Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Offline Data Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, F.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Yu, Y.; Lowe, A.; Kalra, A. Bioimpedance analysis as a tool for hemodynamic monitoring: Overview, methods and challenges. Physiol. Meas. 2021, 42, 03TR01. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.M.; Hail, M.D.; Li, J.; Young, J.B. Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am. J. Crit. Care. 2003, 41, 211. [Google Scholar] [CrossRef]
- Baumert, M.; Porta, M.; Vos, M.A.; Malik, M.; Couderc, J.P.; Laguna, P.; Piccirillo, G.; Smith, G.L.; Tereshchenko, L.G.; Volders, P.G.A. QT interval variability in body surface ECG: Measurement, physiological basis, and clinical value: Position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016, 19, 925–944. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Mariani, M.V.; Di Iorio, C.; Fabietti, M.; Mastropietri, F.; Crapanzano, D.; Bertani, G.; Sabatino, T.; Zaccagnini, G.; et al. Hospital mortality in decompensated heart failure. A pilot study. J. Electrocardiol. 2020, 61, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Bertani, G.; Lospinuso, I.; Mastropietri, F.; Fabietti, M.; Sabatino, T.; Zaccagnini, G.; Crapanzano, D.; Di Diego, I.; et al. Short-Period Temporal Dispersion Repolarization Markers Predict 30-Days Mortality in Decompensated Heart Failure. J. Clin. Med. 2020, 9, 1879. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Bertani, G.; Lospinuso, I.; Sabatino, T.; Zaccagnini, G.; Crapanzano, D.; Di Diego, I.; Corrao, A.; Rossi, P.; et al. Short-period temporal repolarization dispersion in subjects with atrial fibrillation and decompensated heart failure. Pacing Clin. Electrophysiol. 2021, 44, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef]
