Nutraceutical Difference between Two Popular Thai Namwa Cultivars Used for Sun Dried Banana Products
Abstract
1. Introduction
2. Results and Discussion
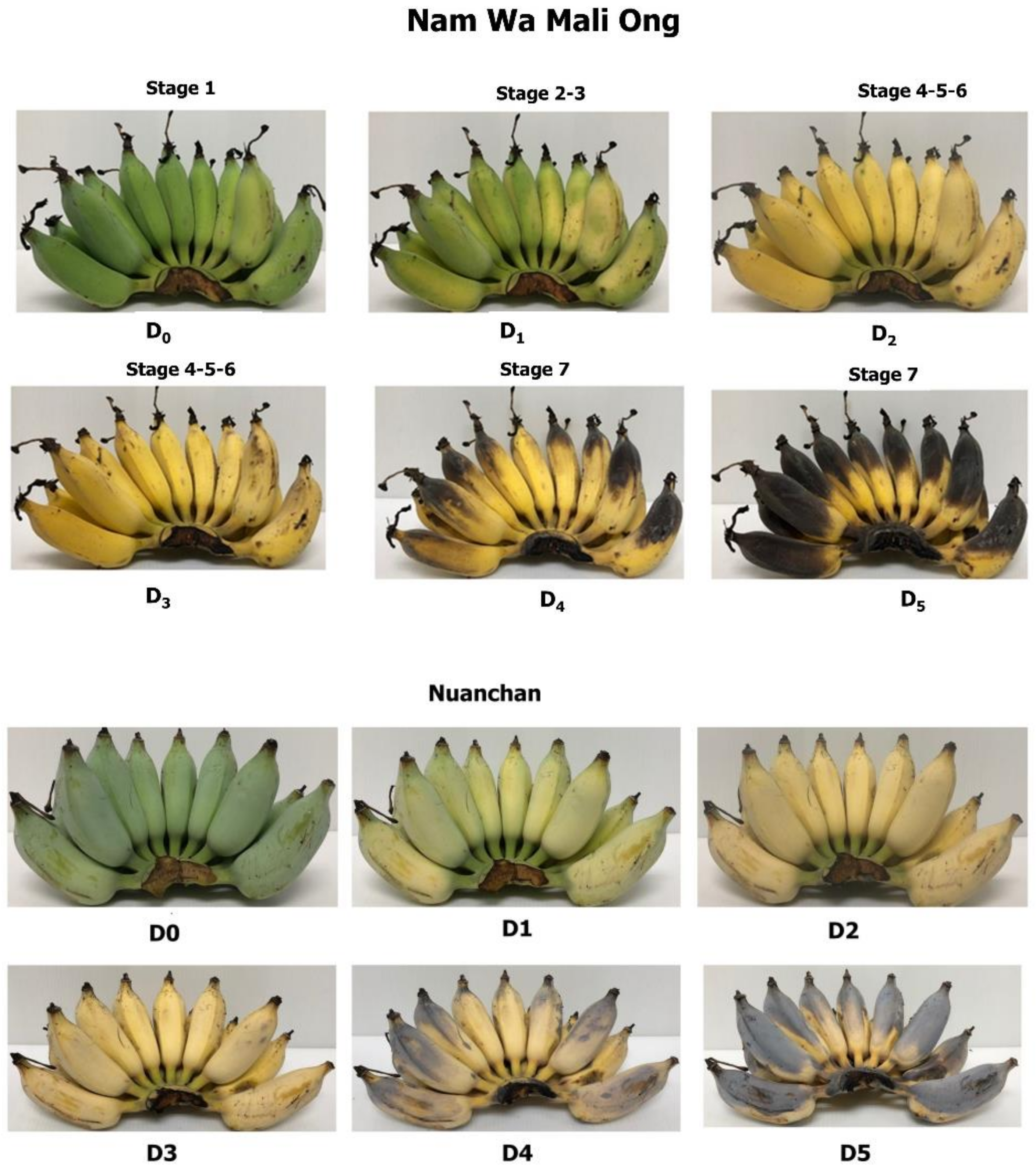
2.1. Physicochemical Properties of Namwa Mali Ong and Nuanchan Varieties during the Ripening Stage
2.2. Flavonoid Contents of Namwa Mali Ong and Nuanchan Varieties during the Ripening Stage
2.3. Phenolic Acid Contents of Namwa Mali Ong and Nuanchan Varieties during the Ripening Stage
2.4. Dopamine and FOS Contents of Namwa Mali Ong and Nuanchan Varieties during the Ripening Stage
2.5. Antioxidant Activities of Namwa Mali Ong and Nuanchan Varieties during the Ripening Stage
2.6. Nutraceutical Contents of Commercial Dried Banana Products and Dried Banana Products from Namwa Mali Ong and Nuanchan Varieties
2.6.1. Flavonoid Contents of Commercial Dried Banana Products and Dried Banana Products from Namwa Mali Ong and Nuanchan Varieties
2.6.2. Phenolic Acid Contents of Commercial Dried Banana Products and Dried Banana Products from Namwa Mali Ong and Nuanchan Varieties
2.6.3. Dopamine and Inulin Contents of Commercial Dried Banana Products and Dried Banana Products from Namwa Mali Ong and Nuanchan Varieties
2.6.4. Antioxidant Activities of Dried Banana Products from Commercial Source and Namwa Mali Ong and Nuanchan Varieties
3. Materials and Methods
3.1. Materials
3.1.1. Experimental Design of Banana Ripening Stage
3.1.2. Experimental Design of Solar Dried Banana Products
3.2. Moisture, Texture, Total Soluble Solids and Sugar
3.3. Flavonoid Content and Phenolic Acid Analysis by HPLC/DAD/MS
3.3.1. Extraction Method
3.3.2. HPLC-DAD and MS Detection Conditions
3.3.3. HPLC-DAD and MS Detection Conditions for Gallocatechin Gallate, Gallocatechin, and Nariginin
3.4. Dopamine Analysis
3.4.1. Extraction of Dopamine
3.4.2. HPLC and Detector Condition
3.5. Inulin-Type Fructans and Fructoolisaccharide Determination
3.6. Total Phenolic Contents (TPC) and Antioxidant Activities Analysis
3.6.1. Sample Extraction
3.6.2. Total Phenolic Contents (TPC)
3.6.3. Total Flavonoid Contents (TFC)
3.6.4. Tannin Content
3.6.5. DPPH Free Radical Scavenging
3.6.6. Ferric Reducing Antioxidant Power (FRAP)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kidoń, M.; Uwineza, P.A. New Smoothie Products Based on Pumpkin, Banana, and Purple Carrot as a Source of Bioactive Compounds. Molecules 2022, 27, 3049. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Zafar, T.A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188. [Google Scholar] [CrossRef]
- Kundapura Venkataramana, R.; Hastantram Sampangi-Ramaiah, M.; Ajitha, R.; Khadke, G.N.; Chellam, V. Insights into Musa balbisiana and Musa acuminata species divergence and development of genic microsatellites by transcriptomics approach. Plant Gene 2015, 4, 78–82. [Google Scholar] [CrossRef]
- Simmonds, N.W.; Shepherd, K. The taxonomy and origins of the cultivated bananas. J. Linn. Soc. Lond. Bot. 1955, 55, 302–312. [Google Scholar] [CrossRef]
- Yin, X.; Quan, J.; Kanazawa, T. Banana Prevents Plasma Oxidative Stress in Healthy Individuals. Plant Foods Hum. Nutr. 2008, 63, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tanjor, S.; Judprasong, K.; Chaito, C.; Jogloy, S. Inulin and Fructooligosacharides in Different Varieties of Jerusalem Artichoke (Helianthus tuberosus L.). KKU Res. J. 2012, 17, 25–34. [Google Scholar]
