Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis cyanocarpa (Blume) Spreng
Abstract
:1. Introduction
2. Results
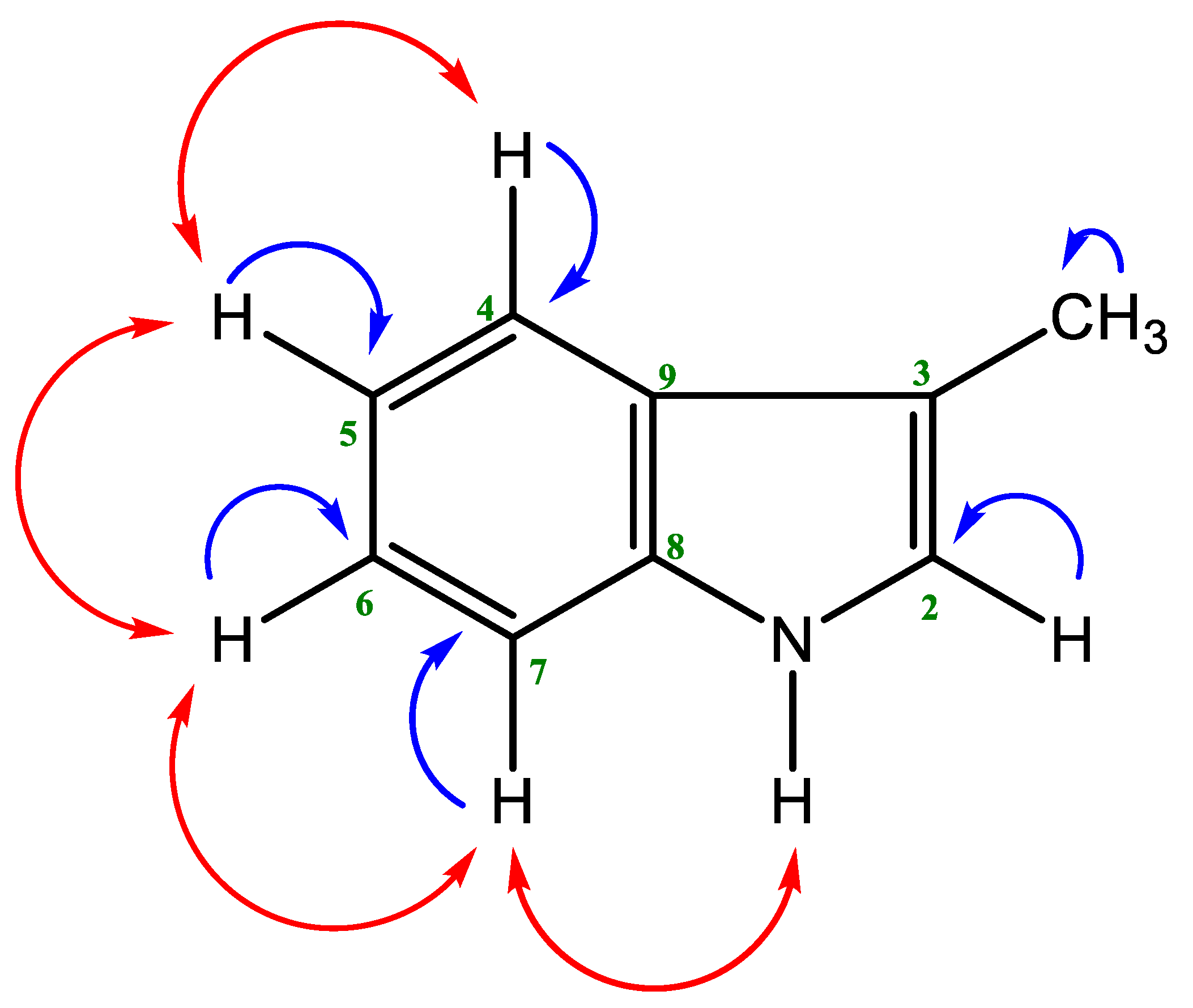
2.1. Isolated Phytochemicals from G. cyanocarpa
2.2. Effect of Isolated Phytochemicals from G. cyanocarpa on DPPH Free Radical Scavenging Activity
2.3. Effect of Isolated Phytochemicals from G. cyanocarpa on Brine Shrimp Lethality Bioassay
2.4. Effect of Isolated Phytochemicals from G. cyanocarpa on Disc Diffusion Assay
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Instrumentations, Drugs and Chemicals
4.3. Test Microorganism
4.4. Experimental Design
4.4.1. Extraction of Plant Material
4.4.2. Isolation of Compounds
4.4.3. Structural Identification of the Compounds
4.5. Antioxidant Assay
DPPH Free Radical Scavenging Assay
4.6. Cytotoxicity Assay (Brine Shrimp Lethality Bioassay)
4.7. Antibacterial Assay (Disc Diffusion Test)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Emon, N.U.; Alam, S.; Rudra, S.; Al Haidar, I.K.; Farhad, M.; Rana, M.E.H.; Ganguly, A. Antipyretic Activity of Caesalpinia digyna (Rottl.) Leaves Extract along with Phytoconstituent’s Binding Affinity to COX-1, COX-2, and MPGES-1 Receptors: In Vivo and in Silico Approaches. Saudi J. Biol. Sci. 2021, 28, 5302–5309. [Google Scholar] [CrossRef] [PubMed]
- Emon, N.U.; Rudra, S.; Alam, S.; Al Haidar, I.K.; Paul, S.; Richi, F.T.; Shahriar, S.; Sayeed, M.A.; Tumpa, N.I.; Ganguly, A. Chemical, Biological and Protein-Receptor Binding Profiling of Bauhinia Scandens L. Stems Provide New Insights into the Management of Pain, Inflammation, Pyrexia and Thrombosis. Biomed. Pharmacother. 2021, 143, 112185. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Emon, N.U.; Alam, S.; Rudra, S.; Akhter, N.; Mamun, M.M.R.; Ganguly, A. Assessment of Pharmacological Activities of Lygodium microphyllum Cav. Leaves in the Management of Pain, Inflammation, Pyrexia, Diarrhea, and Helminths: In Vivo, in Vitro and in Silico Approaches. Biomed. Pharmacother. 2021, 139, 111644. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. (Lausanne) 2022, 13, 800714. [Google Scholar] [CrossRef]
- Chhetri, H.; Yogol, N.; Sherchan, J.; Anupa, K.C.; Mansoor, S.; Thapa, P. Phytochemical and Antimicrobial Evaluations of Some Medicinal Plants of Nepal. Kathmandu Univ. J. Sci. Eng. Technol. 2008, 4, 49–54. [Google Scholar] [CrossRef]
- Ashrafi, S.; Rahman, M.; Ahmed, P.; Alam, S.; Hossain, M.A. Prospective Asian Plants with Corroborated Antiviral Potentials: Position Standing in Recent Years. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Adebesin, O.; Okpuzor, J.; Adenekan, O. Free Radical Scavenging Capacity and Antioxidant Properties of Leaf Extract of Senna Alata and Senna Podocarpa. NISEB J. 2019, 11. [Google Scholar]
- Sultana, N.; Chung, H.-J.; Emon, N.U.; Alam, S.; Taki, M.T.I.; Rudra, S.; Tahamina, A.; Alam, R.; Ahmed, F.; Mamun, A. Al Biological Functions of Dillenia pentagyna Roxb. Against Pain, Inflammation, Fever, Diarrhea, and Thrombosis: Evidenced From in Vitro, in Vivo, and Molecular Docking Study. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, N.; Chung, H.-J.; Alam, R.; Emon, N.U.; Alam, S.; Kabir, M.F.; Islam, M.M.; Hong, S.