- Hsu, A.R.; Barnholt, K.E.; Grundmann, N.K.; Lin, J.H.; McCallum, S.W.; Friedlander, A.L. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. Appl. Physiol. 2006, 100, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Lepretre, P.M.; Koralsztein, J.P.; Billat, V.L. Effect of exercise intensity on relationship between VO2max and cardiac output. Med. Sci. Sports Exerc. 2004, 36, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, A.R.; Alnuaimat, H.; Li, N.; Carrie, R.; Mubarak, K.K. Value of impedance cardiography in patients studied for pulmonary hypertension. Lung 2011, 189, 369–375. [Google Scholar] [CrossRef]
- Pickett, B.R.; Buell, J.C. Usefulness of the impedance cardiogram to reflect left ventricular diastolic function. Am. J. Cardiol. 1993, 71, 1099–1103. [Google Scholar] [CrossRef]
- Gordon, N.; Abbiss, C.R.; Maiorana, A.J.; Marston, K.J.; Peiffer, J.J. Intrarater reliability and agreement of the physioflow bioimpedance cardiography device during rest, moderate and high-intensity exercise. Kinesiology 2018, 50 (Suppl. S1), 140–149. [Google Scholar]
- Van der Meer, N.J.; Oomen, M.W.; Vonk Noordegraaf, A.; Pijpers, R.J.; Plaizier, M.A.; de Vries, P.M. Does impedance cardiography reliably estimate left ventricular ejection fraction? J. Clin. Monit. 1996, 12, 5–9. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet 1995, 346, 1085–1087. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Altman Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Restivo, A.; D’Amario, D.; Paglianiti, D.A.; Laborante, R.; Princi, G.; Cappannoli, L.; Iaconelli, A.; Galli, M.; Aspromonte, N.; Locorotondo, G.; et al. A 3-Year Single Center Experience With Left Atrial Pressure Remote Monitoring: The Long and Winding Road. Front. Cardiovasc. Med. 2022, 9, 899656. [Google Scholar] [CrossRef]