- Schieber, A. By-Products of Plant Food Processing as a Source of Valuable Compounds. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Presannakumar, G.; Vijayalakshmi, N.R. Antioxidant activity of banana flavonoids. Fitoterapia 2008, 79, 279–282. [Google Scholar] [CrossRef]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; Ric De Vos, C.H.; Van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef]
- Dong, C.; Hu, H.; Hu, Y.; Xie, J. Metabolism of Flavonoids in Novel Banana Germplasm during Fruit Development. Front. Plant Sci. 2016, 7, 1291. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Fang, B.; Zhao, Q.; Jiao, B.; Zhou, Z. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014, 161, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid Intake and Long-Term Risk in the Seven Countries Study. Arch. Intern Med. 1995, 155, 381–386. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Feskens, E.J.M.; Kromhout, D.; Hertog, M.G.L.; Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Kanazawa, K.; Sakakibara, H. High Content of Dopamine, a Strong Antioxidant, in Cavendish Banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.A.; Suprasanna, P.; Ganapathi, T.R.; Rao, P.S. In Vitro Production of L-DOPA in Tissue Cultures of Mucuna pruriens L. Pharm. Biol. 2000, 38, 271–273. [Google Scholar] [CrossRef]
- Shalini, R.; Antony, U. Fructan distribution in banana cultivars and effect of ripening and processing on Nendran banana. J. Food Sci. Technol. 2015, 52, 8244–8251. [Google Scholar] [CrossRef][Green Version]
- Der Agopian, R.G.; Soares, C.A.; Purgatto, E.; Cordenunsi, B.R.; Lajolo, F.M. Identification of Fructooligosaccharides in Different Banana Cultivars. J. Agric. Food Chem. 2008, 56, 3305–3310. [Google Scholar] [CrossRef]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Recent trends in the microbial production, analysis and application of Fructooligosaccharides. Trends Food Sci. Technol. 2005, 16, 442–457. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S. Profiles of prebiotic fructooligosaccharides, inulin and sugars as well as physicochemical properties of banana and its snacks as affected by ripening stage and applied drying methods. Dry. Technol. 2019, 38, 724–734. [Google Scholar] [CrossRef]
- L’Homme, C.; Peschet, J.L.; Puigserver, A.; Biagini, A. Evaluation of fructans in various fresh and stewed fruits by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. A 2001, 920, 291–297. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Changes of phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. 2019, 253, 255–262. [Google Scholar] [CrossRef]
- Fatemeh, S.R.; Saifullah, R.; Abbas, F.M.A.; Azhar, M.E. Total Phenolics, Flavonoids and Antioxidant Activity of Banana Pulp and Peel Flours: Influence of Variety and Stage of Ripeness. Int. Food Res. J. 2012, 19, 1041–1046. [Google Scholar]
- Sundaram, S.; Anjum, S.; Dwivedi, P.; Rai, G.K. Antioxidant Activity and Protective effect of Banana Peel against Oxidative Hemolysis of Human Erythrocyte at Different Stages of Ripening. Appl. Biochem. Biotechnol. 2011, 164, 1192–1206. [Google Scholar] [CrossRef]
- Soltani, M.; Alimardani, R.; Omid, M. Prediction of Banana Quality during Ripening Stage Using Capacitance Sensing System. Aust. J. Crop Sci. 2010, 4, 443–447. [Google Scholar]
- Mohapatra, A.; Yuvraj, B.K.; Shanmugasundaram, S. Physicochemical Changes during Ripening of Red Banana. Int. J. Sci. Environ. Technol. 2016, 5, 1340–1348. [Google Scholar]
