Passionfruit (Passiflora edulis) Peel Powder Stimulates the Immune and Antioxidant Defense System in Nile Tilapia, Oreochromis niloticus, Cultivated in a Biofloc System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powdered Passionfruit Peel and Experimental Diets
2.2. Culture Conditions
2.3. Experimental Design
2.4. Growth Rates
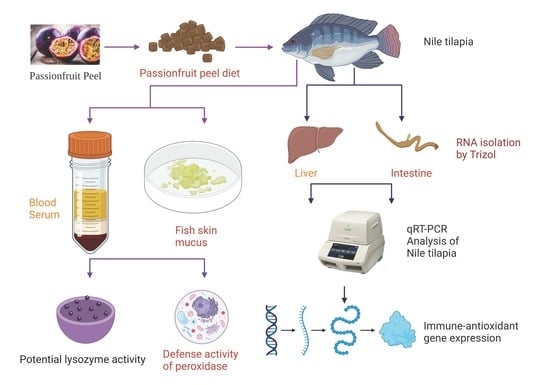
2.5. Immune Response Analysis
2.5.1. Sample Collection
2.5.2. Lysozyme and Peroxidase Assay
2.6. Total RNA Extraction and Real-Time PCR (qPCR) Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance Analysis
3.2. Analysis of Skin Mucus Immune Responses
3.3. Analysis of Serum Immune Responses
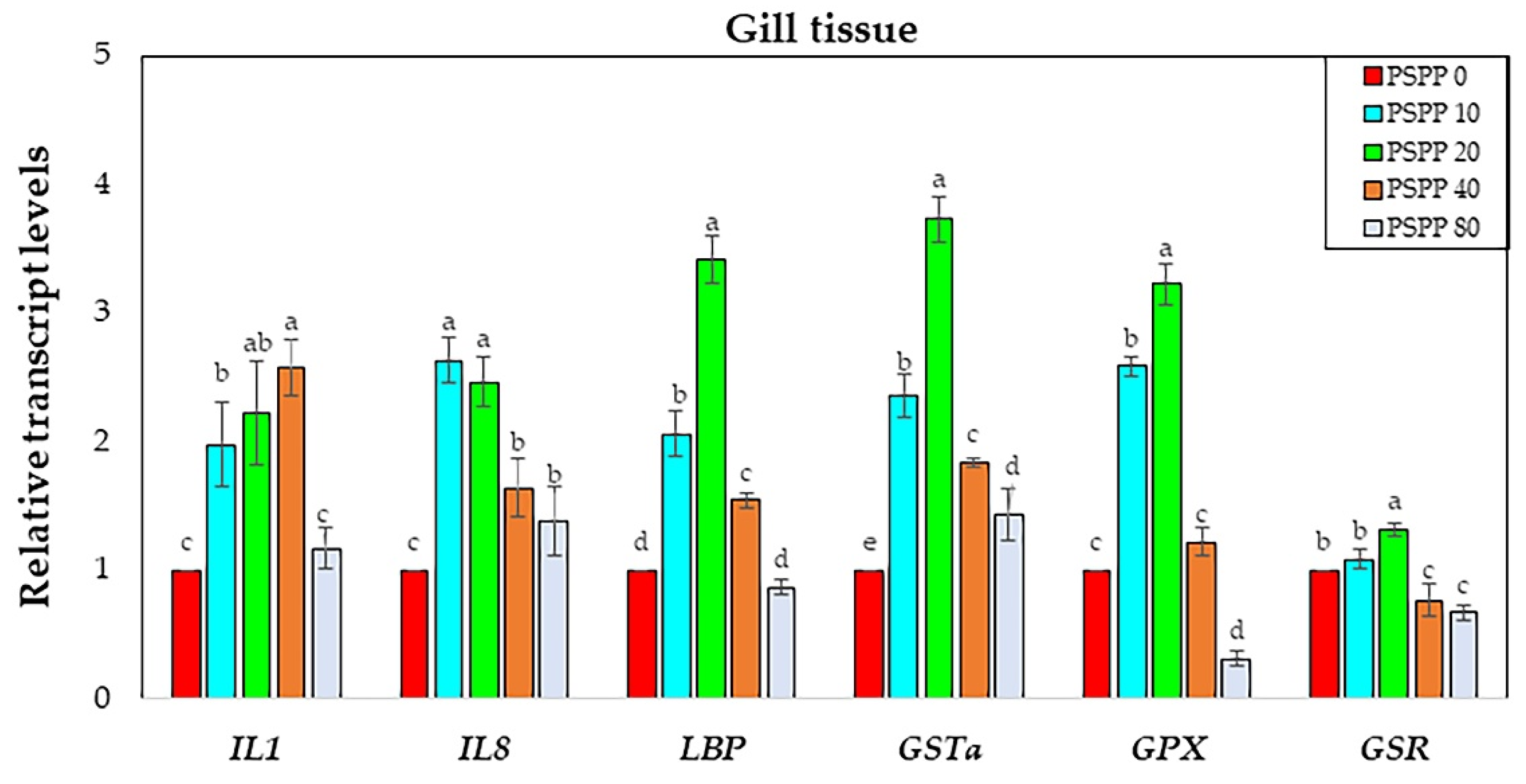
3.4. Analysis of Immune and Antioxidant-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magbanua, T.O.; Ragaza, J.A. Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus. Aquac. Fish. 2022. [Google Scholar] [CrossRef]
- Fialho, N.S.; Valenti, W.C.; David, F.S.; Godoy, E.M.; Proença, D.C.; Roubach, R.; Wolff Bueno, G. Environmental sustainability of Nile tilapia net-cage culture in a neotropical region. Ecol. Indic. 2021, 129, 108008. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Soontara, C.; Kerddee, P.; Nguyen, D.H.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; et al. Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines 2022, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Munguti, J.M.; Nairuti, R.; Iteba, J.O.; Obiero, K.O.; Kyule, D.; Opiyo, M.A.; Abwao, J.; Kirimi, J.G.; Outa, N.; Muthoka, M.; et al. Nile tilapia (Oreochromis niloticus Linnaeus, 1758) culture in Kenya: Emerging production technologies and socio-economic impacts on local livelihoods. Aquac. Fish Fish. 2022, 2, 265–276. [Google Scholar] [CrossRef]
- Paredes-Trujillo, A.; Mendoza-Carranza, M. A systematic review and meta-analysis of the relationship between farm management, water quality and pathogen outbreaks in tilapia culture. J. Fish Dis. 2022, 00, 1–20. [Google Scholar] [CrossRef]
- Alazab, A.; Sadat, A.; Younis, G. Prevalence, antimicrobial susceptibility, and genotyping of Streptococcus agalactiae in Tilapia fish (Oreochromis niloticus) in Egypt. J. Adv. Vet. Anim Res. 2022, 9, 95–103. [Google Scholar] [CrossRef]
- Chen, S.-W.; Liu, C.-H.; Hu, S.-Y. Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 84, 695–703. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Payne, C.J.; Turnbull, J.F.; MacKenzie, S.; Crumlish, M. Investigating the Effect of an Oxytetracycline Treatment on the Gut Microbiome and Antimicrobial Resistance Gene Dynamics in Nile Tilapia (Oreochromis niloticus). Antibiotics 2021, 10, 1213. [Google Scholar] [CrossRef]
