A Pyridazine-Containing Phthalonitrile Resin for Heat-Resistant and Flame-Retardant Polymer Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BCPD Monomer
2.3. Preparation of Poly(BCPD) Resins
2.4. Characterizations
3. Results
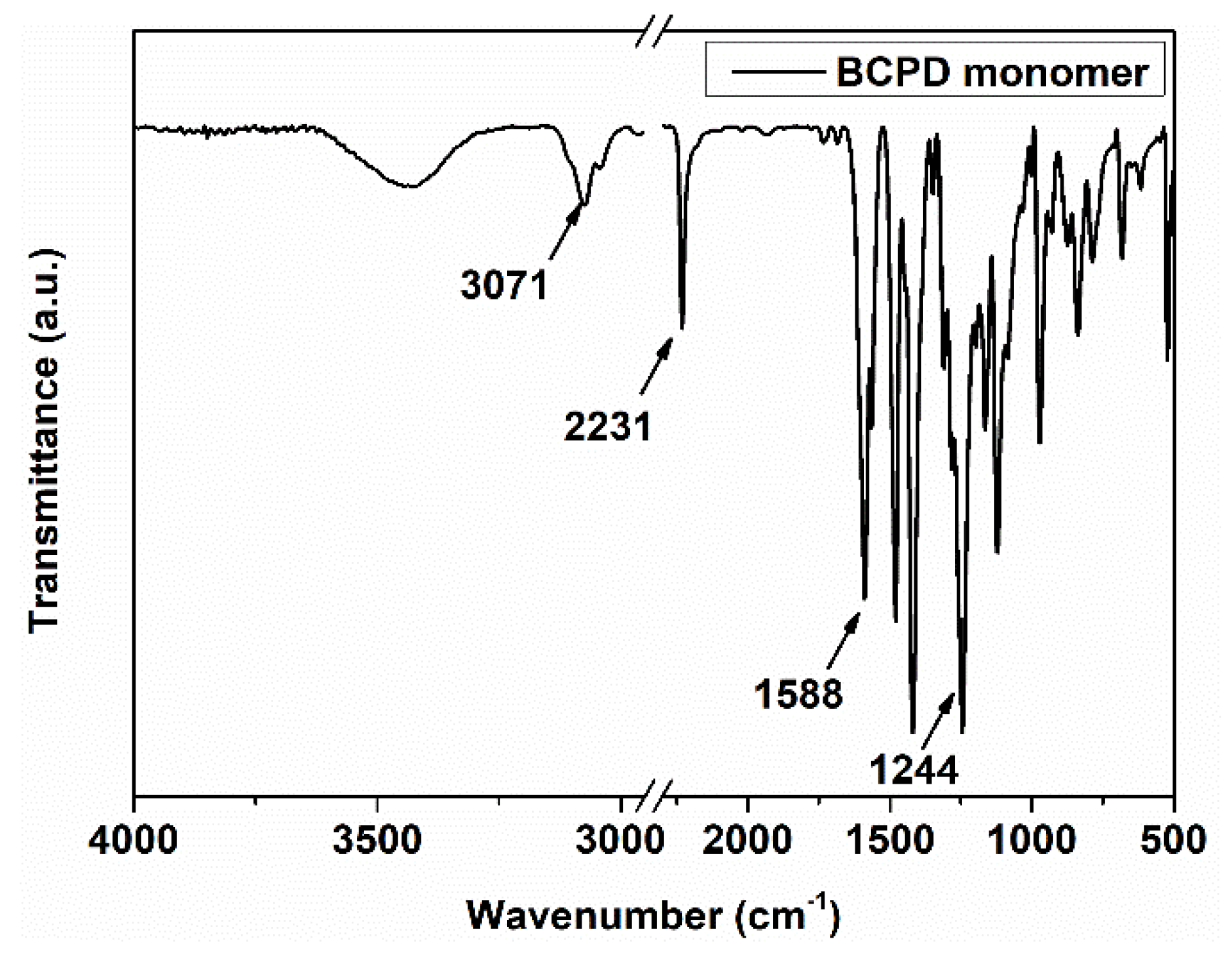
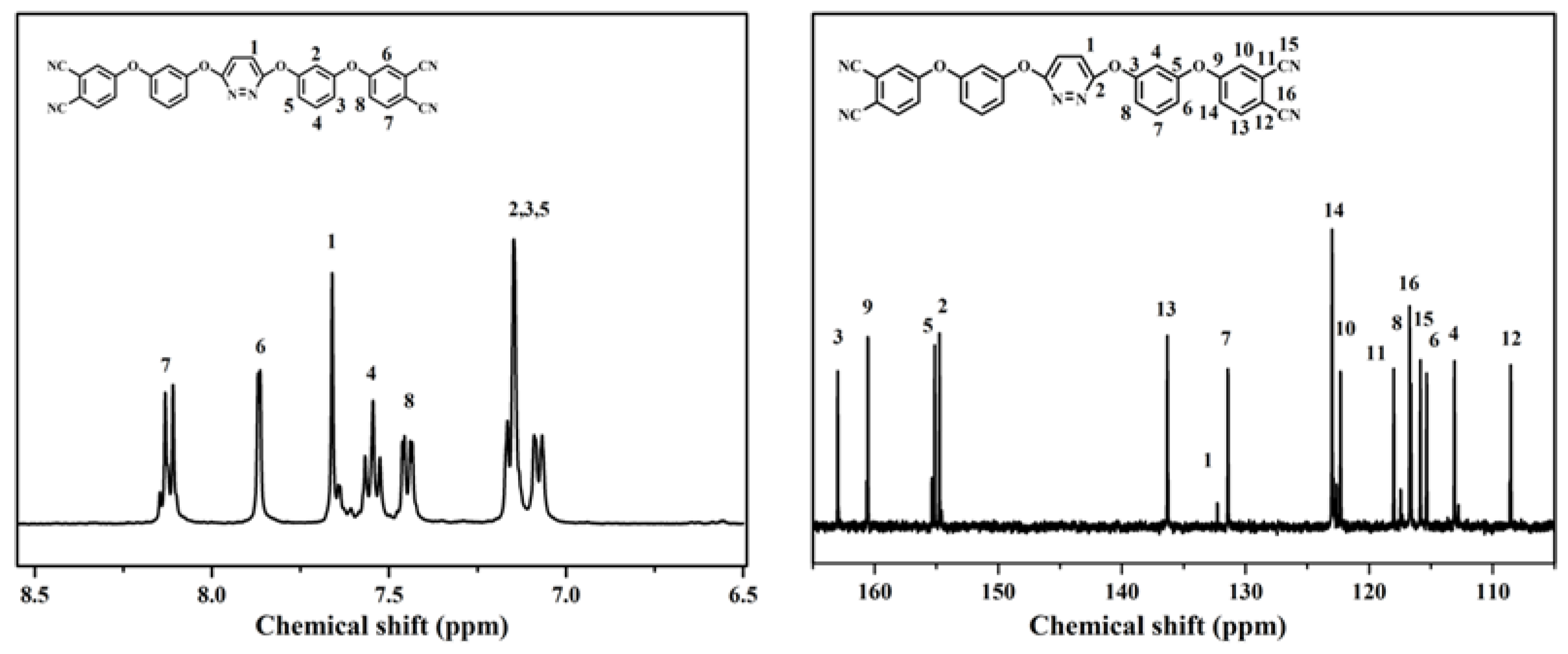
3.1. Characterization of BCPD Monomer
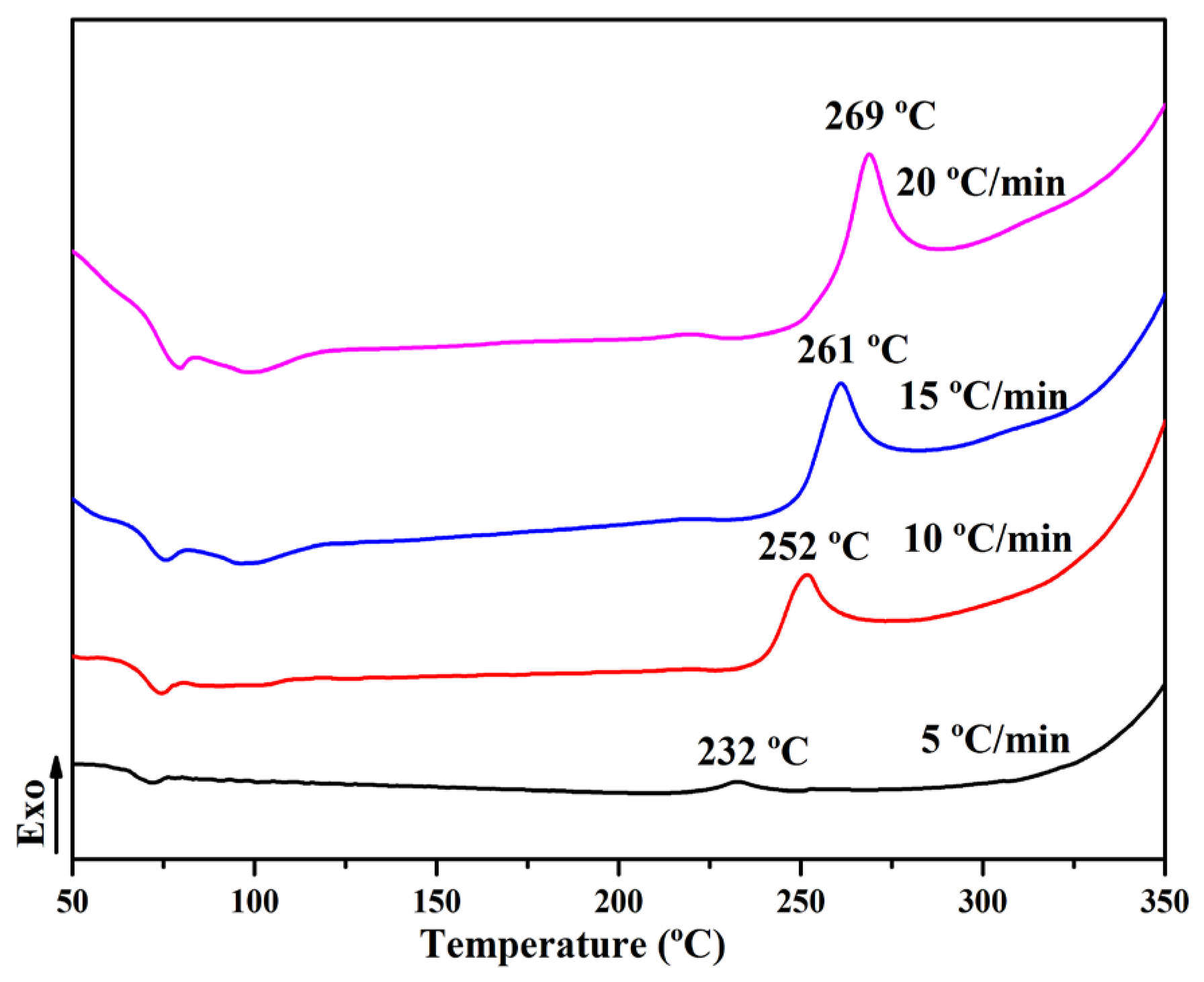
3.2. Curing Behaviors
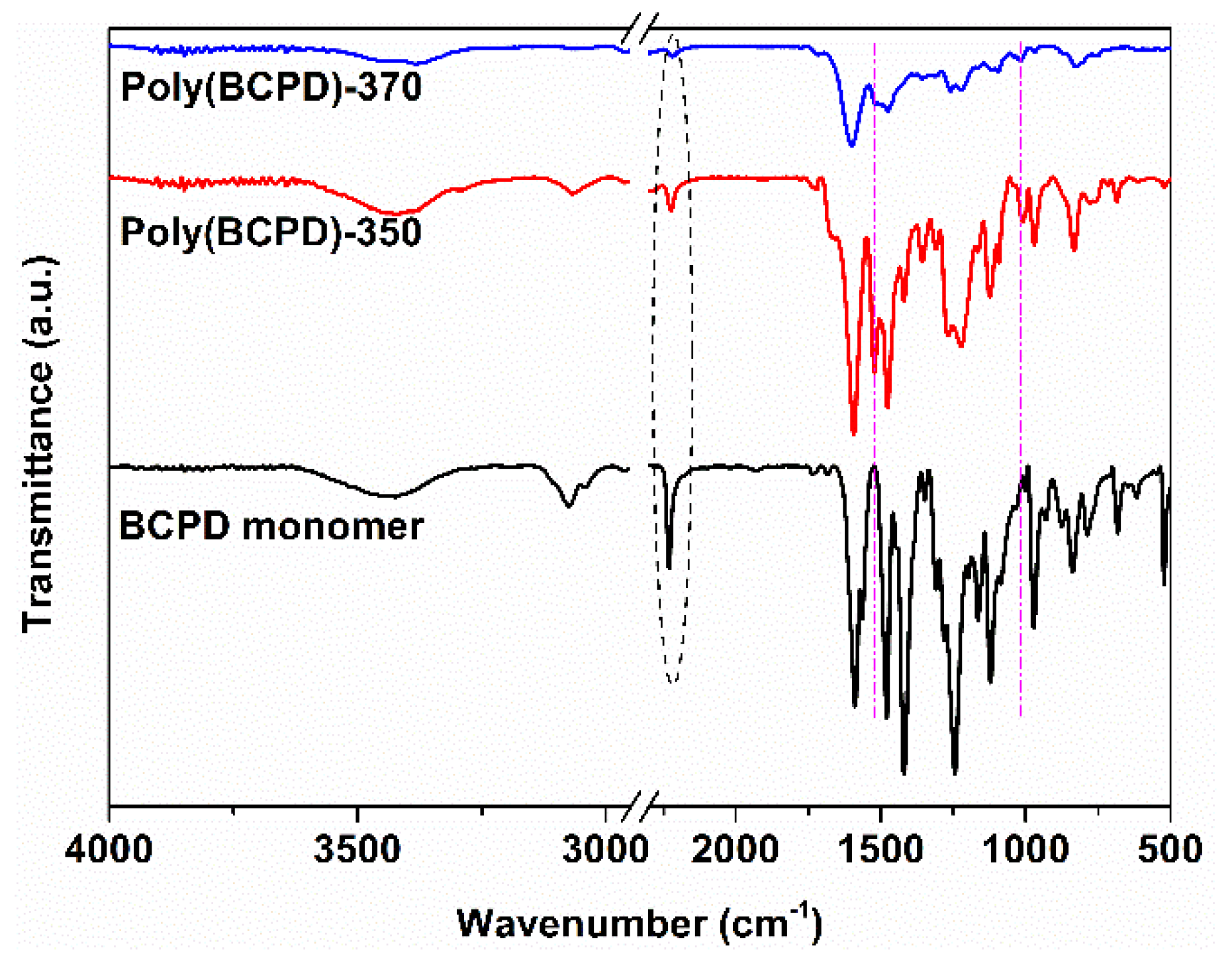
3.3. Structural Characterization of Poly(BCPD) Resin
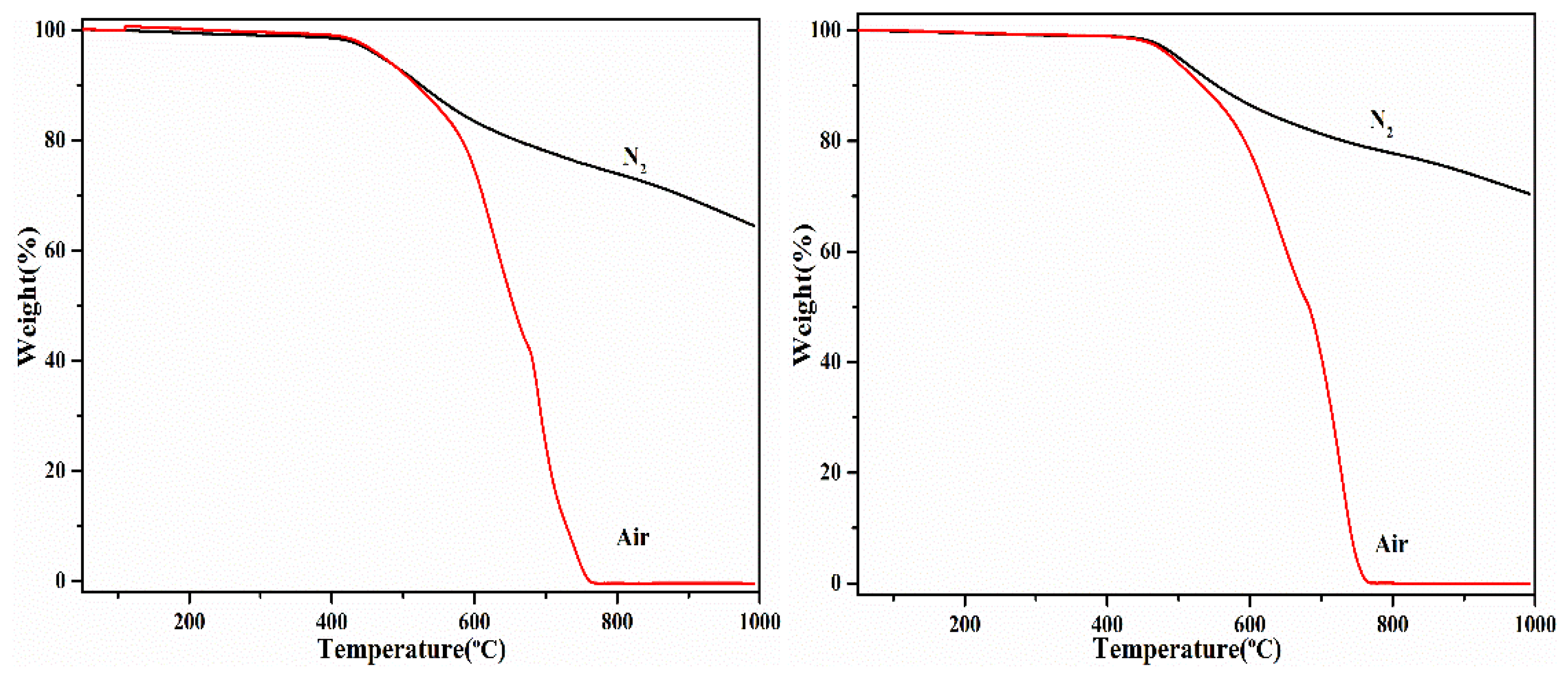
3.4. Thermal and Thermal-Oxidative Stabilities
3.5. Dynamic Mechanical Analysis
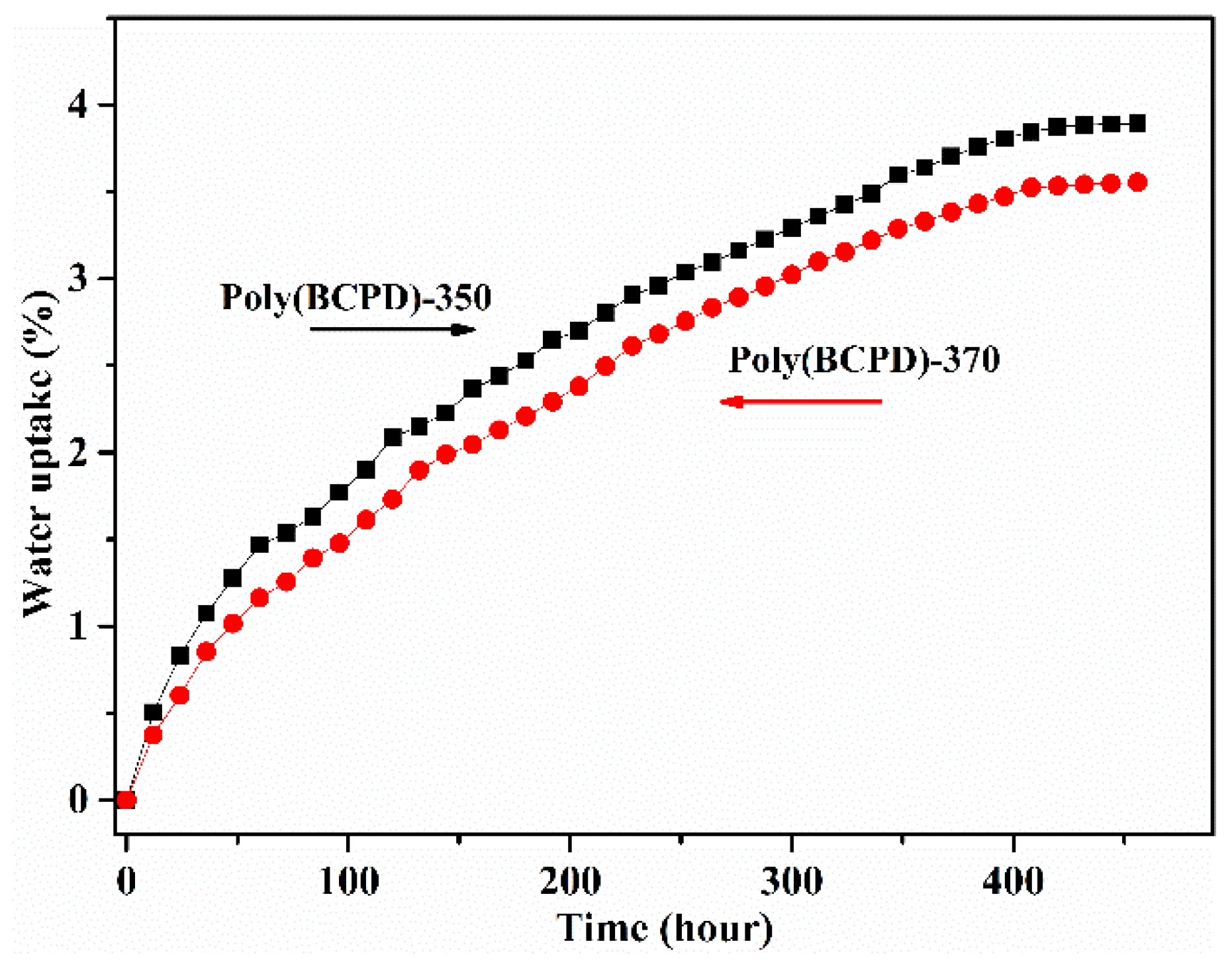
3.6. Water Uptake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keller, T.M.; Price, T.R. Amine-Cured Bisphenol-Linked Phthalonitrile Resins. J. Macrom. Sci. Part A Poly. Chem. 1982, 18, 931–937. [Google Scholar] [CrossRef]
- Wang, A.R.; Dayo, A.Q.; Zu, L.W.; Lv, D.; Song, S.; Tang, T.; Gao, B.C. Bio-based phthalonitrile compounds: Synthesis, curing behavior, thermomechanical and thermal properties. React. Funct. Polym. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Han, Y.; Guo, Y.; Wang, S.; Sun, J.; Zhou, H.; Zhao, T. Phthalonitrile-terminated silicon-containing oligomers: Synthesis, polymerization, and properties. Ind. Eng. Chem. Res. 2019, 58, 9921–9930. [Google Scholar] [CrossRef]