- Sgreccia, D.; Mauro, E.; Vitolo, M.; Manicardi, M.; Valenti, A.C.; Imberti, J.F.; Ziacchi, F.; Boriani, G. Implantable cardioverter defibrillators and devices for cardiac resynchronization therapy: What perspective for patients’ apps combined with remote monitoring? Expert Rev. Med. Devices 2022, 19, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Radhoe, S.P.; Veenis, J.F.; Brugts, J.J. Invasive Devices and Sensors for Remote Care of Heart Failure Patients. Sensors 2021, 21, 2014. [Google Scholar] [CrossRef] [PubMed]
- Burdese, E.; Testa, M.; Raucci, P.; Ferreri, C.; Giovannini, G.; Lombardo, E.; Avogadri, E.; Feola, M. Usefulness of a Telemedicine Program in Refractory Older Congestive Heart Failure Patients. Diseases 2018, 6, 10. [Google Scholar] [CrossRef]
- Perez, M.V. Getting Smart About Wearable ECG Interpretation in the Clinic. JACC Clin. Electrophysiol. 2022, 8, 792–794. [Google Scholar] [CrossRef]
- Kemps, H.M.; Thijssen, E.J.; Schep, G.; Sleutjes, B.T.; de Vries, W.R.; Hoogeveen, A.R.; Wijn, P.F.; Doevendans, P.A. Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J. Appl. Physiol. 2008, 105, 1822–1829. [Google Scholar] [CrossRef]
- Kamath, S.A.; Drazner, M.H.; Tasissa, G.; Rogers, J.G.; Stevenson, L.W.; Yancy, C.W. Correlation of impedance cardiography with invasive hemodynamic measurements in patients with advanced heart failure: The BioImpedance CardioGraphy (BIG) substudy of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial. Am. Heart J. 2009, 158, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, A.; Kansara, B.; Karlekar, A. Comparison of transthoracic electrical bioimpedance cardiac output measurement with thermodilution method in post coronary artery bypass graft patients. Ann. Card. Anaesth. 2011, 14, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Bhavya, G.; Nagaraja, P.S.; Singh, N.G.; Ragavendran, S.; Sathish, N.; Manjunath, N.; Ashok Kumar, K.; Nayak, B. Comparison of continuous cardiac output monitoring derived from regional impedance cardiography with continuous thermodilution technique in cardiac surgical patients. Ann. Card. Anaesth. 2020, 23, 189–192. [Google Scholar] [CrossRef]