- Sun, B.-Y.; He, W.; Yang, H.-X.; Tian, D.-Y.; Jian, P.-Y.; Wu, K.; Yang, C.-G.; Song, X.-H. Increased susceptibility to Aeromonas hydrophila infection in grass carp with antibiotic-induced intestinal dysbiosis. Aquaculture 2022, 552, 737969. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M.R. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Iwashita, M.K.P.; Addo, S.; Terhune, J.S. 9—Use of pre- and probiotics in finfish aquaculture. In Feed and Feeding Practices in Aquaculture, 2nd ed.; Davis, D.A., Ed.; Woodhead Publishing: Oxford, UK, 2022; pp. 269–289. [Google Scholar]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2022, 14, 130–157. [Google Scholar] [CrossRef]
- Buruiana, C.-T.; Gómez, B.; Vizireanu, C.; Garrote, G. Manufacture and evaluation of xylooligosaccharides from corn stover as emerging prebiotic candidates for human health. LWT 2017, 77, 449–459. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Deehan, E.C.; Zhang, Z.; Riva, A.; Armet, A.M.; Perez-Muñoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.-Y.; Cole, J.; et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022, 10, 77. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and Biofunctional properties with Emphasis on Recent Trends and Advances. Trends Food Sci. Technol. 2020, 99, 507–519. [Google Scholar] [CrossRef]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; de Vries, H. Critical success and risk factors for circular business models valorising agricultural waste and by-products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Thakur, N.; Nigam, M.; Tewary, R.; Rajvanshi, K.; Kumar, M.; Shukla, S.K.; Mahmoud, G.A.-E.; Gupta, S. Drivers for the behavioural receptiveness and non-receptiveness of farmers towards organic cultivation system. J. King Saud Univ. Sci. 2022, 34, 102107. [Google Scholar] [CrossRef]
- Yan, C.; Muhammad Rizwan, H.; Liang, D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F. The effect of the root-colonizing Piriformospora indica on passion fruit (Passiflora edulis) development: Initial defense shifts to fitness benefits and higher fruit quality. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Hu, Y.; Jiao, L.; Jiang, M.-H.; Yin, S.; Dong, P.; Zhao, Z.-M.; Yang, D.-P.; Ho, P.-T.; Wang, D.-M. A new C-glycosyl flavone and a new neolignan glycoside from Passiflora edulis Sims peel. Nat. Prod. Res. 2018, 32, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- FMI. Passion Fruit Extract Market projected to Reach USD 1,028.6 Million by 2029—Comprehensive Research Report by FMI; FMI: Maharashtra, India, 2022. [Google Scholar]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Abboud, K.Y.; Iacomini, M.; Simas, F.F.; Cordeiro, L.M.C. High methoxyl pectin from the soluble dietary fiber of passion fruit peel forms weak gel without the requirement of sugar addition. Carbohydr. Polym. 2020, 246, 116616. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Abdul Karim Shah, N.N.; Sulaiman, A.; Mohamed Amin Tawakkal, I.S.; Mohd Nor, M.Z.; Ariffin, S.H.; Abdul Ghani, N.H.; Mohd Salleh, F.S. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers 2021, 13, 3503. [Google Scholar] [CrossRef]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokóvić, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Manap, N.W.A.; Bhuyar, P.; Azelee, N.I.W. Passion fruit (Passiflora edulis) peel powder extract and its application towards antibacterial and antioxidant activity on the preserved meat products. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Cao, Q.; Teng, J.; Wei, B.; Huang, L.; Xia, N. Phenolic compounds, bioactivity, and bioaccessibility of ethanol extracts from passion fruit peel based on simulated gastrointestinal digestion. Food Chem. 2021, 356, 129682. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.d.P.; Teixeira Tarley, C.R. Bioaccessibility estimation of metallic macro and micronutrients Ca, Mg, Zn, Fe, Cu and Mn in flours of oat and passion fruit peel. LWT 2021, 150, 111880. [Google Scholar] [CrossRef]
- Júnior, J.E.L.; da Costa, J.M.; Neiva, J.N.M.; Rodriguez, N.M. Physical-chemical characterization of tropical fruit by-products for use in animal feed. Rev. Ciência Agronômica 2006, 37, 70. [Google Scholar]
- Fachinello, M.R.; Pozza, P.C.; Moreira, I.; Carvalho, P.L.O.; Castilha, L.D.; Pasquetti, T.J.; Esteves, L.A.C.; Huepa, L.M.D. Effect of passion fruit seed meal on growth performance, carcass, and blood characteristics in starter pigs. Trop. Anim. Health Prod. 2015, 47, 1397–1403. [Google Scholar] [CrossRef]
- Perondi, D.; Moreira, I.; Pozza, P.C.; Carvalho, P.L.d.O.; Pasquetti, T.J.; Huepa, L.M.D. Passion fruit seed meal at growing and finishing pig (30-90 kg) feeding. Ciência Agrotecnologia 2014, 38, 390–400. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.; Makkar, H.P. Waste to worth: fruit wastes and by-products as animal feed. CAB Rev. 2015, 10, 1–26. [Google Scholar] [CrossRef]
- Fachinello, M.R.; Pozza, P.C.; Furlan, A.C.; Paula, V.R.C.d.; Bonagurio, L.P.; Marcato, S.M.; Leal, I.F.; Huepa, L.M.D. Nutritional evaluation of passion fruit seed meal for meat quails. Rev. Bras. De Saúde Produção Anim. 2016, 17, 202–213. [Google Scholar] [CrossRef]
- Togashi, C.K.; Fonseca, J.B.; Soares, R.d.T.R.N.; da Costa, A.P.D.; da Silveira, K.F.; Detmann, E. Subprodutos do maracujá em dietas para frangos de corte. Acta Sci. Anim. Sci. 2008, 30, 395–400. [Google Scholar] [CrossRef]
- Togashi, C.K.; Fonseca, J.B.; Soares, R.d.T.R.N.; Gaspar, A.; Detmann, E. Composição em ácidos graxos dos tecidos de frangos de corte alimentados com subprodutos de maracujá. Rev. Bras. De Zootec. 2007, 36, 2063–2068. [Google Scholar] [CrossRef]
- De Souza, A.M.; Campeche, D.F.B.; Moraes, G.; de Melo, F.; da Cruz Neto, M.A.; Melo, J.F.B. Replacing cornmeal with mango meal in diets for juvenile tambaqui Colossoma macropomum: Growth and metabolic parameters. Bol. Inst. Pesca 2018, 44, e248. [Google Scholar] [CrossRef]
- Wegbecher, F.X. Bactérias Celulolíticas e o Uso de Resíduo de Maracujá (Passiflora edulis) em Rações Extrusadas Para Juvenis de Tambaqui (Colossoma macropomum); Instituto Nacional de Pesquisas da Amazônia: Petrópolis, Brazil, 2010. [Google Scholar]