-T.; Sarkar, T.; Sarker, M.M.R.; et al. Chemico-Pharmacological Screening of the Methanol Extract of Gynura nepalensis D.C. Deciphered Promising Antioxidant and Hepatoprotective Potentials: Evidenced from in Vitro, in Vivo, and Computer-Aided Studies. Molecules 2022, 27, 3474. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Afrin, S.; Richi, F.T.; Zhao, C.; Zhou, J.R.; Mohamed, I.N. Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products Against COVID-19: Update on Clinical Trials and Mechanism of Actions. Front. Pharmacol. 2021, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Alam, R.; Chung, H.J.; Emon, N.U.; Kabir, M.F.; Rudra, S.; Alam, S.; Ullah, A.; Hong, S.T.; Aktar Sayeed, M. Chemical, Pharmacological and Computerized Molecular Analysis of Stem’s Extracts of Bauhinia Scandens L. Provide Insights into the Management of Diarrheal and Microbial Infections. Nutrients 2022, 14, 265. [Google Scholar] [CrossRef]
- Isah, T.; Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary Metabolites in Plant Innate Immunity: Conserved Function of Divergent Chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Al-Suod, H.; Ratiu, I.A.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Supercritical Fluid Extraction in Isolation of Cyclitols and Sugars from Chamomile Flowers. J. Sep. Sci. 2019, 42, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial Activity of Some Ethnomedicinal Plants Used by Paliyar Tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35. [Google Scholar] [CrossRef]
- Yasir, M.; Tripathi, M.K.; Singh, P.; Tripathi, M.K.; Shrivastava, R. The Genus Glycosmis: A Comprehensive Review on Its Phytochemical and Pharmacological Perspectives. Nat. Prod. J. 2019, 9, 98–124. [Google Scholar] [CrossRef]
- Greger, H. Phytocarbazoles: Alkaloids with Great Structural Diversity and Pronounced Biological Activities. Phytochem. Rev. 2017, 16, 1095–1153. [Google Scholar] [CrossRef]
- Greger, H.; Hadacek, F.; Hofer, O.; Wurz, G.; Zechner, G. Different Types of Sulphur-Containing Amides from Glycosmis Cf. Chlorosperma. Phytochemistry 1993, 32, 933–936. [Google Scholar] [CrossRef]
- Greger, H.; Zechner, G.; Hofer, O.; Hadacek, F.; Wurz, G. Sulphur-Containing Amides from Glycosmis Species with Different Antifungal Activity. Phytochemistry 1993, 34, 175–179. [Google Scholar] [CrossRef]
- Sarkar, M.; Kundu, S.; Chakraborty, D.P. Glycarpine, a New Alkaloid from Glycosmis cyanocarpa. Phytochemistry 1978, 17, 2145–2146. [Google Scholar] [CrossRef]
- Wurz, G.; Hofer, O.; Greger, H. Structure and Synthesis of Phenaglydon, a New Quinolone Derived Phenanthridine Alkaloid from Glycosmis Cyanocarpa. Nat. Prod. Lett. 2006, 3, 177–182. [Google Scholar] [CrossRef]
- Xiong, L.; Gao, Y.; Niu, C.; Wang, H.B.; Li, W.H. Synthesis and in Vitro Anticancer Activity of Novel 2-((3-Thioureido) Carbonyl) Phenyl Acetate Derivatives. Lett. Drug Des. Discov. 2014, 11, 132–137. [Google Scholar] [CrossRef]
- D’Ischia, M.; Manini, P.; Napolitano, A. Oxidative Damage to Carbohydrates and Amino Acids. In Oxidative Stress, Disease and Cancer; World Scientific: Singapore, 2006; pp. 333–356. [Google Scholar] [CrossRef]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Marzouni, H.Z. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Luo, M.L.; Huang, W.; Zhu, H.P.; Peng, C.; Zhao, Q.; Han, B. Advances in Indole-Containing Alkaloids as Potential Anticancer Agents by Regulating Autophagy. Biomed. Pharmacother. 2022, 149, 112827. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.M. A Review on Medicinal Plants with Anticancer Activity Available in Bangladesh. Mod. Appl. Pharm. Pharmacol. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 283. [Google Scholar]
