matei, E.; et al. In vitro anticancer activity and oxidative stress biomarkers status determined by Usnea barbata (L.) f.h. wigg. dry extracts. Antioxidants 2021, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Spisák, S.; Solymosi, N.; Ittzés, P.; Bodor, A.; Kondor, D.; Vattay, G.; Barták, B.K.; Sipos, F.; Galamb, O.; Tulassay, Z.; et al. Complete Genes May Pass from Food to Human Blood. PLoS ONE 2013, 8, e69805. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar] [CrossRef]
- Cazacu, B.C.; Buzgar, N.; Iancu, O.G. Geochemical and spatial distribution of heavy metals in forest soils adjacent to the Tinovul Mare Poiana Stampei peat bog. Rev. Chim. 2018, 69, 434–438. [Google Scholar] [CrossRef]
- Culicov, O.A.; Yurukova, L. Comparison of element accumulation of different moss- and lichen-bags, exposed in the city of Sofia (Bulgaria). J. Atmos. Chem. 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Jayasekera, R.; Rossbach, M. Background levels of heavy metals in plants of different taxonomic groups from a montane rain forest in Sri Lanka. Environ. Geochem. Health 1996, 18, 55–62. [Google Scholar] [CrossRef]
- Conti, M.E.; Finoia, M.G.; Bocca, B.; Mele, G.; Alimonti, A.; Pino, A. Atmospheric background trace elements deposition in Tierra del Fuego region (Patagonia, Argentina), using transplanted Usnea barbata lichens. Environ. Monit. Assess. 2012, 184, 527–538. [Google Scholar] [CrossRef]
- World Health Organisation. WHO Guidelines on Good Manufacturing Practices (GMP) for Herbal Medicines 2007; IRIS (Institutional Repository for Information Sharing); World Health Organisation: Geneva, Switzerland, 2007; Available online: https://apps.who.int/iris/handle/10665/43672 (accessed on 20 May 2022).
- Popovici, V.; Bucur, L.; Popescu, A.; Schröder, V.; Costache, T.; Rambu, D.; Cucolea, I.E.; Gîrd, C.E.; Caraiane, A.; Gherghel, D.; et al. Antioxidant and cytotoxic activities of Usnea barbata (L.) f.h. wigg. dry extracts in different solvents. Plants 2021, 10, 909. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Determination of the content in usnic acid and polyphenols from the extracts of Usnea barbata L. And the evaluation of their antioxidant activity. Farmacia 2018, 66, 337–341. [Google Scholar]
- Popovici, V.; Bucur, L.; Costache, T.; Gherghel, D.; Vochita, G.; Mihai, C.T.; Rotinberg, P.; Schroder, V.; Badea, F.C.; Badea, V. Studies on Preparation and UHPLC Analysis of the Usnea Barbata (L) F.H.Wigg Dry acetone extract. Rev. Chim. 2019, 70, 3775–3777. [Google Scholar] [CrossRef]
- Stoicescu, I.; Lupu, E.C.; Radu, M.D.; Popescu, A.; Mihai, S. High-Performance Liquid Chromatography–Diode Array Detection (HPLC-DAD) Method for the Determination of Phenolic Compounds of Water Chesnut (Trapa natans L.). Anal. Lett. 2022, 55, 2147–2159. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary metabolite profiling of species of the genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen Metabolites: An Overview of Some Secondary Metabolites and Their Biological Potential. In Reference Series in Phytochemistry; Springer Science and Business Media B.V.: Berlin, Germany, 2020; pp. 175–209. [Google Scholar]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A Comparative Study of Isolated Secondary Metabolites from Lichens and Their Antioxidative Properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Caraiane, A.; Botnarciuc, M. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Evaluation of the Antibacterial Action of the Usnea barbata L. Extracts on Streptococcus Species from the Oro-Dental Cavity. In Proceedings of the 17th Romanian National Congress of Pharmacy, Bucharest, Romania, 26–29 September 2018. [Google Scholar]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Comparative study regarding antibacterial action if the Usnea barbata L. extracts on Gram-positive bacteria from the oro-dental cavity. In Proceedings of the 5th SGEM International Multidisciplinary Scientific Conferences on Social Sciences and Arts, Albena, Bulgaria, 24 August–2 September 2018. [Google Scholar]
- Bucur, L.; Badea, V. Comparative Study between Antioxidant Activity and Antibacterial Effect of Usnea barbata (L.) F.H. Wigg Extracts and Volatile Oils Marked in Romania. Ovidius. Univ. Ann. Econ. Sci. Ser. 2020, 2, 246–251. [Google Scholar]
- Lykkesfeldt, J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta 2007, 380, 50–58. [Google Scholar] [CrossRef]
- Cherian, D.; Peter, T.; Narayanan, A.; Madhavan, S.; Achammada, S.; Vynat, G. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci. 2019, 11, S297–S300. [Google Scholar] [CrossRef]
- Patel, V.M.; Prajapati, B.G.; Patel, M.M. Effect of hydrophilic polymers on buccoadhesive Eudragit patches of propranolol hydrochloride using factorial design. AAPS Pharm. Sci. Tech. 2007, 8, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Diaz del Consuelo, I.; Falson, F.; Guy, R.H.; Jacques, Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J. Control. Release 2007, 122, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gavriloaia, M.R.; Budura, E.A.; Toma, C.C.; Mitu, M.A.; Karampelas, O.; Arama, C.; Lupuleasa, D. In vitro evaluation of diffusion and rheological profiles for dexamethasone inclusion complexes with β-cyclodextrin or hydroxypropyl β-cyclodextrin. Farmacia 2012, 60, 895–904. [Google Scholar]
- Mǎnescu, O.; Lupuleasa, D.; Miron, D.S.; Budura, E.A.; Rǎdulescu, F.Ş. In vitro drug release from topical antifungal pharmaceutical formulations. Farmacia 2011, 59, 15–23. [Google Scholar]
- Guo, R.; Du, X.; Zhang, R.; Deng, L.; Dong, A.; Zhang, J. Bioadhesive film formed from a novel organic-inorganic hybrid gel for transdermal drug delivery system. Eur. J. Pharm. Biopharm. 2011, 79, 574–583. [Google Scholar] [CrossRef]
- Nesseem, D.I.; Eid, S.F.; El-Houseny, S.S. Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory drug. Life Sci. 2011, 89, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Ramezani, V.; Seyedabadi, M.; Ranjbar, A.M.; Jafari, H.; Honarvar, M.; Fanaei, H. Formulation and optimization of oral mucoadhesive patches of Myrtus communis by box behnken design. Adv. Pharm. Bull. 2017, 7, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Esim, Ö. Preparation and in vitro evaluation of methylene blue films for treatment of oral mucosal diseases. Gulhane Med. J. 2019, 61, 109–113. [Google Scholar] [CrossRef]
- Cao, N.; Yang, X.; Fu, Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Bharkatiya, M.; Nema, R.K.; Bhatnagar, M. Designing and Characterization of Drug Free Patches for Transdermal Application. Int. J. Pharm. Sci. Drug Res. 2010, 2, 35. [Google Scholar]
- Shaikh, R.; Raj Singh, T.; Garland, M.; Woolfson, A.; Donnelly, R. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [PubMed]
- Tedesco, M.P.; Monaco-Lourenço, C.A.; Carvalho, R.A. Characterization of oral disintegrating film of peanut skin extract—Potential route for buccal delivery of phenolic compounds. Int. J. Biol. Macromol. 2017, 97, 418–425. [Google Scholar] [CrossRef]