- Yu, X.Y.; Naito, K.; Kang, C.; Qu, X.W.; Zhang, Q.X. Synthesis and Properties of a High-Temperature Naphthyl-Based Phthalonitrile Polymer. Macrom. Chem. Phys. 2013, 214, 361–369. [Google Scholar] [CrossRef]
- Keller, T.M. Imide-containing phthalonitrile resin. Polymer 1993, 34, 952–955. [Google Scholar] [CrossRef]
- Wang, A.R.; Dayo, A.Q.; Lv, D.; Xu, Y.L.; Wang, J.; Liu, W.B.; Derradji, M. Novel amino-containing fluorene-based bisphthalonitrile compounds with flexible group: Synthesis, curing behavior, and properties. High Perform. Polym. 2018, 30, 767–775. [Google Scholar] [CrossRef]
- Keller, T.M.; Dominguez, D.D.; Laskoski, M. Oligomeric bisphenol A-based PEEK-like phthalonitrile-cure and polymer properties. J. Macrom. Sci. Part A Poly. Chem. 2016, 54, 3769–3781. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, J.; Zhan, Y.; Yang, X.; Zhong, J.; Zhao, R.; Liu, X. Effect of curing behaviors on the properties of poly (arylene ether nitrile) end-capped with phthalonitrile. J. Appl. Polym. Sci. 2012, 125, 3829–3835. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, L.; Zhou, H.; Lin, X.; Tong, Z.; Zhang, M.; Xu, C. Self-catalyzed silicon-containing phthalonitrile resins with low melting point, excellent solubility and thermal stability. J. Appl. Polym. Sci. 2014, 131, 40919. [Google Scholar] [CrossRef]