- Kannel, W.B.; Kannel, C.; Paffenbarger, R.S., Jr.; Cupples, L.A. Heart rate and cardiovascular mortality: The Framingham Study. Am. Heart J. 1987, 113, 1489–1494. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]
- Seviiri, M.; Lynch, B.M.; Hodge, A.M.; Yang, Y.; Liew, D.; English, D.R.; Giles, G.G.; Milne, R.L.; Dugué, P.A. Resting heart rate, temporal changes in resting heart rate, and overall and cause-specific mortality. Heart 2018, 104, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Cikes, M.; Solomon, S.D. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J. 2016, 37, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Sciomer, S.; Moscucci, F.; Salvioni, E.; Marchese, G.; Bussotti, M.; Corrà, U.; Piepoli, M.F. Role of gender, age and BMI in prognosis of heart failure. Eur. J. Prev. Cardiol. 2020, 27 (Suppl. S2), 46–51. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Sciomer, S.; Moscucci, F.; Maiello, M.; Cugusi, L.; Gallina, S.; Dei Cas, A.; Lombardi, C.; Pengo, M.; Parati, G.; et al. J Cardiovascular prevention in women: A narrative review from the Italian Society of Cardiology working groups on ‘Cardiovascular Prevention, Hypertension and peripheral circulation’ and on ‘Women Disease’. Cardiovasc. Med. 2019, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Sciomer, S.; Moscucci, F.; Maffei, S.; Gallina, S.; Mattioli, A.V. Prevention of cardiovascular risk factors in women: The lifestyle paradox and stereotypes we need to defeat. Eur. J. Prev Cardiol. 2019, 26, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Moscucci, F.; Lavalle, F.; Politi, C.; Campanale, A.; Baggio, G.; Sciomer, S. Acute Coronary Syndrome in Women: A new and specific approach is needed. Eur. J. Prev. Cardiol 2022, 29, e305–e308. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; A Figtree, G.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
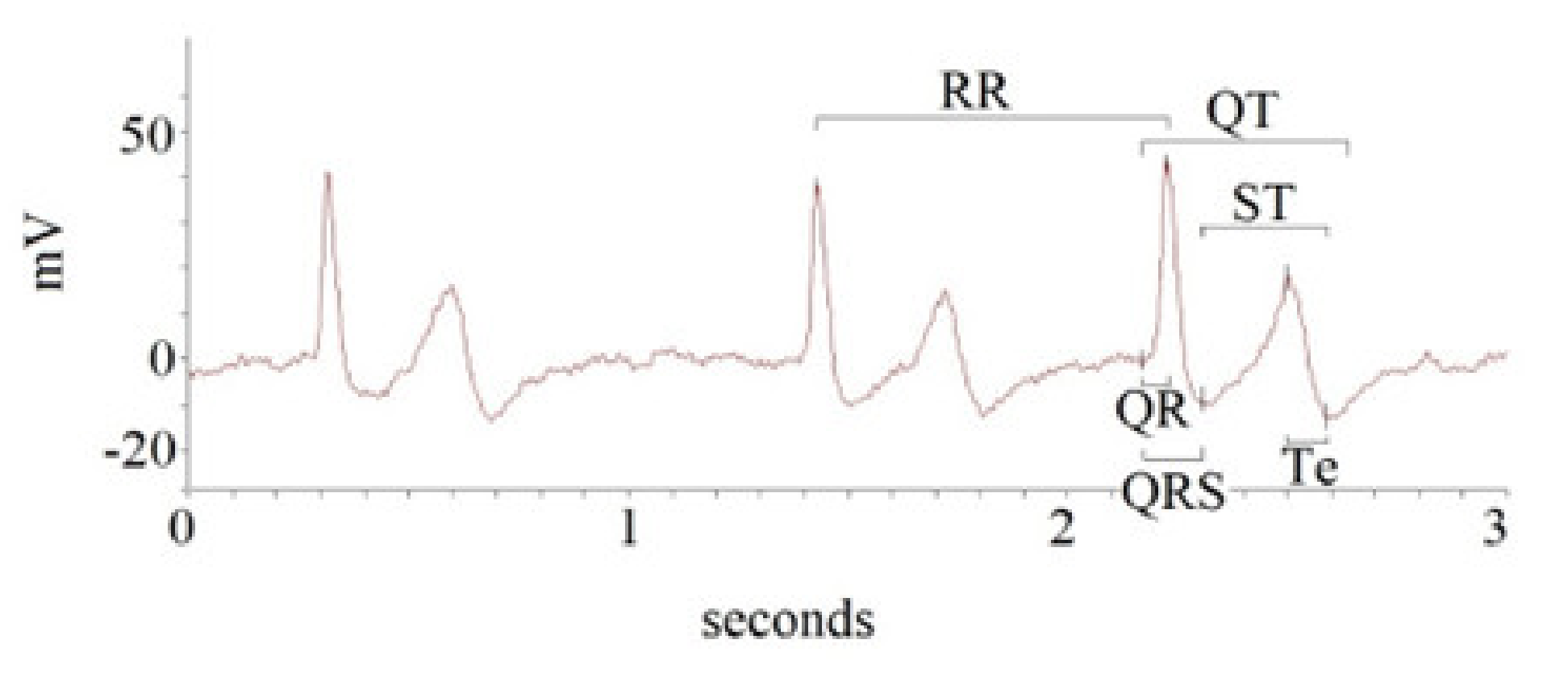
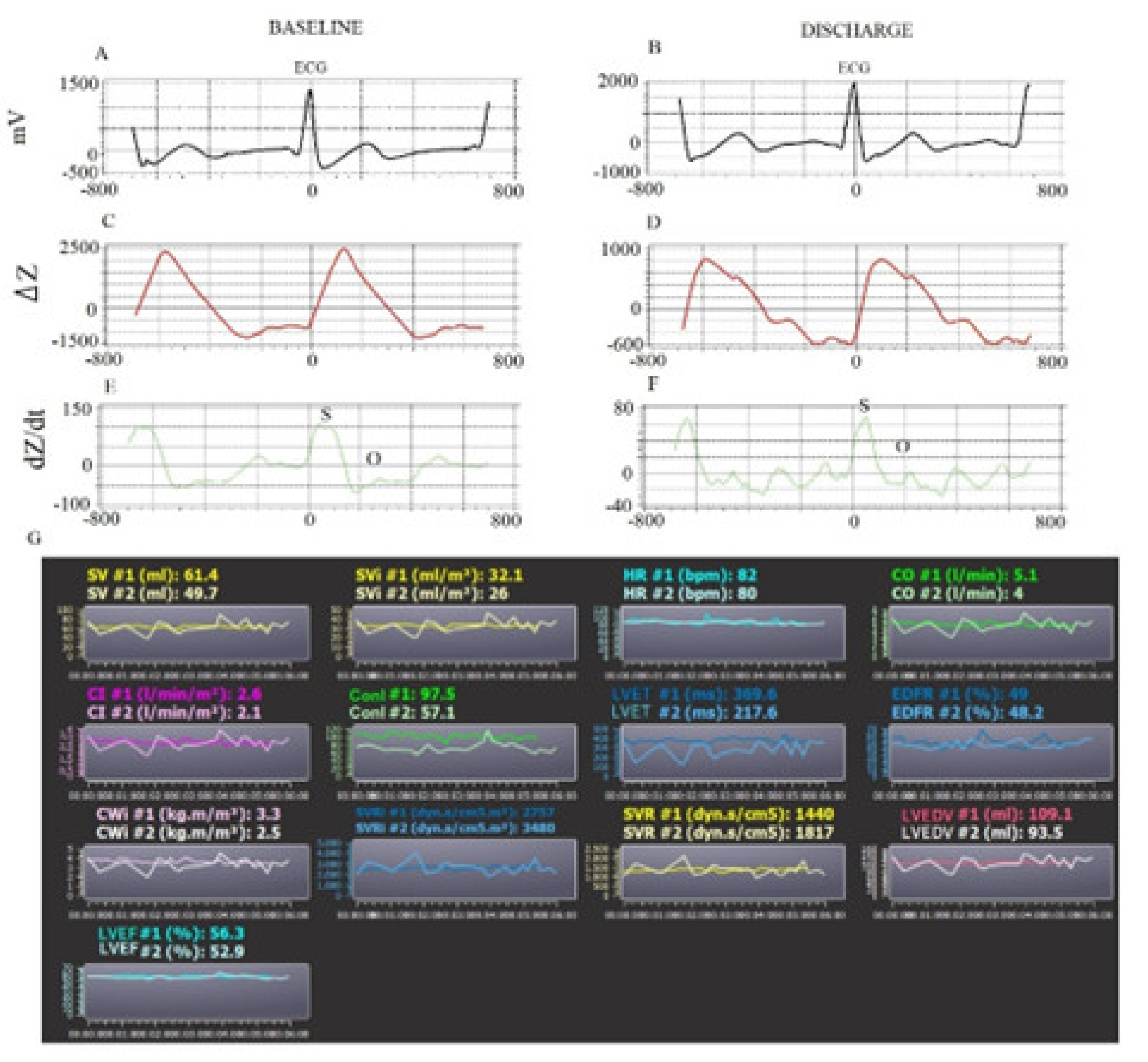

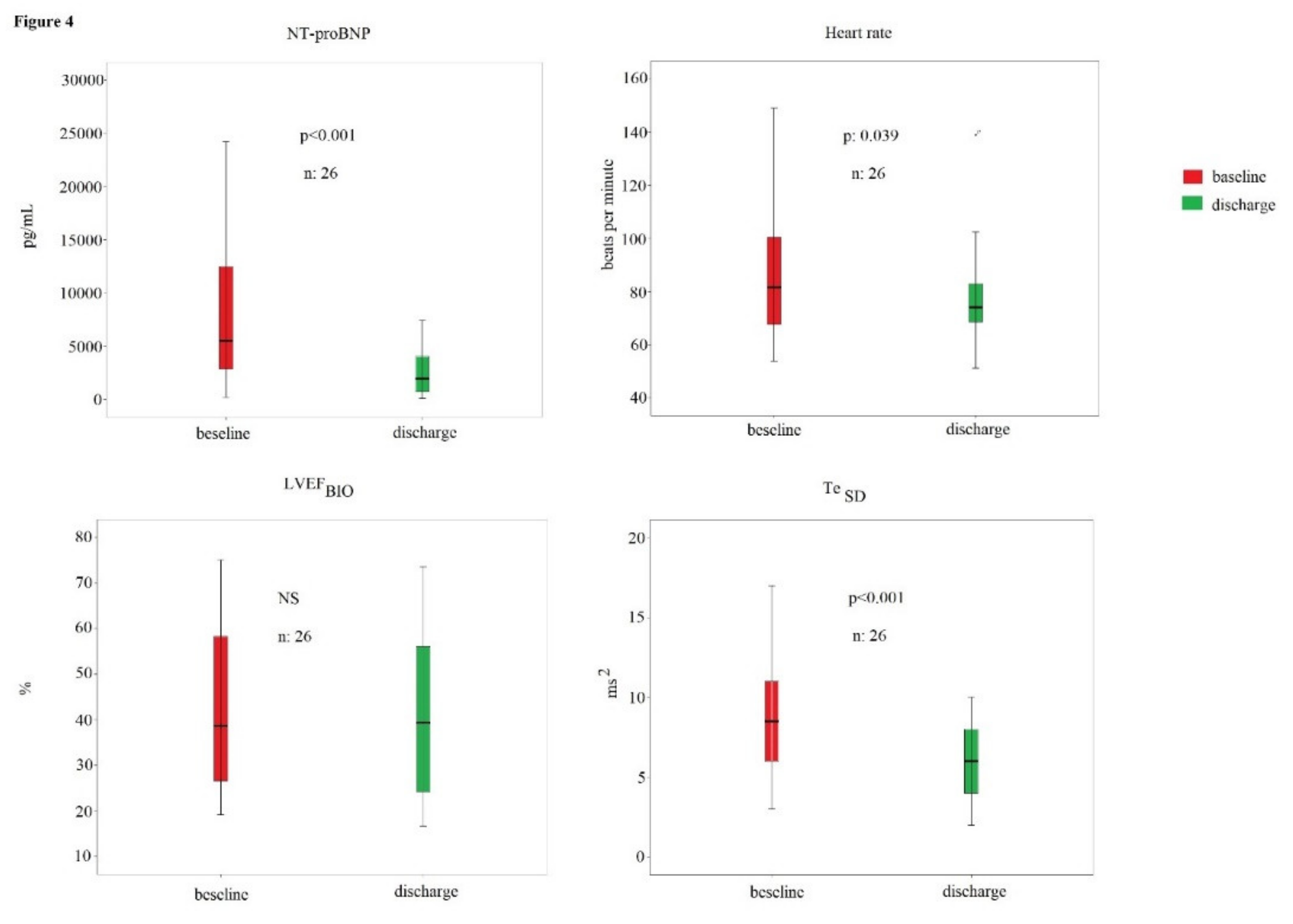