- Agnew, E.; Dolecek, C.; Hasan, R.; Lahra, M.; Merk, H.; Perovic, O.; Sievert, D.; Smith, R.; Taylor, A.; Turner, P. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; WHO: Geneva, Switzerland, 2021; p. 180.
- Ripa, F.; Nahar, L.; Fazal, A.; Khatun, M.H. Antibacterial and Phytochemical Evaluation of Three Medicinal Plants of Bangladesh. Int. J. Pharm. Sci. Res. 2012, 3, 788–792. [Google Scholar]
- Emon, N.U.; Alam, S.; Rudra, S.; Riya, S.R.; Paul, A.; Hossen, S.M.M.; Kulsum, U.; Ganguly, A. Antidepressant, Anxiolytic, Antipyretic, and Thrombolytic Profiling of Methanol Extract of the Aerial Part of Piper nigrum: In Vivo, in Vitro, and in Silico Approaches. Food Sci. Nutr. 2021, 9, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Emon, N.U.; Shahriar, S.; Richi, F.T.; Haque, M.R.; Islam, M.N.; Sakib, S.A.; Ganguly, A. Pharmacological and Computer-Aided Studies Provide New Insights into Millettia Peguensis Ali (Fabaceae). Saudi Pharm. J. 2020, 28, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Emon, N.U.; Alam, S.; Rudra, S.; Chowdhury, S.; Rajbangshi, J.C.; Ganguly, A. Evaluation of Pharmacological Potentials of the Aerial Part of Achyranthes aspera L.: In Vivo, in Vitro and in Silico Approaches. Adv. Tradit. Med. 2022, 22, 141–154. [Google Scholar] [CrossRef]
- Alam, S.; Rashid, M.A.; Sarker, M.M.R.; Emon, N.U.; Arman, M.; Mohamed, I.N.; Haque, M.R. Antidiarrheal, Antimicrobial and Antioxidant Potentials of Methanol Extract of Colocasia gigantea Hook. f. Leaves: Evidenced from in Vivo and in Vitro Studies along with Computer-Aided Approaches. BMC Complement. Med. Ther. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Abraham, R.J.; Reid, M. 1H Chemical Shifts in NMR. Part 18. Ring Currents and π-Electron Effects in Hetero-Aromatics. J. Chem. Soc. Perkin Trans. 2002, 2, 1081–1091. [Google Scholar] [CrossRef]
- Ibata, K.; Mizuno, M.; Takigawa, T.; Tanaka, Y. Long-Chain Betulaprenol-Type Polyprenols from the Leaves of Ginkgo biloba. Biochem. J. 1983, 213, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Pateh; Haruna, A.K.; Garba, M.; Iliya, I.; Sule, I.M.; Abubakar, M.S.; Ambi, A.A. Isolation of Stigmasterol, β-Sitosterol and 2-Hydroxyhexadecanoic Acid Methyl Ester from the Rhizomes of Stylochiton Lancifolius Pyer and Kotchy (Araceae). Niger. J. Pharm. Sci. 2009, 8, 19–25. [Google Scholar]
- Monguchi, Y.; Fujita, Y.; Hashimoto, S.; Ina, M.; Takahashi, T.; Ito, R.; Nozaki, K.; Maegawa, T.; Sajiki, H. Palladium on Carbon-Catalyzed Solvent-Free and Solid-Phase Hydrogenation and Suzuki–Miyaura Reaction. Tetrahedron 2011, 67, 8628–8634. [Google Scholar] [CrossRef]
- Ahsan, M.; Gray, A.I.; Waterman, P.G.; Armstrong, J.A. 4-Quinolinone, 2-Quinolinone, 9-Acridanone, and Furoquinoline Alkaloids from the Aerial Parts of Boronia bowmanii. J. Nat. Prod. 1994, 57, 670–672. [Google Scholar] [CrossRef]