- Reginio, F.C.; Ketnawa, S.; Ogawa, Y. In Vitro Examination of Starch Digestibility of Saba Banana [Musa ‘Saba’(Musa Acu-minata × Musa Balbisiana)]: Impact of Maturity and Physical Properties of Digesta. Sci. Rep. 2020, 10, 1811. [Google Scholar] [CrossRef]
- Hibler, M.; Hardy, D. Breeding a Better Banana; International Development Research Center: Ottawa, ON, Canada, 1994; Volume 22. [Google Scholar]
- Salvador, A.; Sanz, T.; Fiszman, S.M. Changes in colour and texture and their relationship with eating quality during storage of two different dessert bananas. Postharvest Biol. Technol. 2007, 43, 319–325. [Google Scholar] [CrossRef]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Texture of Fresh Fruit. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 20, pp. 121–224. ISBN 9780471189060. [Google Scholar]
- Bennett, R.N.; Shiga, T.M.; Hassimotto, N.M.A.; Rosa, E.A.S.; Lajolo, F.M.; Cordenunsi, B.R. Phenolics and Antioxidant Properties of Fruit Pulp and Cell Wall Fractions of Postharvest Banana (Musa acuminata Juss.) Cultivars. J. Agric. Food Chem. 2010, 58, 7991–8003. [Google Scholar] [CrossRef]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa Cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Youryon, P.; Supapvanich, S. Physicochemical quality and antioxidant changes in ‘Leb Mue Nang’ banana fruit during ripening. Agric. Nat. Resour. 2017, 51, 47–52. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; BK, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef]
- Schwertz, M.C.; Maia, J.R.P.; de Sousa, R.F.S.; Aguiar, J.P.L.; Yuyama, L.K.O.; Lima, E.S. Hypolipidemic effect of camu-camu juice in rats. Rev. Nutr. 2012, 25, 35–44. [Google Scholar] [CrossRef]
- Savi, P.D.R.S.; Dos Santos, L.; Gonçalves, A.M.; Biesek, S.; De Lima, C.P. Analysis of Total Flavonoids Present in Some of the Most Consumed Conventional and Organic Fruits and Vegetables in Southern Brazil. DEMETRA Aliment. Nutr. Saúde. 2017, 12, 275–287. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and Its Derivatives: Chemical Structure and Bioactivity—A Review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Cheigh, C.-I.; Chung, E.-Y.; Chung, M.-S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Ongphimai, N.; Lilitchan, S.; Aryusuk, K.; Bumrungpert, A.; Krisnangkura, K. Phenolic Acids Content and Antioxidant Ca-pacity of Fruit Extracts from Thailand. Chiang Mai J. Sci. 2013, 40, 636–642. [Google Scholar]
- Russell, W.R.; Labat, A.; Scobbie, L.; Duncan, G.J.; Duthie, G.G. Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem. 2009, 115, 100–104. [Google Scholar] [CrossRef]
- Kyamuhangire, W.; Krekling, T.; Reed, E.; Pehrson, R. The microstructure and tannin content of banana fruit and their likely influence on juice extraction. J. Sci. Food Agric. 2006, 86, 1908–1915. [Google Scholar] [CrossRef]
- Chai, F.Y.; Heng, S.S.L.; Asilah, S.M.D.; Adila, I.N.I.; Tan, Y.E.; Chong, H.C. Wild Banana Seed Phytobezoar Rectal Impaction Causing Intestinal Obstruction. Indian J. Surg. 2015, 78, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Ganapathi, T.R. Banana and Plantains: Improvement, Nutrition, and Health. In Reference Series in Phytochemistry; Springer Nature Switzerland: Cham, Switzerland, 2019; pp. 1755–1774. ISBN 9783319780306. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Bi, J.; Yi, J.Y.; Chen, Q.; Wu, X.; Zhou, M. Degradation kinetics of total phenolic compounds, capsaicinoids and antioxidant activity in red pepper during hot air and infrared drying process. Int. J. Food Sci. Technol. 2016, 51, 842–853. [Google Scholar] [CrossRef]