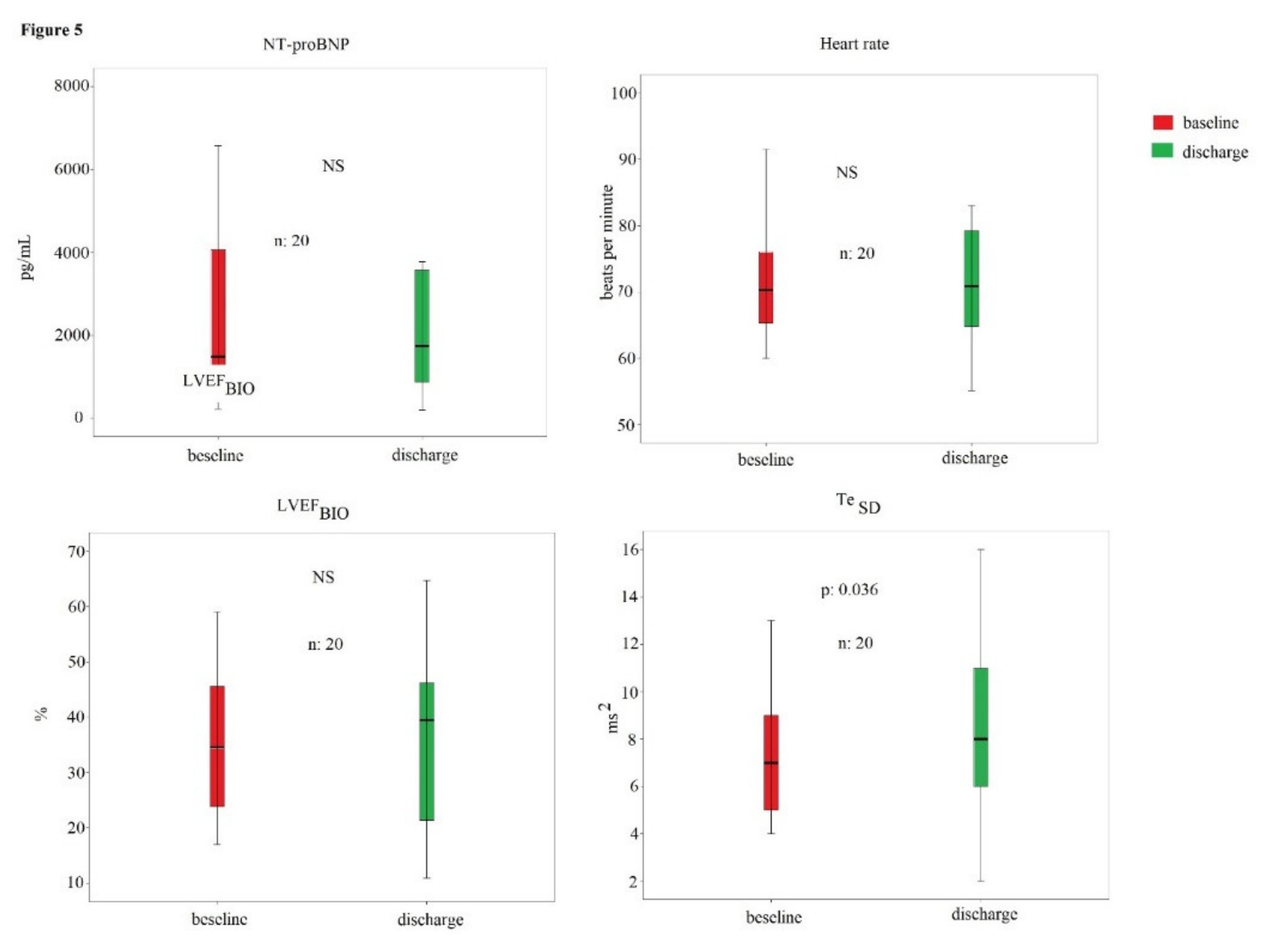
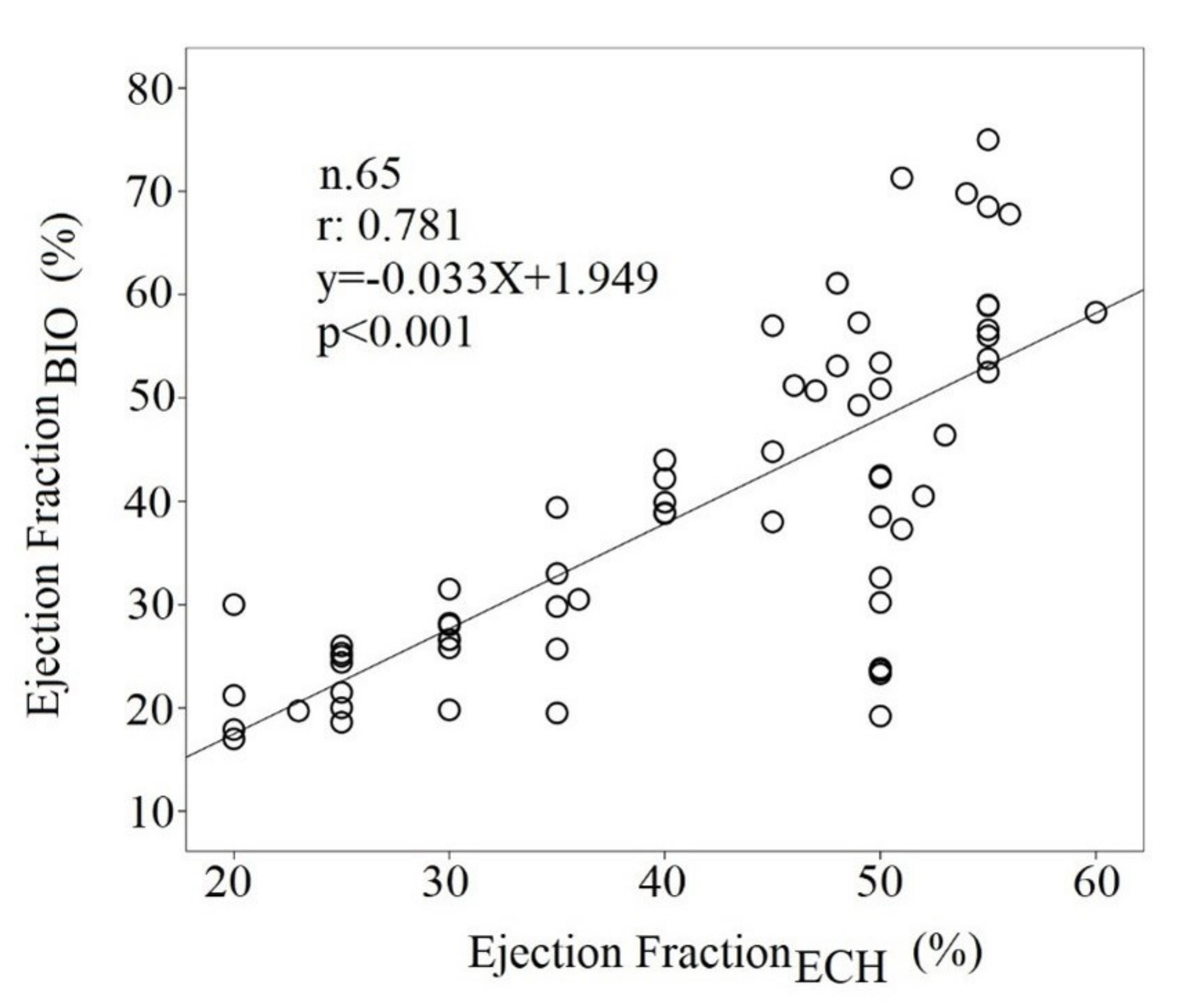
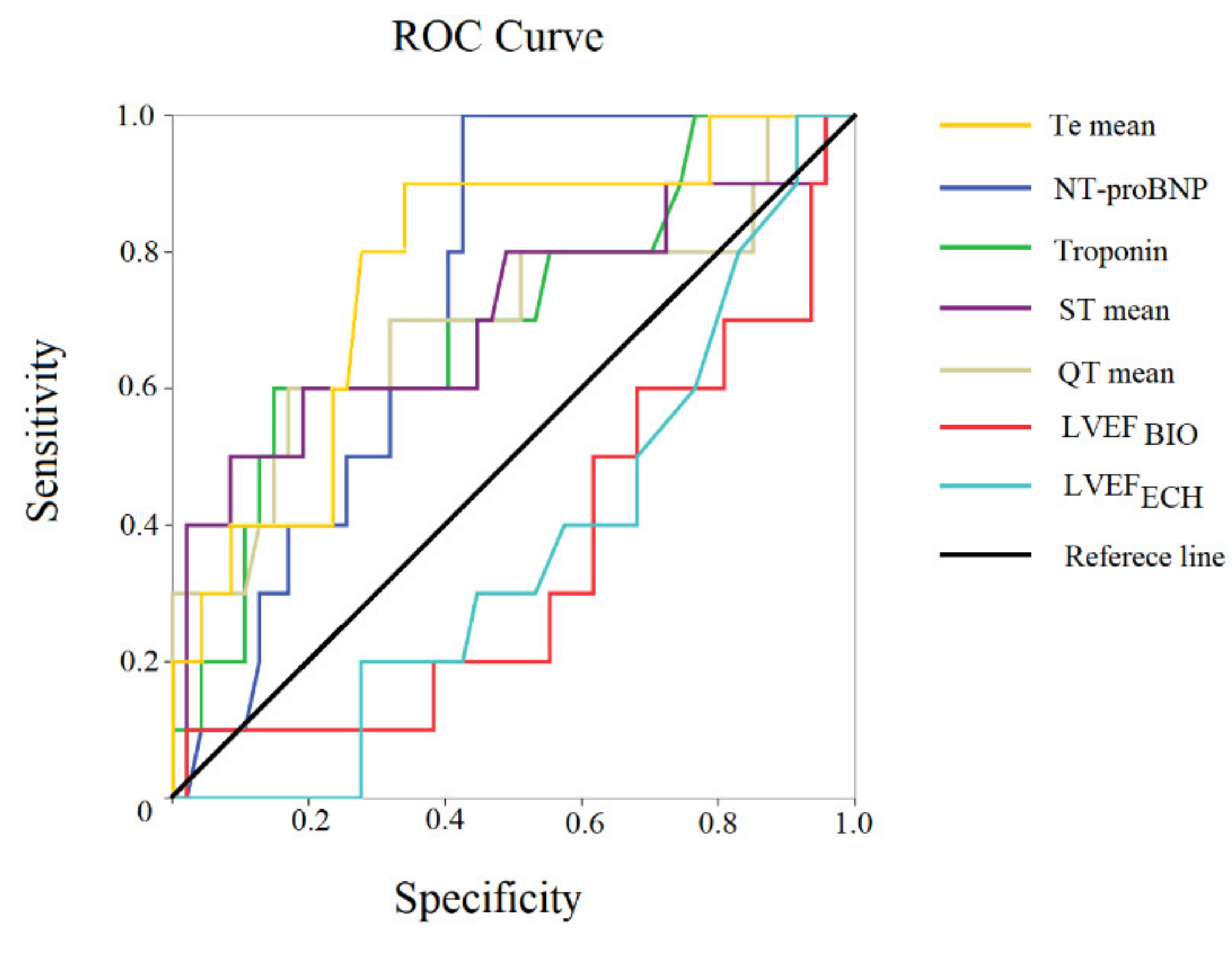
| All Subjects | Heart Failure with Reduced Ejection | Heart Failure with Preserved Ejection | ||
|---|---|---|---|---|
| N:65 | N:39 | N:26 | p-Value | |
| Age, years | 81 ± 10 | 83 ± 9 | 81 ± 10 | 0.445 |
| M/F, n | 35/30 | 24/15 | 11/15 | 0.128 |
| BMI, kg/m2 | 24 ± 4 | 26 ± 5 | 26 ± 5 | 0.655 |
| Echocardiographic findings | ||||
| Left Ventricular Ejection Fraction, % | 43 ± 10 | 35 ± 8 | 52 ± 3 | <0.001 |
| Left Ventricular Mass Index, g/m2 | 144 ± 32 | 157 ± 41 | 124 ± 21 | 0.001 |
| Left Ventricular end-diastolic diameter, mm | 54 ± 7 | 57 ± 8 | 50 ± 5 | 0.001 |
| Left Atrial Transverse Diameter, mm | 47 ± 6 | 49 ± 7 | 46 ± 4 | 0.043 |
| Tricuspid annular plane systolic excursion, | 20 ± 3 | 19 ± 4 | 21 ± 4 | 0.049 |
| Tricuspid regurgitation peak gradient, mm Hg | 45 ± 11 | 44 ± 10 | 44 ± 13 | 0.999 |
| Clinical parameters | ||||
| Arterial O2 saturation, % | 98 ± 2 | 98 ± 2 | 98 ± 2 | 0.539 |
| Fraction of inspired O2,% | 26 ± 9 | 27 ± 9 | 25 ± 7 | 0.538 |
| PaO2/FiO2 ratio | 356 ± 99 | 350 ± 109 | 353 ± 77 | 0.884 |
| A-ADO2, mmHg | 33 (36) | 35 (61) | 30 (32) | 0.659 |
| NT-pro BNP, pg/mL | 3160 (7295) | 4140 (7310) | 2680 (5216) | 0.047 |
| C-reactive protein (mg/dL) | 5.58 (14) | 3.72 (15.25) | 9.18 (13.54) | 0.920 |
| High-sensitivity cardiac troponin (pg/L) | 40 (74) | 52 (72) | 29 (55) | 0.038 |
| Blood potassium (mmol/L) | 4.14 ± 0.69 | 3.99 ± 0.69 | 4.24 ± 0.68 | 0.147 |
| Blood calcium (mmol/L) | 2.17 ± 0.21 | 2.16 ± 0.19 | 2.19 ± 0.23 | 0.530 |
| Creatinine clearance (mL/m) | 53 ± 26 | 55 ± 28 | 54 ± 28 | 0.454 |
| Serum Creatinine, mg/dL | 1.06 (0.84) | 1.05 (0.89) | 1.06 (0.79) | 0.804 |
| Serum Urea, mmol/L | 7.60 (6.9) | 8.20 (8.30) | 7.55 (5.8) | 0.789 |
| Aspartate Aminotraferase, U/L | 22 (15) | 22 (24) | 21 (11) | 0.845 |
| Alanine Aminotrasferase, U/L | 16 (14) | 17 (17) | 15 (10) | 0.924 |