- Babkin, A.V.; Zodbinov, E.B.; Bulgakov, B.A.; Kepman, A.V.; Avdeev, V.V. Low-melting siloxane-bridged phthalonitriles for heat-resistant matrices. Eur. Polym. J. 2015, 66, 452–457. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, J.; Guo, H.M.; Chen, X.G.; Yu, X.Y.; Zhang, Q.X. A novel high temperature vinylpyridine-based phthalonitrile polymer with a low melting point and good mechanical properties. Polym. Chem. 2018, 9, 976–983. [Google Scholar] [CrossRef]
- Laskoski, M.; Clarke, J.S.; Neal, A.; Harvey, B.G.; Ricks-Laskoski, H.L.; Hervey, W.J.; Daftary, M.N.; Shepherd, A.R.; Keller, T.M. Sustainable High-Temperature Phthalonitrile Resins Derived from Resveratrol and Dihydroresveratrol. Chemistryselect 2016, 1, 3423–3427. [Google Scholar] [CrossRef]
- Keller, T.M. Synthesis and Polymerization of Multiple Aromatic Ether Phthalonitriles. Chem. Mater. 1994, 6, 302–305. [Google Scholar] [CrossRef]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and properties of aromatic ether phosphine oxide containing oligomeric phthalonitrile resins with improved oxidative stability. Polymer 2007, 48, 6234–6240. [Google Scholar] [CrossRef]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Alkyne-Containing Phthalonitrile Resins: Controlling Mechanical Properties by Selective Curing. J. Polym. Sci. Part A-Polym. Chem. 2013, 51, 4774–4778. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Keller, T.M. Low-melting Phthalonitrile Oligomers: Preparation, Polymerization and Polymer Properties. High Perform. Polym. 2006, 18, 283–304. [Google Scholar] [CrossRef]