- Repka, M.A.; McGinity, J.W. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. J. Control. Release 2001, 70, 341–351. [Google Scholar] [CrossRef]
- Ravi Kumar Reddy, J.; Indira Muzib, Y.; Chowdary, K.P.R. Development and in-vivo characterization of novel trans buccal formulations of Amiloride hydrochloride. J. Pharm. Res. 2013, 6, 647–652. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Marudova, M. Polyelectrolyte Multilayer Films as a Potential Buccal Platform for Drug Delivery. Polymers 2022, 14, 734. [Google Scholar] [CrossRef]
- Panomsuk, S.P.; Hatanaka, T.; Aiba, T.; Katayama, K.; Koizumi, T. A study of the hydrophilic cellulose matrix: Effect of drugs on swelling properties. Chem. Pharm. Bull. 1996, 44, 1039–1042. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, D.; Jayakumari, L.S. Evaluation of commercial arrowroot starch/CMC film for buccal drug delivery of glipizide. Polimeros 2019, 29, e2019047. [Google Scholar] [CrossRef]
- Waghulde, S.; Kale, M.K.; Patil, V. Brine Shrimp Lethality Assay of the Aqueous and Ethanolic Extracts of the Selected Species of Medicinal Plants. Proceedings 2019, 41, 47. [Google Scholar]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.; Sarkar, D.; Shetty, K. Elicitation of Stress-Induced Phenolic Metabolites for Antimicrobial Applications against Foodborne Human Bacterial Pathogens. Antibiotics 2021, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA. Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-based antioxidant extracts and compounds in the management of oral cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, R.M.; Jovanova, B.; Kadifkova Panovska, T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Popovici, P.C.; Ancuceanu, V.R.; Olaru, T.O.; Stoicescu, C.-S.; Dinu, M. Toxicity Assessment of Nephrolepis exaltata (L.) Schott, Fam. Nephrolepidaceae. Acta Biol. Marisiensis 2018, 1, 27–36. [Google Scholar] [CrossRef]
- Hovaneţ, M.V.; Ancuceanu, R.V.; Dinu, M.; Oprea, E.; Budura, E.A.; Negreş, S.; Velescu, B.Ş.; Duţu, L.E.; Anghel, I.A.; Ancu, I.; et al. Toxicity and anti-inflammatory activity of Ziziphus jujuba Mill. leaves. Farmacia 2016, 64, 802–808. [Google Scholar]
- Iancu, I.M.; Bucur, L.A.; Schroder, V.; Mireșan, H.; Sebastian, M.; Iancu, V.; Badea, V. Phytochemical evaluation and cytotoxicity assay of Lythri herba extracts. Farmacia 2021, 69, 51–58. [Google Scholar] [CrossRef]
- Nazir, S.; Ansari, F.L.; Hussain, T.; Mazhar, K.; Muazzam, A.G.; Qasmi, Z.U.H.; Makhmoor, T.; Noureen, H.; Mirza, B. Brine shrimp lethality assay ‘an effective prescreen’: Microwave-assisted synthesis, BSL toxicity and 3DQSAR studies-based designing, docking and antitumor evaluation of potent chalcones. Pharm. Biol. 2013, 51, 1091–1103. [Google Scholar] [CrossRef]
- Arcanjo, D.; Albuquerque, A.; Melo-Neto, B.; Santana, L.; Medeiros, M.; Citó, A. Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz. J. Biol. 2012, 72, 505–509. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between reactive oxygen species and autophagy: Special issue: Death mechanisms in cellular homeostasis. Biochim. Biophys. Actamol. Cell Res. 2021, 1868, 119041. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Murata, M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1808. [Google Scholar] [CrossRef] [PubMed]