- Sarpong, F.; Yu, X.; Zhou, C.; Amenorfe, L.P.; Bai, J.; Wu, B.; Ma, H. The kinetics and thermodynamics study of bioactive compounds and antioxidant degradation of dried banana (Musa ssp.) slices using controlled humidity convective air drying. J. Food Meas. Charact. 2018, 12, 1935–1946. [Google Scholar] [CrossRef]
- Djendoubi Mrad, N.; Boudhrioua, N.; Kechaou, N.; Courtois, F.; Bonazzi, C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod. Process. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Sorsa, S. Testing the Effects of Drying Methods on Willow Flavonoids, Tannins, and Salicylates. J. Chem. Ecol. 2001, 27, 779–789. [Google Scholar] [CrossRef]
- de Ancos, B.; Ibañez, E.; Reglero, G.; Cano, M.P. Frozen Storage Effects on Anthocyanins and Volatile Compounds of Raspberry Fruit. J. Agric. Food Chem. 2000, 48, 873–879. [Google Scholar] [CrossRef]
- Yousif, A.N.; Durance, T.D.; Scaman, C.H.; Girard, B. Headspace Volatiles and Physical Characteristics of Vacuum-microwave, Air, and Freeze-dried Oregano (Lippia berlandieri Schauer). J. Food Sci. 2000, 65, 926–930. [Google Scholar] [CrossRef]
- Feldman, J.; Lee, E.; Castleberry, C. Catecholamine and Serotonin Content of Foods: Effect on Urinary Excretion of Homo-vanillic and 5-Hydroxyindoleacetic Acid. J. Am. Die. Asso. 1987, 87, 1031–1035. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Bacchiocca, M.; Biagiotti, E.; Ninfali, P. Nutritional and Technological Reasons for Evaluating the Antioxidant Capacity of Vegetable Products. Ital. J. Food Sci. 2006, 18, 209–217. [Google Scholar]
- Boyer, J.; Liu, R.H. Antioxidants of Apples. N. Y. Fruit Q. 2004, 11, 11–15. [Google Scholar]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Bruno Bonnet, C.; Hubert, O.; Mbéguié-A.-Mbéguié, D.; Pallet, D.; Hiol, A.; Reynes, M.; Poucheret, P. Effect of physiological harvest stages on the composition of bioactive compounds in Cavendish bananas. J. Zhejiang Univ. Sci. B 2013, 14, 270–278. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Ramos, A.M.; Stringheta, P.C. Colour and Texture Change during Banana Drying (Musa Spp Nanica). Alimentaria 2002, 337, 153–158. [Google Scholar]
- Xu, R.-Y.; Niimi, Y.; Han, D.-S. Changes in endogenous abscisic acid and soluble sugars levels during dormancy-release in bulbs of Lilium rubellum. Sci. Hortic. 2006, 111, 68–72. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of Phenolic Compounds in White Rice, Brown Rice, and Germinated Brown Rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef]
- Peñarrieta, J.M.; Alvarado, J.A.; Akesson, B.; Bergenståhl, B. Separation of Phenolic Compounds from Foods by Reversed-Phase High Performance Liquid Chromatography. Rev. Boliv. QUÍMICA 2007, 24, 1–4. [Google Scholar]
- Šeremet, D.; Durgo, K.; Jokić, S.; Huđek, A.; Cebin, A.V.; Mandura, A.; Jurasović, J.; Komes, D. Valorization of Banana and Red Beetroot Peels: Determination of Basic Macrocomponent Composition, Application of Novel Extraction Methodology and Assessment of Biological Activity In Vitro. Sustainability 2020, 12, 4539. [Google Scholar] [CrossRef]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. AOAC Int. 2002, 12, 1–38. [Google Scholar]