| γ-Glutamyl trasferase, U/L | 31 (49) | 30 (71) | 31 (37) | 0.516 |
| Alkaline phosphatase, U/L | 82 (55) | 80 (65) | 84 (40) | 0.556 |
| Total Bilirubin, mg/dL | 0.73 (0.51) | 0.80 (0.34) | 0.62 (0.74) | 0.253 |
| Preexisting clinical Conditions | ||||
| Hypertension, n (%) | 58 (89) | 35 (90) | 26 (100) | 0.870 |
| Hypercholesterolemia, n (%) | 34 (52) | 22 (56) | 12 (46) | 0.417 |
| Diabetes, n (%) | 32 (50) | 20 (51) | 12 (46) | 0.798 |
| Renal Insufficiency, n (%) | 35 (54) | 23 (59) | 12 (46) | 0.310 |
| Known Myocardial Ischemia History, n (%) | 29 (45) | 23 (59) | 6 (23) | 0.004 |
| Valve Diseases | 26 (40) | 17 (44) | 9 (35) | 0.469 |
| Premature Supraventricular Complexes, n (%) | 1 (2) | 1 (3) | 0 (0) | 0.411 |
| Premature Ventricular Complexes, n (%) | 7 (11) | 5 (13) | 2 (8) | 0.513 |
| Permanent Atrial fibrillation, n (%) | 22 (34) | 16 (41) | 6 (23) | 0.134 |
| Left Bundle Branch Block, n (%) | 14 (22) | 13 (33) | 1 (4) | 0.005 |
| Right Bundle Branch Block, n (%) | 6 (9) | 4 (10) | 2 (8) | 0.726 |
| Pacemaker- ICD, n (%) | 11 (17) | 10 (26) | 1 (4) | 0.022 |
| Deceased Subjects, n (%) | 10 (15) | 8 (21) | 2 (8) | 0.160 |
| Consolidated Pharmacological therapy | ||||
| β-blockers, n (%) | 40 (62) | 26 (67) | 14 (54) | 0.298 |
| Furosemide, n (%) | 50 (77) | 33 (85) | 17 (65) | 0.071 |
| ACEi/Sartans | 29 (45) | 17 (44) | 12 (46) | 0.839 |
| Aldosterone antagonists, n (%) | 10 (15) | 6 (15) | 4 (15) | 1.000 |
| Potassium, n (%) | 2 (3) | 1 (3) | 1 (4) | 0.769 |
| Nitrates, n (%) | 13 (20) | 9 (23) | 4 (15) | 0.448 |
| Digoxin, n (%) | 3 (5) | 3 (8) | 0 (0) | 0.148 |
| Statins, n (%) | 17 (26) | 11 (29) | 6 (23) | 0.602 |
| Antiplatelet drugs, n (%) | 31 (48) | 17 (44) | 14 (54) | 0.417 |
| Oral Anticoagulants, n (%) | 17 (27) | 12 (32) | 5 (19) | 0.272 |
| Diltiazem or Verapamil, n (%) | 1 (2) | 0 (0) | 1 (4) | 0.217 |
| Dihydropyridine Calcium channel blockers, n (%) | 10 (15) | 6 (15) | 4 (15) | 1.000 |