- dos Santos, E.A.; Ribeiro, A.E.C.; Barcellos, T.T.; Monteiro, M.L.G.; Mársico, E.T.; Caliari, M.; Júnior, M.S.S. Exploitation of byproducts from the passion fruit juice and tilapia filleting industries to obtain a functional meat product. Food Biosci. 2021, 41, 101084. [Google Scholar] [CrossRef]
- Ahmad, I.; Babitha Rani, A.; Verma, A.; Maqsood, M. Biofloc technology: An emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 2017, 25, 1215–1226. [Google Scholar] [CrossRef]
- Durigon, E.G.; Lazzari, R.; Uczay, J.; de Alcântara Lopes, D.L.; Jerônimo, G.T.; Sgnaulin, T.; Emerenciano, M.G.C. Biofloc technology (BFT): Adjusting the levels of digestible protein and digestible energy in diets of Nile tilapia juveniles raised in brackish water. Aquac. Fish. 2020, 5, 42–51. [Google Scholar] [CrossRef]
- Wei, G.; Shan, D.; Li, G.; Li, X.; Tian, R.; He, J.; Shao, Z. Prokaryotic communities vary with floc size in a biofloc-technology based aquaculture system. Aquaculture 2020, 529, 735632. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Abakari, G.; Wu, X.; He, X.; Fan, L.; Luo, G. Bacteria in biofloc technology aquaculture systems: Roles and mediating factors. Rev. Aquac. 2022, 14, 1260–1284. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Van Doan, H.; Hoseinifar, S.H.; Harikrishnan, R.; Khamlor, T.; Punyatong, M.; Tapingkae, W.; Yousefi, M.; Palma, J.; El-Haroun, E. Impacts of pineapple peel powder on growth performance, innate immunity, disease resistance, and relative immune gene expression of Nile tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2021, 114, 311–319. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; World Aquaculture Society: Baton Rouge. LA, USA, 2015. [Google Scholar]
- Panigrahi, A.; Saranya, C.; Sundaram, M.; Vinoth Kannan, S.R.; Das, R.R.; Satish Kumar, R.; Rajesh, P.; Otta, S.K. Carbon: Nitrogen (C:N) ratio level variation influences microbial community of the system and growth as well as immunity of shrimp (Litopenaeus vannamei) in biofloc based culture system. Fish Shellfish. Immunol. 2018, 81, 329–337. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Sringarm, K.; Hoseinifar, S.H.; Dawood, M.A.O.; El-Haroun, E.; Harikrishnan, R.; Jaturasitha, S.; Paolucci, M. Impacts of Amla (Phyllanthus emblica) fruit extract on growth, skin mucosal and serum immunities, and disease resistance of Nile tilapia (Oreochromis niloticus) raised under biofloc system. Aquac. Rep. 2022, 22, 100953. [Google Scholar] [CrossRef]