- Luquerodriguez, J.M.; Luquedecastro, M.; Perezjuan, P. Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemaking residues. Bioresour. Technol. 2007, 98, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- Espinosa, A.; Santacruz, S. Phenolic Compounds from the Peel of Musa Cavendish, Musa Acuminata and Musa Cavandanaish. Rev. Politécnica-Enero 2017, 38, 3–5. [Google Scholar]
- Aboul-Enein, A.M.; Salama, Z.A.; Gaafar, A.A.; Aly, H.F.; Bou-Elella, F.A.; Ahmed, H.A. Identification of Phenolic Compounds from Banana Peel (Musa Paradaisica, L.) as Antioxidant and Antimicrobial Agents. J. Chem. Pharm. Res. 2016, 8, 46–55. [Google Scholar]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]









| Nutrient | Kluai Namwa (Raw Banana) | Sun-Dried Banana Product Made from Kluai Namwa | Unit |
|---|---|---|---|
| Amounts | Amounts | ||
| Energy | 118 | 301 | kcal |
| Water | 68.8 | 19.9 | g |
| Carbohydrates | 27.18 | 67.0 | g |
| Sugars | 18.47 | 63.50 | g |
| Dietary fiber | 2.4 | 6.6 | g |
| Protein | 0.78 | 3.3 | g |
| Fat | 0.15 | 0.7 | g |
| Vitamins | |||
| Vitamin A (retinol activity equivalent) | 3 | mcg | |
| B-carotene | 39 | mcg | |
| Thiamine (B1) | 0.04 | mg | |
| Riboflavin (B2) | 0.03 | mg | |
| Niacin | 0.99 | mg | |
| Vitamin C | 13 | 5 | mg |
| Minerals | |||
| Calcium | 7 | 3 | mg |
| Magnesium | 25 | mg | |
| Phosphorus | 26 | mg | |
| Sodium | 4 | 50 | mg |
| Potassium | 241 | mg | |
| Iron | 0.52 | 0.8 | mg |
| Copper | 0.08 | mg | |
| Zinc | 0.13 | mg | |
| Selenium | 0.3 | mcg |
| Ripening Stage | Flavonoids (mg/kg) | Total (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| Flavonols | Flavanone | Flavanols | ||||||
| Quercetin | Kampherol | Rutin | Naringenin | Catechin | Gallocatechin | Gallocatechin Gallate | ||
| Kluai Namwa Mali Ong | ||||||||
| D0 | 61.82 ± 3.16 dA | 23.81 ± 3.98 cA | 157.40 ± 3.47 bA | 77.09 ± 1.35 eA | 77.22 ± 4.50 bA | 43.38 ± 2.38 aA | 18.74 ± 2.08 A | 459.44 ± 3.93 dA |
| D2 | 96.73 ± 5.24 cA | 26.99 ± 2.23 bcA | 204.89 ± 6.70 aA | 116.42 ± 1.51 cA | 101.74 ± 8.87 aA | 25.99 ± 2.24 cA | 0 | 572.75 ± 22.31 abA |
| D3 | 120.06 ± 0.76 bA | 29.54 ± 1.76 bB | 165.00 ± 0.71 bA | 103.83 ± 7.96 dA | 74.81 ± 4.16 bB | 32.95 ± 0.35 bA | 0 | 526.18 ± 8.55 cA |
| D4 | 116.89 ± 4.24 bA | 28.60 ± 0.79 bcB | 165.79 ± 5.69 bA | 120.12 ± 3.07 cA | 97.67 ± 3.57 aA | 31.60 ± 3.42 bcA | 0 | 560.66 ± 2.26 bA |
| D5 | 129.53 ± 3.97 aA | 35.74 ± 1.58 aA | 167.91 ± 4.89 bA | 134.87 ± 2.06 bA | 91.12 ± 4.04 aA | 35.86 ± 1.44 bA | 0 | 595.01 ± 5.77 aA |
| D6 | 138.76 ± 3.84 aA | 35.75 ± 1.52 aA | 136.00 ± 3.91 cB | 147.23 ± 3.61 aA | 96.77 ± 6.27 aA | 30.18 ± 2.69 bcA | 0 | 584.67 ± 0.96 abA |
| Kluai Namwa Nuanchan | ||||||||
| D0 | 71.97 ± 5.01 dA | 26.92 ± 1.94 eA | 202.49 ± 27.69 bA | 67.88 ± 5.31 cA | 99.84 ± 3.44 bA | 33.77 ± 2.38 aA | 21.40 ± 0.51 A | 524.27 ± 30.89 bA |
| D2 | 98.01 ± 0.72 cA | 28.19 ± 0.48 deA | 154.35 ± 22.54 bcA | 114.36 ± 10.04 aA | 78.62 ± 3.13 dA | 31.65 ± 1.03 aA | 0 | 505.1715.81 bA |
| D3 | 108.25 ± 9.01 cA | 33.96 ± 2.03 cdA | 129.15 ± 7.34 cA | 98.28 ± 6.75 bA | 87.80 ± 4.19 cA | 27.28 ± 0.98 bA | 0 | 484.71 ± 8.42 bA |
| D4 | 121.87 ± 0.93 bA | 36.74 ± 0.85 cA | 260.38 ± 40.39 aA | 113.07 ± 3.10 aB | 97.21 ± 1.27 bA | 23.33 ± 0.70 bA | 0 | 652.59 ± 34.95 aA |
| D5 | 135.00 ± 4.36 aA | 44.18 ± 5.06 bA | 187.39 ± 11.31 bcA | 115.77 ± 1.11 aA | 102.61 ± 3.45 abA | 16.94 ± 2.26 cA | 0 | 601.88 ± 14.31 aA |
| D6 | 138.14 ± 3.27 aA | 50.37 ± 1.86 aA | 165.60 ± 5.86 bcA | 123.45 ± 2.84 aBA | 109.22 ± 2.34 aA | 15.65 ± 1.99 cA | 0 | 602.41 ± 7.64 aA |