- Laskoski, M.; Schear, M.B.; Neal, A.; Dominguez, D.D.; Ricks-Laskoski, H.L.; Hervey, J.; Keller, T.M. Improved synthesis and properties of aryl ether-based oligomeric phthalonitrile resins and polymers. Polymer 2015, 67, 185–191. [Google Scholar] [CrossRef]
- Laskoski, M.; Neal, A.; Schear, M.B.; Ricks-Laskoski, H.L.; Saab, A.P. Oligomeric aliphatic-aromatic ether containing phthalonitrile resins. J. Macrom. Sci. Part A Poly. Chem. 2015, 54, 2186–2191. [Google Scholar] [CrossRef]
- Laskoski, M.; Clarke, J.S.; Neal, A.; Ricks-Laskoski, H.L.; Hervey, W.J.; Keller, T.M. Synthesis of bisphenol A-free oligomeric phthalonitrile resins with sulfone and sulfone-ketone containing backbones. J. Macrom. Sci. Part A Poly. Chem. 2016, 54, 1639–1646. [Google Scholar] [CrossRef]
- Yang, K.X.; Chen, X.G.; Zhang, Z.J.; Yu, X.Y.; Naito, K.; Zhang, Q.X. Introducing rigid pyrimidine ring to improve the mechanical properties and thermal-oxidative stabilities of phthalonitrile resin. Polym. Adv. Technol. 2020, 31, 328–337. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, P.; Zhang, Z.; Yu, X.; Naito, K.; Zhang, Q. Synthesis and properties of pyrazine-based oligomeric phthalonitrile resins. High Perform. Polym. 2019, 31, 1075–1084. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Ji, P.; Yu, X.; Zhang, Q. Synthesis and properties of a novel high-temperature vinylpyridine-based phthalonitrile polymer. High Perform. Polym. 2018, 31, 820–830. [Google Scholar] [CrossRef]