| Propafenone, n (%) | 1 (2) | 0 (0) | 1 (4) | 0.217 |
| Amiodarone, n (%) | 3 (5) | 3 (8) | 0 (0) | 0.148 |
| Valsartan/Sacubitril, n (%) | 1 (2) | 1 (3) | 0 (0) | 0.411 |
| All Subjects | Heart Failure with Reduced Ejection | Heart Failure with Preserved Ejection | ||
|---|---|---|---|---|
| N:65 | N:39 | N:26 | p-Value | |
| Heart Rate, b/m | 77 ± 19 | 80 ± 22 | 73 ± 13 | 0.170 |
| Stroke Volume, mL | 65 ± 19 | 60 ± 18 | 73 ± 18 | 0.003 |
| Stroke Volume Index, mL/m2 | 37 ± 11 | 33 ± 10 | 42 ± 10 | 0.002 |
| Cardiac Output, L/m | 4.87 ± 1.38 | 4.59 ± 1.38 | 5.29 ± 1.29 | 0.046 |
| Cardiac Index, L/m/m2 | 2.72 ± 0.81 | 2.54 ± 0.79 | 2.99 ± 079 | 0.027 |
| Systemic Vascular Resistance, Dyn.s/cm2 | 1531 ± 647 | 1812 ± 683 | 1580 ± 573 | 0.159 |
| Systemic Vascular Resistance Index, Dyn.s/cm2.m2 | 3070 ± 1138 | 3259 ± 1213 | 2786 ± 969 | 0.101 |
| SBP, mm Hg | 123 ± 17 | 120 ± 17 | 127 ± 15 | 0.131 |
| MBP, mm Hg | 92 ± 11 | 90 ± 11 | 94 ± 11 | 0.221 |
| DBP, mm Hg | 69 ± 10 | 67 ± 9 | 7 ± 11 | 0.126 |
| Left Ventricular Ejection Fraction, % | 39 ± 16 | 33 ± 13 | 48 ± 16 | <0.001 |
| Contractility Index, | 79 ± 51 | 63 ± 35 | 104 ± 61 | 0.001 |
| Left Ventricular Ejection Time, ms | 270 ± 83 | 249 ± 75 | 303 ± 85 | 0.010 |
| Left Cardiac Work Index, kg.m/m2 | 3.24 ± 1.20 | 2.97 ± 1.18 | 3.65 ± 1.14 | 0.026 |
| Left Ventricular End Diastolic Volume, mL | 180 ± 72 | 196 ± 85 | 156 ± 35 | 0.025 |
| Early Diastolic Filling Ratio | 85 ± 35 | 93 ± 40 | 72 ± 21 | 0.017 |
| All Subjects | Heart Failure with Reduced Ejection | Heart Failure with Preserved Ejection | ||
|---|---|---|---|---|
| N:65 | N:39 | N:26 | p-Value | |
| QR mean, ms | 45 ± 18 | 50 ± 20 | 37 ± 8 | 0.005 |
| QRSD, ms2 | 5 (5) | 6 (5) | 4 (4) | 0.012 |
| QRS mean, ms | 104 ± 33 | 114 ± 36 | 86 ± 19 | 0.001 |
| QRSSD, ms2 | 7 (5) | 8 (6) | 6 (4) | 0.093 |
| QT mean, ms | 475 ± 97 | 509 ± 95 | 420 ± 53 | 0.002 |