| Ripening Stage | Phenolic Acids (mg/g) | |||||
|---|---|---|---|---|---|---|
| Tannic Acid | Gallic Acid | Ellagic Acid | Ferulic Acid | Chlorogenic Acid | Total (mg/g) | |
| Namwa Mali Ong | ||||||
| D0 | 112.02 ± 6.68 aA | 49.12 ± 1.51 cA | 96.60 ± 3.20 bA | 33.01 ± 3.03 aA | 47.09 ± 0.28 aA | 337.84 ± 11.67 aA |
| D2 | 98.32 ± 3.04 bA | 32.81 ± 1.82 eA | 74.96 ± 5.19 cA | 20.86 ± 2.38 bA | 47.66 ± 2.86 aA | 274.61 ± 4.45 bA |
| D3 | 84.07 ± 6.65 cA | 42.22 ± 1.78 dB | 81.37 ± 0.91 cA | 22.79 ± 1.78 bA | 45.33 ± 2.91 aB | 275.78 ± 8.65 bA |
| D4 | 89.81 ± 0.64 bcA | 61.51 ± 1.22 bA | 114.31 ± 5.55 aA | 23.22 ± 2.80 bA | 44.76 ± 4.54 aB | 333.61 ± 0.06 aA |
| D5 | 98.45 ± 6.70 bA | 69.77 ± 4.99 aA | 103.93 ± 3.60 bA | 21.31 ± 1.84 bA | 46.10 ± 0.98 aB | 339.56 ± 6.17 aA |
| D6 | 90.33 ± 1.80 bcA | 51.20 ± 2.67 cB | 97.08 ± 1.53 bA | 0 | 45.22 ± 2.64 aA | 283.82 ± 0.30 bB |
| Namwa Nuanchan | ||||||
| D0 | 102.53 ± 5.61 bA | 45.44 ± 1.80 cB | 103.95 ± 1.55 abA | 19.75 ± 0.81 bcA | 50.12 ± 0.43 bA | 321.78 ± 4.12 bA |
| D2 | 68.34 ± 2.69 dA | 46.42 ± 4.65 cA | 106.88 ± 4.79 abA | 17.70 ± 0.42 cA | 53.79 ± 1.99 bA | 293.13 ± 0.13 cA |
| D3 | 67.02 ± 3.49 dA | 59.50 ± 2.53 bA | 112.07 ± 9.40 aA | 22.67 ± 1.44 abA | 52.45 ± 3.66 bA | 313.70 ± 3.36 bA |
| D4 | 131.07 ± 10.51 aA | 56.39 ± 0.70 bA | 97.23 ± 3.44 bA | 22.14 ± 1.69 abA | 65.85 ± 3.94 aA | 372.66 ± 7.63 aA |
| D5 | 96.14 ± 3.18 cA | 68.87 ± 3.45 aA | 67.27 ± 3.70 cB | 24.35 ± 1.01 aA | 66.56 ± 2.64 aA | 323.18 ± 6.68 bB |
| D6 | 85.63 ± 0.86 cA | 69.00 ± 1.99 aA | 72.49 ± 3.95 cB | 20.54 ± 1.26 bc | 66.95 ± 1.21 aA | 314.60 ± 2.87 bA |
| Ripening Stage | TPC (mg GAE/100 g) | Tannin (mg TAE/100 g) | TFC (mg QE/100 g) | DPPH (mg TE/100 g) | FRAP (mg FeSO4/100 g) |
|---|---|---|---|---|---|
| Kluai Namwa Mali Ong | |||||
| D0 | 160.01 ± 8.76 bB | 206.18 ± 10.86 bB | 176.06 ± 9.64 aA | 119.44 ± 12.45 dB | 970.28 ± 107.20 cA |
| D2 | 195.61 ± 14.11 bB | 254.51 ± 25.57 bB | 97.30 ± 10.93 bB | 251.18 ± 22.79 cB | 2660.19 ± 140.09 bA |
| D3 | 228.80 ± 15.57 bA | 287.66 ± 11.24 bA | 89.21 ± 6.44 bA | 437.30 ± 38.67 bA | 961.34 ± 104.33 cA |
| D4 | 589.97 ± 25.71 aA | 801.38 ± 37.24 aA | 78.42 ± 11.80 bA | 732.40 ± 16.21 aA | 2392.84 ± 81.86 bA |
| D5 | 601.38 ± 228.57 aA | 817.95 ± 266.34 aA | 157.03 ± 39.57 aA | 847.37 ± 232.27 aA | 5280.64 ± 1904.99 aA |
| Kluai Namwa Nuanchan | |||||
| D0 | 207.30 ± 36.32 bcA | 274.97 ± 30.61 cdA | 184.29 ± 18.68 bA | 238.84 ± 49.56 dA | 917.74 ± 67.66 cA |
| D2 | 728.57 ± 213.55 aA | 899.42 ± 261.50 aA | 211.62 ± 21.92 aA | 834.48 ± 161.08 bA | 2190.79 ± 191.58 aB |
| D3 | 117.71 ± 29.72 cB | 145.69 ± 33.92 dB | 19.63 ± 8.87 dB | 50.89 ± 1.63 eB | 496.44 ± 179.09 dB |
| D4 | 270.15 ± 25.60 bB | 378.46 ± 20.83 bcB | 82.33 ± 12.05 cA | 473.05 ± 29.82 cB | 1110.08 ± 134.81 bB |
| D5 | 329.32 ± 18.03 bB | 459.16 ± 18.80 bB | 77.95 ± 3.95 cB | 1028.18 ± 28.32 aA | 1153.30 ± 51.45 bB |
| Samples | Fructose | Glucose | Sucrose | Total |
|---|---|---|---|---|
| Kluai Namwa Mali Ong | ||||
| Before drying | 32.62 ± 0.37 a | 30.31 ± 0.29 a | 0 | 62.93 ± 0.10 a |
| After drying | 24.20 ± 0.06 b | 24.23 ± 0.16 b | 0.04 ± 0.03 | 48.47 ± 0.20 b |
| Kluai Namwa Nuanchan | ||||
| Before drying | 35.23 ± 0.13 a | 37.67 ± 0.18 a | 0 | 72.96 ± 0.09 a |
| After drying | 22.82 ± 0.12 b | 24.39 ± 0.94 b | 0 | 47.21 ± 1.05 b |