- Xuan, C.L.; Wannavijit, S.; Outama, P.; Lumsangkul, C.; Tongsiri, S.; Chitmanat, C.; Doan, H.V. Dietary inclusion of rambutan (Nephelium lappaceum L.) seed to Nile tilapia (Oreochromis niloticus) reared in biofloc system: Impacts on growth, immunity, and immune-antioxidant gene expression. Fish Shellfish. Immunol. 2022, 122, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.M., Jr.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Cordero, H.; Cuesta, A.; Meseguer, J.; Esteban, M.Á. Changes in the levels of humoral immune activities after storage of gilthead seabream (Sparus aurata) skin mucus. Fish Shellfish. Immunol. 2016, 58, 500–507. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Le Xuan, C.; Wannavijit, S.; Outama, P.; Montha, N.; Lumsangkul, C.; Tongsiri, S.; Chitmanat, C.; Hoseinifar, S.H.; van Doan, H. Effects of dietary rambutan (Nephelium lappaceum L.) peel powder on growth performance, immune response and immune-related gene expressions of striped catfish (Pangasianodon hypophthalmus) raised in biofloc system. Fish Shellfish. Immunol. 2022, 124, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Hamajima, N.; Okada, R.; Naito, M.; Morita, E.; Hishida, A.; Kawai, S.; Nishio, K.; Yin, G.; Asai, Y. SAS/STAT 9.1 user’s guide SAS/STAT 9.1 user’s guide, 2004. J. Epidemiol. 2009, 19, 72–80. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Badawy, M.T.; Ahmed, F.K.; Kalia, A.; Abd-Elsalam, K.A. Chapter 10—Fruit peel waste-to-wealth: Bionanomaterials production and their applications in agroecosystems. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Abd-Elsalam, K.A., Periakaruppan, R., Rajeshkumar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–257. [Google Scholar]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Perar, K.; Fonseca, F.; Affonso, E.; Nobre, A. Passion Fruit (Passiflora edulis) Seed Cake as a Feed Ingredient for Jaraqui (Semaprochilodus insignis) and Tambaqui (Colossoma macropomum). J. Aquac. Mar. Biol. 2017, 6, 173. [Google Scholar] [CrossRef]
- Lazzari, R.; Uczay, J.; Henriques, J.; Durigon, E.; Kunz, D.; Peixoto, N.; Fronza, D. Growth and digestive enzymes of silver catfish fed diets containing fruit residue. Arq. Bras. De Med. Veterinária E Zootec. 2019, 71, 323–330. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; ElHady, M.; Shalaby, S.I. Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 2019, 505, 92–97. [Google Scholar] [CrossRef]
- Conrado, A.; Iunes, R.; Neyrão, I. Performance of Nile tilapia (Oreochromis niloticus) juveniles fed diets with different levels of passion fruit seed oil (Passiflora edulis). Livest. Res. Rural. Dev. 2020, 32, 8. [Google Scholar]
- Hamed, H.S.; Abdel-Tawwab, M. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquaculture 2021, 540, 736742. [Google Scholar] [CrossRef]
- Chekani, R.; Akrami, R.; Ghiasvand, Z.; Chitsaz, H.; Jorjani, S. Effect of dietary dehydrated lemon peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 2021, 539, 736597. [Google Scholar] [CrossRef]
- Zhuo, L.-C.; Chen, C.-F.; Lin, Y.-H. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish. Immunol. 2021, 117, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.-C.; Abang Zamhari, D.N.J.b.; Yong, A.S.K.; Shapawi, R.; Lin, Y.-H. Effects of fermented lemon peel supplementation in diet on growth, immune responses, and intestinal morphology of Asian sea bass, Lates calcarifer. Aquac. Rep. 2021, 21, 100801. [Google Scholar] [CrossRef]