- Kardeh, S.; Ashkani-Esfahani, S.; Alizadeh, A.M. Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur. J. Pharmacol. 2014, 735, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Actamol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, Á.; Cook, A.W.; Gough, R.E.; Schilling, M.; Olszok, N.A.; Brown, I.; Wang, L.; Aaron, J.; Martin-Fernandez, M.L.; Rehfeldt, F.; et al. DNA damage alters nuclear mechanics through chromatin reorganization. Nucleic Acids Res. 2021, 49, 340–353. [Google Scholar] [CrossRef]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Coman, F.; Ozon, E.A.; Gherghiceanu, F.; Andronic, O.; Ion, D.; Stănescu, M.; Bolocan, A. The use of nutritional supplement in romanian patients–attitudes and beliefs. Farmacia 2019, 67, 1060–1065. [Google Scholar] [CrossRef]
- Favreau, J.T.; Ryu, M.L.; Braunstein, G.; Orshansky, G.; Park, S.S.; Coody, G.L.; Love, L.A.; Fong, T.L. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Ann. Intern. Med. 2002, 136, 590–595. [Google Scholar] [CrossRef]
- Sanchez, W.; Maple, J.T.; Burgart, L.J.; Kamath, P.S. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin. Proc. 2006, 81, 541–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.A.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Healpart C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar]












| Ingredients | Quantity (g) | |
|---|---|---|
| F-UBE-HPC | R | |
| UBE | 0.30 | - |
| Ethyl alcohol 96% (v/v) | 10.00 | 10.00 |
| PEG 400 | 5.00 | 5.00 |
| HPC 20% water dispersion (w/w) | 84.70 | 85.00 |
| Film | 1st Step (%) | 2nd Step (%) | 3rd Step (%) |
|---|---|---|---|
| TG (%) | TG(%)/Tmax (°C) | TG(%)/Tmax (°C) | |
| R | 2.4% | 88.4%/357.6 °C | 9.2%/468.3 °C |
| F-UBE-HPC | 2.3% | 85.6%/352.3 °C | 12.1%/471.8 °C |
| Parameter * | Formulation Code | |
|---|---|---|
| F-UBE-HPC | R | |
| Weight uniformity (mg) | 110 ± 4.77 | 107 ± 5.25 |
| Thickness (mm) | 0.093 ± 0.002 | 0.093 ± 0.003 |
| Folding endurance value | >300 | >300 |
| Tensile strength (kg/mm2) | 2.48 ± 1.58 | 2.57 ± 1.71 |
| Elongation % | 63.14 ± 1.94 | 62.75 ± 1.52 |
| Moisture content % (w/w) | 6.58 ± 0.44 | 6.23 ± 0.56 |
| pH | 7.10 ± 0.02 | 7.07 ± 0.01 |
| Disintegration time (seconds) | 118 ± 3.16 | 115 ± 4.19 |
| Swelling ratio (% after 6 h) | 289 ± 5.82 | 288 ± 6.13 |
| Ex vivo biooadhesion time (minutes) | 98 ± 3.58 | 98 ± 4.17 |
| Microdilution | CTR | TRF 10.1 mg/mL | F-UBE-HPC 110 mg/mL | |
|---|---|---|---|---|
| 30 mg/mL | 122 mg/mL | |||
| 1 | 1.5 | 6.10 | 0.5 | 5.5 |
| 2 | 0.75 | 4.88 | 0.25 | 2.75 |
| 3 | 0.375 | 3.904 | 0.125 | 1.375 |
| 4 | 0.187 | 3.123 | 0.062 | 0.685 |
| 5 | 0.093 | 2.498 | 0.031 | 0.342 |
| 6 | 0.046 | 1.998 | 0.015 | 0.171 |
| 7 | 0.024 | 1599 | 0.007 | 0.085 |
| Dil. | S. aureus | P. aeruginosa | ||||||
|---|---|---|---|---|---|---|---|---|
| CTR F-UBE | F-UBE-HPC | CTR | F-UBE-HPC | |||||
| A | B | C | D | E | F | G | H | |
| 1 |  |  |  |  |  |  |  |  |
| 2 | ||||||||
| 3 | ||||||||
| 4 | ||||||||
| 5 | ||||||||
| 6 | ||||||||
| 7 | ||||||||
 * * | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Matei, E.; Cozaru, G.-C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. https://doi.org/10.3390/pharmaceutics14091808
Popovici V, Matei E, Cozaru G-C, Bucur L, Gîrd CE, Schröder V, Ozon EA, Sarbu I, Musuc AM, Atkinson I, et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics. 2022; 14(9):1808. https://doi.org/10.3390/pharmaceutics14091808
Chicago/Turabian StylePopovici, Violeta, Elena Matei, Georgeta-Camelia Cozaru, Laura Bucur, Cerasela Elena Gîrd, Verginica Schröder, Emma Adriana Ozon, Iulian Sarbu, Adina Magdalena Musuc, Irina Atkinson, and et al. 2022. "Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy" Pharmaceutics 14, no. 9: 1808. https://doi.org/10.3390/pharmaceutics14091808