| QTSD, ms2 | 10 (5) | 11 (5) | 8 (6) | 0.021 |
| ST mean, ms | 369 ± 77 | 395 ± 79 | 328 ± 53 | 0.001 |
| STSD, ms2 | 9 (4) | 9 (4) | 9 (4) | 0.232 |
| Te mean, ms | 108 ± 33 | 116 ± 31 | 95 ± 24 | 0.005 |
| TeSD, ms2 | 8 (5) | 8 (5) | 7 (5) | 0.179 |
| Deceased CHF | Survivor CHF Subjects | ||
|---|---|---|---|
| N:10 | N:55 | p-Value | |
| QR mean, ms | 53 ± 22 | 43 ± 16 | 0.093 |
| QRSD, ms2 | 6 (5) | 5 (6) | 0.268 |
| QRS mean, ms | 108 ± 28 | 103 ± 34 | 0.680 |
| QRSSD, ms2 | 7 (4) | 7 (5) | 0.665 |
| QT mean, ms | 533 ± 116 | 462 ± 89 | 0.036 |
| QTSD, ms2 | 11 (7) | 10 (5) | 0.287 |
| ST mean, ms | 426 ± 101 | 357 ± 66 | 0.009 |
| STSD, ms2 | 10 (4) | 9 (4) | 0.264 |
| Te mean, ms | 136 ± 40 | 103 ± 25 | 0.001 |
| TeSD, ms2 | 9 (7] | 7 (4] | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, G.; Moscucci, F.; Corrao, A.; Carnovale, M.; Di Diego, I.; Lospinuso, I.; Caltabiano, C.; Mezzadri, M.; Rossi, P.; Magrì, D. Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments. Biomedicines 2022, 10, 2407. https://doi.org/10.3390/biomedicines10102407
Piccirillo G, Moscucci F, Corrao A, Carnovale M, Di Diego I, Lospinuso I, Caltabiano C, Mezzadri M, Rossi P, Magrì D. Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments. Biomedicines. 2022; 10(10):2407. https://doi.org/10.3390/biomedicines10102407
Chicago/Turabian StylePiccirillo, Gianfranco, Federica Moscucci, Andrea Corrao, Myriam Carnovale, Ilaria Di Diego, Ilaria Lospinuso, Cristina Caltabiano, Martina Mezzadri, Pietro Rossi, and Damiano Magrì. 2022. "Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments" Biomedicines 10, no. 10: 2407. https://doi.org/10.3390/biomedicines10102407
APA StylePiccirillo, G., Moscucci, F., Corrao, A., Carnovale, M., Di Diego, I., Lospinuso, I., Caltabiano, C., Mezzadri, M., Rossi, P., & Magrì, D. (2022). Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments. Biomedicines, 10(10), 2407. https://doi.org/10.3390/biomedicines10102407