- Whangchai, N.; Yaibouathong, D.; Junluthin, P.; Balakrishnan, D.; Unpaprom, Y.; Ramaraj, R.; Pimpimol, T. Effect of biogas sludge meal supplement in feed on growth performance molting period and production cost of giant freshwater prawn culture. Chemosphere 2022, 301, 134638. [Google Scholar] [CrossRef]
- Abboud, K.Y.; da Luz, B.B.; Dallazen, J.L.; Werner, M.F.d.P.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Iacomini, M.; Cordeiro, L.M.C. Gastroprotective effect of soluble dietary fibres from yellow passion fruit (Passiflora edulis f. flavicarpa) peel against ethanol-induced ulcer in rats. J. Funct. Foods 2019, 54, 552–558. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, G.C.; Batista, Â.G.; Carazin, C.B.B.; Cintra, D.E.; Prado, M.A.; Júnior, M.R.M. Passion fruit peel intake decreases inflammatory response and reverts lipid peroxidation and adiposity in diet-induced obese rats. Nutr. Res. 2020, 76, 106–117. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Sganzerla, M.; Jacques, A.C.; Barcia, M.T.; Zambiazi, R.C. Carotenoids, tocopherols and ascorbic acid content in yellow passion fruit (Passiflora edulis) grown under different cultivation systems. LWT Food Sci. Technol. 2015, 64, 259–263. [Google Scholar] [CrossRef]
- Shabir, U.; Dar, J.S.; Bhat, A.H.; Ganai, B.A.; Khan, I.A. Isolation and characterization of β-defensin-like protein 1 from epidermal mucus of fungal infected fish (Cyprinus carpio) and assessment of its antimicrobial potencies. Aquac. Rep. 2022, 23, 101056. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Ding, L.G.; Huang, Z.Y.; Xu, H.Y.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Wannavijit, S.; Outama, P.; Le Xuan, C.; Lumsangkul, C.; Lengkidworraphiphat, P.; Tongsiri, S.; Chitmanat, C.; Doan, H.V. Modulatory effects of longan seed powder on growth performance, immune response, and immune-antioxidant related gene expression in Nile tilapia (Oreochromis niloticus) raised under biofloc system. Fish Shellfish. Immunol. 2022, 123, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Lee, C.-H.; Kim, K.-D.; Lim, H.J.; Kim, H.S. Effects of diet supplementation with plant juice processing by-products on juvenile black rockfish (Sebastes schlegelii) growth performance, feed utilization, non-specific immunity, and disease resistance against Vibrio harveyi. Aquac. Rep. 2021, 21, 100831. [Google Scholar] [CrossRef]
- Silva-Brito, F.; Cardoso, A.; Machado, M.; Ramos-Pinto, L.; Hinzmann, M.; Abreu, H.; Costas, B.; Magnoni, L. Dietary supplementation with Gracilaria gracilis by-products modulates the immune status and oxidative stress response of gilthead seabream (Sparus aurata) stimulated with Photobacterium damselae subsp. piscicida. Fish Shellfish. Immunol. 2022, 126, 164–177. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef]