- Peng, X.; Sheng, H.; Guo, H.; Naito, K.; Yu, X.; Ding, H.; Qu, X.; Zhang, Q. Synthesis and properties of a novel high-temperature diphenyl sulfone-based phthalonitrile polymer. High Perform. Polym. 2014, 26, 837–845. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Zhang, Z.; Yang, K.; Li, P.; Chen, X.; Yu, X.; Naito, K.; Zhang, Q. Synthesis and properties of a high-performance pyrimidine-containing self-catalyzed phthalonitrile polymer. J. Macrom. Sci. Part A Poly. Chem. 2019, 57, 2287–2294. [Google Scholar] [CrossRef]
- Wu, M.; Xu, J.; Bai, S.; Chen, X.; Yu, X.; Naito, K.; Zhang, Z.; Zhang, Q. A high-performance functional phthalonitrile resin with a low melting point and a low dielectric constant. Soft Matter 2020, 16, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zong, L.; Wang, J.; Jian, X. Enhanced thermal property via tunable bisphenol moieties in branched phthalonitrile thermoset. Polymer 2019, 172, 372–381. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Cao, X.M.; Cai, W.A.; Song, S.; Wang, J.; Zegaoui, A.; Gong, L.D. Synthesis of benzophenone-center bisphenol-A containing phthalonitrile monomer (BBaph) and its copolymerization with Pa benzoxazine. React. Funct. Polym. 2018, 129, 46–52. [Google Scholar] [CrossRef]