- Tabassum, F.; Hasan, C.M.; Masud, M.M.; Jamshidi, S.; Rahman, K.M.; Ahsan, M. Indole Alkaloids from the Leaves of Ravenia spectabilis Engl. with Activity against Pancreatic Cancer Cell Line. Phytochemistry 2021, 186, 112744. [Google Scholar] [CrossRef]
- Rios, M.Y.; Delgado, G. Terpenoids and Alkaloids from Esenbeckia belizencis. Spontaneous Oxidation of Furoquinoline Alkaloids. J. Nat. Prod. 1992, 55, 1307–1309. [Google Scholar] [CrossRef]
- Hosaka, S.; Obuki, M.; Nakajima, J.; Suzuki, M. Comparative Study of Antioxidants as Quenchers or Scavengers of Reactive Oxygen Species Based on Quenching of MCLA-Dependent Chemiluminescence. Luminescence 2005, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. Bioact. Compd. Health Benefits Potential Appl. 2019, 33–50. [Google Scholar] [CrossRef]
- Singh, T.P.; Singh, O.M. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini-Rev. Med. Chem. 2017, 18, 9–25. [Google Scholar] [CrossRef]
- Süzen, S. Antioxidant Activities of Synthetic Indole Derivatives and Possible Activity Mechanisms. Bioact. Heterocycles 2007, V, 145–178. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Baskar, A.A.; Al Numair, K.S.; Gabriel Paulraj, M.; Alsaif, M.A.; Al Muamar, M.; Ignacimuthu, S. β-Sitosterol Prevents Lipid Peroxidation and Improves Antioxidant Status and Histoarchitecture in Rats with 1,2-Dimethylhydrazine-Induced Colon Cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.A.; Khan, A.; Farooq, U.; Khan, S. Alkaloids as Cyclooxygenase Inhibitors in Anticancer Drug Discovery. Curr. Protein Pept. Sci. 2017, 19, 292–301. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the Target-Based Design of Anticancer Agents: A Versatile Scaffold with Diverse Mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Brancale, A.; Silvestri, R. Indole, a Core Nucleus for Potent Inhibitors of Tubulin Polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant Derived and Dietary Phenolic Antioxidants: Anticancer Properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Sumardi, S.; Nuryawan, A.; Wati, R. Cytotoxic and Antiproliferative Activity of Polyisoprenoids in Seventeen Mangroves Species Against WiDr Colon Cancer Cells. Asian Pacific J. Cancer Prev. 2018, 19, 3393. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Y.; Zhang, H.; Xia, L. Molecular Mechanism of β-Sitosterol and Its Derivatives in Tumor Progression. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Nascimento, G.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Vital, P.; Rivera, W.L. Antimicrobial Activity and Cytotoxicity of Chromolaena odorata (L. f.) King and Robinson and Uncaria perrottetii (A. Rich) Merr. Extracts. J. Med. Plants Res. 2009, 3, 511–518. [Google Scholar]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-Trans-Coumaroyl-2-Hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Wang, C.; Ye, J.; Zhou, H.; Chen, H. Polyprenols of Ginkgo biloba Enhance Antibacterial Activity of Five Classes of Antibiotics. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, A.; Seepersaud, M.; Maxwell, A.; Jayaraman, J.; Ramsubhag, A.; Editors, A.; Barona-Gomez, F.; Licona-Cassani, C.; Diana Ceapă, C. Isolation and Antibacterial Activity of Indole Alkaloids from Pseudomonas aeruginosa UWI-1. Mol. 2020, 25, 3744. [Google Scholar] [CrossRef] [PubMed]