- Lall, S.P.; Dumas, A. 3—Nutritional requirements of cultured fish: Formulating nutritionally adequate feeds. In Feed and Feeding Practices in Aquaculture, 2nd ed.; Davis, D.A., Ed.; Woodhead Publishing: Oxford, UK, 2022; pp. 65–132. [Google Scholar]
- Cruvinel, W.d.M.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; Souza, A.W.S.d.; Silva, N.P.d.; Andrade, L.E.C. Immune system: Part I. Fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev. Bras. De Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef]
- Ahlf, W.; Heise, S. Sediment Toxicity Assessment: Rationale for effect classes (5 pp). J. Soils Sediments 2005, 5, 16–20. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free. Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Panigrahi, A.; Esakkiraj, P.; Das, R.R.; Saranya, C.; Vinay, T.; Otta, S.K.; Shekhar, M.S. Bioaugmentation of biofloc system with enzymatic bacterial strains for high health and production performance of Penaeus indicus. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S.H.; Harikrishnan, R.; Balasundaram, C.; Jaturasitha, S. Effects of coffee silverskin on growth performance, immune response, and disease resistance of Nile tilapia culture under biofloc system. Aquaculture 2021, 543, 736995. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Jaturasitha, S.; Meidong, R.; Hoseinifar, S.H.; Dawood, M.A. Modulation of growth, skin mucus and serum immunities, and disease resistance of Nile tilapia fed host-associated probiotic (Lactobacillus paracasei l61-27b). Aquac. Nutr. 2021, 27, 3–12. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.; Dawood, M.A. The enrichment of diet with beneficial bacteria (single-or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]



| PSPP | PSPP0 | PSPP10 | PSPP20 | PSPP40 | PSPP80 | |
|---|---|---|---|---|---|---|
| Fish meal | - | 150 | 150 | 150 | 150 | 150 |
| Corn meal | - | 200 | 200 | 200 | 200 | 200 |
| Soybean meal | - | 390 | 387 | 384 | 383 | 380 |
| Wheat flour | - | 70 | 70 | 70 | 70 | 70 |
| Rice bran | - | 150 | 150 | 150 | 135 | 100 |
| PSPP | - | 0 | 10 | 20 | 40 | 80 |
| Cellulose | - | 20 | 13 | 6 | 2 | 0 |
| Soybean oil | - | 5 | 5 | 5 | 5 | 5 |
| Premix | - | 10 | 10 | 10 | 10 | 10 |
| Vitamin C (98%) | - | 5 | 5 | 5 | 5 | 5 |
| Total | - | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate composition of the experimental diets (%) | ||||||
| Crude protein | 10.1 | 32.80 | 35.2 | 34.5 | 33.6 | 32.3 |
| Crude lipid | 1.01 | 2.85 | 3.45 | 4.18 | 3.62 | 3.60 |
| Fiber | 25.73 | 3.68 | 4.36 | 5.45 | 5.21 | 6.44 |
| Ash | 8.02 | 7.59 | 8.38 | 7.96 | 7.87 | 7.99 |
| Dry matter | 95.13 | 99.16 | 97.05 | 96.16 | 98 | 97.69 |
| Gross Energy (cal/g) | 2731 | 4273 | 4278 | 4203 | 4185 | 4085 |
| Target Genes | Sequence (5′-3′) | Tm (°C) | Product Size (bp) | Ref. |
|---|---|---|---|---|
| 18S rRNA | GTGCATGGCCGTTCTTAGTT CTCAATCTCGTGTGGCTGAA | 60 | 150 | [61] |