- Peng, W.; Yao, F.; Hu, J.; Liu, Y.; Lu, Z.; Liu, Y.; Liu, Z.; Zeng, K.; Yang, G. Renewable protein-based monomer for thermosets: A case study on phthalonitrile resin. Green Chem. 2018, 20, 5158–5168. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Perez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Han, Y.; Liao, G.; Xu, Y.; Yu, G.; Jian, X. Cure kinetics, phase behaviors, and fracture properties of bismaleimide resin toughened by poly(phthalazinone ether ketone). Polym. Eng. Sci. 2010, 49, 2301–2308. [Google Scholar] [CrossRef]
- Teo, K.C.; Pan, B.; Xiao, Y.; Lu, X. Epoxy/polyhedral oligomeric silsesquioxane (POSS) hybrid networks cured with an anhydride: Cure kinetics and thermal properties. Polymer 2007, 48, 5671–5680. [Google Scholar]
- Yue, J.; Zhao, C.; Dai, Y.; Li, H.; Li, Y. Catalytic effect of exfoliated zirconium phosphate on the curing behavior of benzoxazine. Thermochim. Acta 2017, 650, 18–25. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Zhang, J.; Yang, X.; Zhao, R.; Liu, X. Self-promoted curing phthalonitrile with high glass transition temperature for advanced composites. J. Polym. Res. 2012, 19, 9918. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Li, Z.; Xu, S.; Han, Y.; Luo, Z.; Zhao, T. Synthesis and properties of phthalonitrile terminated polyaryl ether nitrile containing fluorene group. J. Appl. Polym. Sci. 2018, 135, 46606. [Google Scholar] [CrossRef]