| Commercial products | ||||
| DBPM | 19.49 ± 0.11 c | 20.50 ± 0.03 d | 0 | 40.00 ± 0.14 d |
| DBPJ | 20.93 ± 0.16 b | 22.39 ± 0.06 b | 0 | 43.32 ± 0.11 b |
| DBPA | 23.05 ± 0.09 a | 24.93 ± 0.10 a | 0 | 47.98 ± 0.08 a |
| DBPS | 19.60 ± 0.05 c | 20.99 ± 0.08 c | 0.05 ± 0.01 | 40.65 ± 0.06 c |
| Samples | Nutraceuticals | ||||||
|---|---|---|---|---|---|---|---|
| Quercetin | Kampherol | Rutin | Naringenin | Catechin | Gallo- Catechin | Total mg/kg | |
| Kluai Namwa Mali Ong | |||||||
| Before drying | 89.81 ± 1.01 a | 37.35 ± 2.83 a | 176.66 ± 6.92 a | 132.29 ± 3.99 a | 45.14 ± 3.42 a | 33.96 ± 3.80 a | 518.21 ± 8.13 a |
| After drying | 40.74 ± 0.87 b (55%) | 16.55 ± 2.15 b (56%) | 40.14 ± 2.37 b (77%) | 31.18 ± 1.72 b (76%) | 35.59 ± 2.19 a (21%) | 14.02 ± 1.64 b (59%) | 178.21 ± 6.21 b (66%) |
| Kluai Namwa Nuanchan | |||||||
| Before drying | 110.54 ± 2.96 a | 28.67 ± 4.62 a | 166.39 ± 10.50 a | 82.41 ± 8.10 a | 84.84 ± 6.36 a | 23.71 ± 0.88 a | 496.54 ± 20.71 a |
| After drying | 39.53 ± 4.41 b (64%) | 14.20 ± 1.24 a (50%) | 54.23 ± 8 (67%) | 43.34 ± 2.69 a (47%) | 20.97 ± 1.53 a (75%) | 9.85 ± 0.45 b (58%) | 182.10 ± 1.35 b (63%) |
| The four commercial and two products from Mali Ong and Nuanchan | |||||||
| DBPM | 39.03 ± 2.10 b | 16.49 ± 1.65 ab | 38.34 ± 2.28 b | 45.22 ± 2.74 a | 22.18 ± 0.28 b | 10.42 ± 0.99 bc | 172.30 ± 5.30 c |
| DBPJ | 25.48 ± 3.37 c | 22.13 ± 4.21 a | 22.08 ± 2.29 c | 36.93 ± 3.14 cd | 32.66 ± 6.90 a | 9.16 ± 1.05 c | 148.44 ± 5.85 d |
| DBPA | 55.41 ± 3.06 a | 10.21 ± 1.62 c | 44.75 ± 1.16 ab | 40.66 ± 1.74 abc | 22.73 ± 2.03 b | 13.37 ± 1.38 abc | 187.14 ± 5.42 ab |
| DBPS | 55.72 ± 2.37 a | 12.92 ± 1.53 bc | 41.54 ± 2.65 b | 37.10 ± 2.44 bcd | 32.23 ± 1.63 a | 17.66 ± 3.52 a | 197.16 ± 1.45 a |
| DBML | 40.74 ± 0.87 b | 16.55 ± 2.15 ab | 40.14 ± 2.37 b | 31.18 ± 1.72 d | 35.59 ± 2.19 a | 14.02 ± 1.64 ab | 178.21 ± 6.20 bc |
| DBNJ | 39.96 ± 4.75 b | 14.20 ± 1.25 bc | 54.23 ± 8.70 a | 43.33 ± 2.69 ab | 20.97 ± 1.53 b | 9.85 ± 0.45 bc | 182.53 ± 1.97 bc |
| Sample | Phenolic Acids | Total mg/kg | ||||
|---|---|---|---|---|---|---|
| Tannic Acid | Gallic Acid | Ellagic Acid | Ferulic Acid | Chlorogenic Acid | ||
| Kluai Namwa Mali Ong | ||||||
| Before drying | 90.99 ± 3.63 a | 84.61 ± 5.29 a | 107.03 ± 2.83 a | 0 | 44.38 ± 4.41 a | 327.00 ± 0.38 a |
| After drying | 21.81 ± 2.31 b (76%) | 22.17 ± 2.86 b (74%) | 29.88 ± 1.79 b (72%) | 0 | 22.19 ± 1.79 a (50%) | 96.06 ± 0.55 b (70%) |
| Kluai Namwa Nuanchan | ||||||
| Before drying | 82.47 ± 8.73 a | 66.33 ± 3.52 a | 69.05 ± 2.91 a | 20.43 ± 1.69 | 47.84 ± 8.93 a | 286.12 ± 12.92 a |
| After drying | 32.35 ± 4.54 a (61%) | 14.92 ± 3.39 a (76%) | 36.38 ± 1.34 b (47%) | 0 (100%) | 18.54 ± 2.95 a (61%) | 102.09 ± 0.46 b (64%) |
| The four commercial and two products from Mali Ong and Nuanchan | ||||||
| DBPM | 22.81 ± 2.08 cd | 18.35 ± 3.27 ab | 40.06 ± 2.64 b | 7.69 ± 0.95 b | 13.18 ± 2.54 bc | 102.09 ± 4.49 bc |
| DBPJ | 29.83 ± 0.83 bc | 14.07 ± 2.60 bc | 26.94 ± 3.97 c | 7.58 ± 0.02 b | 10.09 ± 0.79 c | 88.49 ± 0.23 d |
| DBPA | 32.03 ± 3.24 b | 18.42 ± 1.36 ab | 29.39 ± 1.44 c | 7.71 ± 0.89 b | 16.41 ± 2.82 b | 103.96 ± 2.36 b |
| DBPS | 46.49 ± 3.37 a | 8.79 ± 0.33 c | 46.29 ± 1.99 a | 10.09 ± 0.74 a | 14.42 ± 1.47 bc | 126.06 ± 3.48 a |
| DBML | 21.82 ± 2.31 d | 22.18 ± 2.86 a | 29.88 ± 1.79 c | 0 | 22.20 ± 1.79 a | 96.06 ± 0.54 c |
| DBNJ | 32.35 ± 4.54 b | 14.92 ± 3.39 bc | 36.39 ± 1.34 b | 0 | 18.54 ± 2.96 ab | 102.19 ± 0.47 bc |