| il-1 | GTCTGTCAAGGATAAGCGCTG ACTCTGGAGCTGGATGTTGA | 59 | 200 | [61] |
| il-8 | CTGTGAAGGCATGGGTGTG GATCACTTTCTTCACCCAGGG | 59 | 196 | [61] |
| lbp | ACCAGAAACTGCGAGAAGGA GATTGGTGGTCGGAGGTTTG | 59 | 200 | [61] |
| gst-α | ACTGCACACTCATGGGAACA TTAAAAGCCAGCGGATTGAC | 60 | 190 | [61] |
| gpx | GGTGGATGTGAATGGAAAGG CTTGTAAGGTTCCCCGTCAG | 60 | 190 | [61] |
| gsr | CTGCACCAAAGAACTGCAAA CCAGAGAAGGCAGTCCACTC | 60 | 172 | [61] |
| PSPP 0 | PSPP 10 | PSPP 20 | PSPP 40 | PSPP 80 | |
|---|---|---|---|---|---|
| IW (g) | 14.22 ± 0.04 a | 14.23 ± 0.04 a | 14.25 ± 0.05 a | 14.15 ± 0.03 a | 14.23 ± 0.03 a |
| FW (g) | |||||
| 4 weeks | 28.60 ± 1.64 a | 29.05 ± 1.11 a | 28.69 ± 0.64 a | 29.73 ± 1.23 a | 29.67 ± 0.37 a |
| 8 weeks | 55.03 ± 0.64 a | 55.01 ± 0.91 a | 53.84 ± 1.35 a | 55.93 ± 1.36 a | 54.05 ± 0.79 a |
| SGR (%) | |||||
| 4 weeks | 2.32 ± 0.18 a | 2.37 ± 0.13 a | 2.33 ± 0.06 a | 2.47 ± 0.13 a | 2.45 ± 0.05 a |
| 8 weeks | 2.26 ± 0.02 a | 2.25 ± 0.03 a | 2.21 ± 0.04 a | 2.29 ± 0.04 a | 2.22 ± 0.03 a |
| WG (g) | |||||
| 4 weeks | 14.38 ± 1.61 a | 14.82 ± 1.13 a | 14.44 ± 0.59 a | 15.58 ± 1.21 a | 15.44 ± 0.39 a |
| 8 weeks | 40.81 ± 0.64 a | 40.78 ± 0.90 a | 39.59 ± 1.33 a | 41.78 ± 1.34 a | 39.82 ± 0.82 a |
| FCR | |||||
| 4 weeks | 1.00 ± 0.07 a | 1.03 ± 0.03 a | 1.11 ± 0.08 a | 0.98 ± 0.06 a | 1.01 ± 0.00 a |
| 8 weeks | 0.96 ± 0.03 a | 1.01 ± 0.03 a | 1.01 ± 0.02 a | 0.98 ± 0.02 a | 1.01 ± 0.02 a |
| SR (%) | |||||
| 4 weeks | 100 | 98 | 97 | 100 | 98 |
| 8 weeks | 98 | 95 | 95 | 98 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Outama, P.; Linh, N.V.; Xuan, C.L.; Wannavijit, S.; Tongsiri, S.; Chitmanat, C.; Montha, N.; Van Doan, H. Passionfruit (Passiflora edulis) Peel Powder Stimulates the Immune and Antioxidant Defense System in Nile Tilapia, Oreochromis niloticus, Cultivated in a Biofloc System. Fishes 2022, 7, 233. https://doi.org/10.3390/fishes7050233
Outama P, Linh NV, Xuan CL, Wannavijit S, Tongsiri S, Chitmanat C, Montha N, Van Doan H. Passionfruit (Passiflora edulis) Peel Powder Stimulates the Immune and Antioxidant Defense System in Nile Tilapia, Oreochromis niloticus, Cultivated in a Biofloc System. Fishes. 2022; 7(5):233. https://doi.org/10.3390/fishes7050233
Chicago/Turabian StyleOutama, Piyatida, Nguyen Vu Linh, Chinh Le Xuan, Supreya Wannavijit, Sudaporn Tongsiri, Chanagun Chitmanat, Napatsorn Montha, and Hien Van Doan. 2022. "Passionfruit (Passiflora edulis) Peel Powder Stimulates the Immune and Antioxidant Defense System in Nile Tilapia, Oreochromis niloticus, Cultivated in a Biofloc System" Fishes 7, no. 5: 233. https://doi.org/10.3390/fishes7050233
APA StyleOutama, P., Linh, N. V., Xuan, C. L., Wannavijit, S., Tongsiri, S., Chitmanat, C., Montha, N., & Van Doan, H. (2022). Passionfruit (Passiflora edulis) Peel Powder Stimulates the Immune and Antioxidant Defense System in Nile Tilapia, Oreochromis niloticus, Cultivated in a Biofloc System. Fishes, 7(5), 233. https://doi.org/10.3390/fishes7050233