- Alawode, T.T.; Lajide, L.; Olaleye, M.; Owolabi, B. Stigmasterol and β-Sitosterol: Antimicrobial Compounds in the Leaves of Icacina Trichantha Identified by GC–MS. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 1–8. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Chokshi, H.P.; Desai, H.K. Separation of Diterpenoid Alkaloid Mixtures Using Vacuum Liquid Chromatography. J. Nat. Prod. 1986, 49, 892–900. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C.L. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Islam, A.; Emon, N.U.; Islam, Q.S.; Ahsan, M. Chemico-Biological Profiling of Blumea lacera (Burm.f.) DC. (Family: Asteraceae) Provides New Insights as a Potential Source of Antioxidant, Cytotoxic, Antimicrobial, and Antidiarrheal Agents. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Bauer, A.W. Antibiotic Susceptibility Testing by a Standardized Single Disc Method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]






| Test Microorganisms | Diameter of Zone of Inhibition (mm) | Vancomycin | Azithromycin | Tetracycline | Levofloxacin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | |||||||||
| 1 | 2 | 3 | 4 | 5 | |||||
| Gram-Positive Bacteria | |||||||||
| Staphylococcus aureus | 21 | 17 | 19 | 16 | 17 | 41 | 39 | 37 | 40 |
| Sarcina lutea | 20 | 19 | - | 17 | 15 | 42 | 40 | 38 | 39 |
| Gram-Negative Bacteria | |||||||||
| Salmonella typhi | 18 | - | 17 | - | - | 39 | 34 | 41 | 36 |
| Klebsiella spp. | - | - | - | - | - | 43 | 37 | 39 | 38 |
| Shigella flexneri | - | - | - | - | - | 38 | 41 | 38 | 41 |
| Escherichia coli | 19 | - | 18 | - | - | 42 | 40 | 41 | 42 |
| Compounds. | VLC Fraction No. | Sephadex Fraction No. | Further Purification Steps | Rf | UV Visualization | Color upon Spraying Vanillin Sulphate |
|---|---|---|---|---|---|---|
| Compound 1 | 15 | 56–69 | PTLC using E: T = 25:75 | 0.14 | Quenching | Yellow, turns black upon heating |
| Compound 2 | 14–15 | 77–101 | PTLC using E: T = 15:85 | 0.35 | Dark Quenching | Orange red, turns purple upon heating |
| Compound 3 | 1–3 | 3–13 | PTLC using E: T = 1:99 | 0.84 | Quenching | Pale yellow |
| Compound 4 | 1–3 | 26–30 | Crystals obtained from concentrated sephadex fraction | 0.30 | Not observed | Purple, turns black upon heating |
| Compound 5 | 4–9 | * 192–231 | Solid crystal obtained | 0.34 | Not observed | Purple, turns black upon heating |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafi, S.; Alam, S.; Emon, N.U.; Ahsan, M. Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis cyanocarpa (Blume) Spreng. Molecules 2022, 27, 5972. https://doi.org/10.3390/molecules27185972
Ashrafi S, Alam S, Emon NU, Ahsan M. Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis cyanocarpa (Blume) Spreng. Molecules. 2022; 27(18):5972. https://doi.org/10.3390/molecules27185972
Chicago/Turabian StyleAshrafi, Sania, Safaet Alam, Nazim Uddin Emon, and Monira Ahsan. 2022. "Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis cyanocarpa (Blume) Spreng" Molecules 27, no. 18: 5972. https://doi.org/10.3390/molecules27185972
APA StyleAshrafi, S., Alam, S., Emon, N. U., & Ahsan, M. (2022). Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis cyanocarpa (Blume) Spreng. Molecules, 27(18), 5972. https://doi.org/10.3390/molecules27185972