| Sample | Dopamine (mg/kg) | Dopamine (mg/kg) (dw) |
|---|---|---|
| Nam Wa Mali Ong | ||
| Before drying | 30.39 ± 1.61 a | 101.44 ± 5.38 a |
| After drying | 18.00 ± 1.72 a | 24.52 ± 2.34 b |
| Nam Wa Nuanchan | ||
| Before drying | 20.21 ± 2.86 a | 69.91 ± 9.91 a |
| After drying | 28.14 ± 1.29 a | 38.52 ± 1.76 a |
| The four commercial and two products from Mali Ong and Nuanchan | ||
| DBPM | 24.37 ± 2.55 ab | 33.74 ± 3.54 ab |
| DBPJ | 26.52 ± 2.26 a | 35.87 ± 3.05 a |
| DBPA | 20.73 ± 2.12 bc | 28.59 ± 2.93 bc |
| DBPS | 15.20 ± 1.39 d | 22.26 ± 2.03 c |
| DBML | 18.00 ± 1.72 cd | 24.52 ± 2.34 c |
| DBNJ | 28.14 ± 1.29 a | 38.52 ± 1.76 a |
| Sample | TPC (mg GAE/100 g) | Tannin (mg TAE/100 g) | DPPH (mg TE/100 g) | FRAP (mg FeSO4/100 g) | TFC (mg QE/100 g) |
|---|---|---|---|---|---|
| Kluai Namwa Mali Ong | |||||
| Before drying | 1276.74 ± 181.51 a | 23.82 ± 2.68 a | 5675.32 ± 266.95 a | 7593.33 ± 1670.99 a | 495.45 ± 93.66 a |
| After drying | 246.91 ± 16.25 b (81%) | 4.95 ± 0.39 b (79%) | 662.74 ± 14.65 b (88%) | 1215.46 ± 50.02 b (84%) | 120.78 ± 11.42 b (76%) |
| Kluai Namwa Nuanchan | |||||
| Before drying | 737.75 ± 226.40 a | 14.98 ± 3.97 a | 3292.75 ± 155.58 a | 4404.39 ± 811.75 a | 255.04 ± 67.09 a |
| After drying | 249.22 ± 16.07 b (66%) | 5.88 ± 0.28 b (61%) | 632.33 ± 35.68 b (81%) | 1280.07 ± 81.51 b (71%) | 104.54 14.82 b (59%) |
| The four commercial and two products from Mali Ong and Nuanchan | |||||
| DBPM | 266.05 ± 40.56 a | 5.33 ± 0.76 ab | 617.37 ± 45.92 a | 1110.58 ± 72.98 c | 44.71 ± 9.08 c |
| DBPJ | 286.62 ± 36.66 a | 5.84 ± 0.46 a | 643.85 ± 20.61 a | 1151.72 ± 34.13 bc | 45.03 ± 5.36 c |
| DBPA | 248.61 ± 62.23 a | 4.75 ± 0.92 b | 639.68 ± 29.73 a | 1129.19 ± 63.14 c | 44.82 ± 15.51 c |
| DBPS | 193.47 ± 19.77 b | 4.04 ± 0.19 c | 635.41 ± 19.63 a | 1108.55 ± 39.01 c | 59.16 ± 17.68 c |
| DBML | 246.91 ± 16.25 a | 4.95 ± 0.38 b | 662.74 ± 14.65 a | 1215.46 ± 50.02 ab | 120.79 ± 11.42 a |
| DBNJ | 249.22 ± 16.07 a | 5.88 ± 0.28 a | 632.33 ± 35.68 a | 1280.07 ± 81.51 a | 104.54 14.82 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongwaiwech, D.; Kamchonemenukool, S.; Ho, C.-T.; Li, S.; Thongsook, T.; Majai, N.; Premjet, D.; Sujipuli, K.; Weerawatanakorn, M. Nutraceutical Difference between Two Popular Thai Namwa Cultivars Used for Sun Dried Banana Products. Molecules 2022, 27, 5675. https://doi.org/10.3390/molecules27175675
Wongwaiwech D, Kamchonemenukool S, Ho C-T, Li S, Thongsook T, Majai N, Premjet D, Sujipuli K, Weerawatanakorn M. Nutraceutical Difference between Two Popular Thai Namwa Cultivars Used for Sun Dried Banana Products. Molecules. 2022; 27(17):5675. https://doi.org/10.3390/molecules27175675
Chicago/Turabian StyleWongwaiwech, Donporn, Sudthida Kamchonemenukool, Chi-Tang Ho, Shiming Li, Tipawan Thongsook, Nutthaporn Majai, Duangporn Premjet, Kawee Sujipuli, and Monthana Weerawatanakorn. 2022. "Nutraceutical Difference between Two Popular Thai Namwa Cultivars Used for Sun Dried Banana Products" Molecules 27, no. 17: 5675. https://doi.org/10.3390/molecules27175675
APA StyleWongwaiwech, D., Kamchonemenukool, S., Ho, C.-T., Li, S., Thongsook, T., Majai, N., Premjet, D., Sujipuli, K., & Weerawatanakorn, M. (2022). Nutraceutical Difference between Two Popular Thai Namwa Cultivars Used for Sun Dried Banana Products. Molecules, 27(17), 5675. https://doi.org/10.3390/molecules27175675