- Kacher, J.; Landon, C.; Adams, B.L.; Fullwood, D. Bragg’s Law diffraction simulations for electron backscatter diffraction analysis. Ultramicroscopy 2009, 109, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, L.R. Characterization of nano-layered multilayer coatings using modified Bragg law. Mater. Charact. 2008, 59, 1285–1291. [Google Scholar] [CrossRef]
- Krevelen, D. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Wang, C.; Chu, M.C.; Lin, T.L.; Lai, S.M.; Shih, H.H.; Yang, J.C. Microstructures of a highly short-chain branched polyethylene. Polymer 2001, 42, 1733–1741. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Keller, T.M. Properties of phthalonitrile monomer blends and thermosetting phthalonitrile copolymers. Polymer 2007, 48, 91–97. [Google Scholar] [CrossRef]
- Liao, S.; Wu, H.; He, X.; Hu, J.H.; Li, R.; Liu, Y.; Yang, G. Promoting effect of methyne/methylene moiety of bisphenol E/F on phthalonitrile resin curing: Expanding the structural design route of phthalonitrile resin. Polymer 2020, 210, 123001. [Google Scholar] [CrossRef]
- He, X.; Liao, S.; Chen, M.; Li, R.; Liu, Y.; Liang, B.; Yang, G. Study on the phthalonitrile cured via bio-tyrosine cyclic peptide: Achieving good thermal properties under low post-curing temperature. Polym. Degrad. Stab. 2020, 181, 109289. [Google Scholar] [CrossRef]
- Tian, Y.; Pu, Z.; Xia, J.; Hu, L.; Cheng, J.; Zhong, J. Research on the relationship between structure and properties of the soluble polyaryl ether ketone terminated with phthalonitrile. J. Polym. Res. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Chen, M.; He, X.; Guo, Y.; Hu, J.; Liang, B.; Zeng, K.; Yang, G. A new molecular design platform for high-performance polymers from versatile bio-based tyramine: A case study of tyramine-derived phthalonitrile resin. Polym. Chem. 2021, 12, 408–422. [Google Scholar] [CrossRef]






| Sample | Tm (°C) | Crystalline or Amorphous | Ref |
|---|---|---|---|
| BDCN | 194 | crystalline | [4] |
| BCPM | 159 | crystalline | [20] |
| BDSP | 189 | crystalline | [22] |
| BCPP | 156 | crystalline | [21] |
| BDS | 221 | crystalline | [23] |
| BCSP | 92 | amorphous | [11] |
| ACPP | 84 | amorphous | [24] |
| PBDP | 96 | amorphous | [25] |
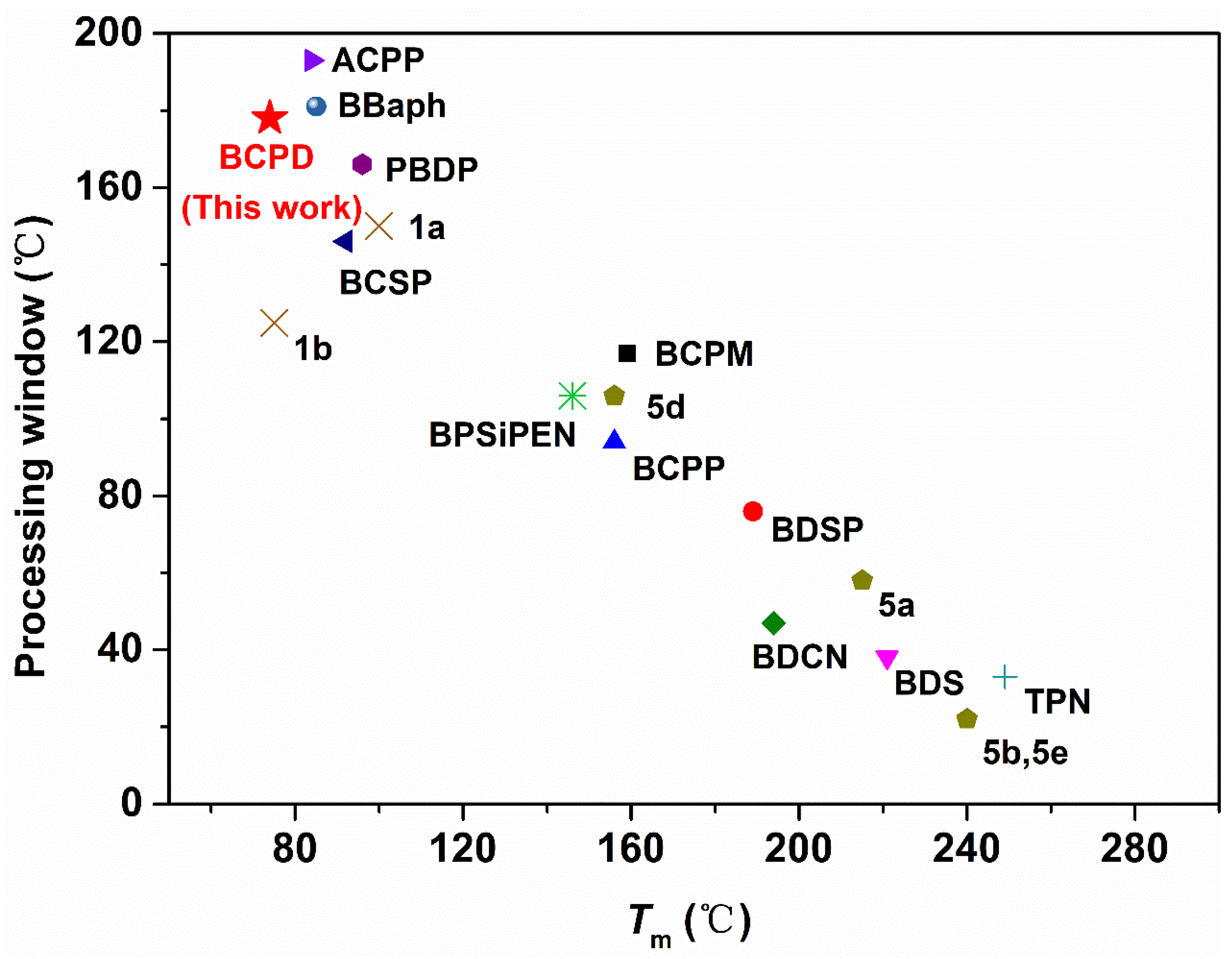
| BCPD | 74 | amorphous | This work |
| β/(°C/min−1) | Tp/(K) | ln(β/) | 1/Tp/(K−1) |
|---|---|---|---|
| 5 | 494.65 | −10.84 | 1.98 × 103 |
| 10 | 509.75 | −10.22 | 1.90 × 103 |
| 15 | 520.25 | −9.85 | 1.87 × 103 |
| 20 | 528.25 | −9.60 | 1.84 × 103 |
| Sample | In Nitrogen | In Air | ||||
|---|---|---|---|---|---|---|
| T5%/°C | T10%/°C | CR/% | T5%/°C | T10%/°C | CR/% | |
| Polymer A | 470 | 525 | 64.4 | 472 | 518 | 0 |
| Polymer B | 501 | 553 | 70.4 | 492 | 533 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Yang, K.; Li, Y.; Rong, J.; Jia, D.; Jia, Z.; Naito, K.; Yu, X.; Zhang, Q. A Pyridazine-Containing Phthalonitrile Resin for Heat-Resistant and Flame-Retardant Polymer Materials. Polymers 2022, 14, 4144. https://doi.org/10.3390/polym14194144
Wu M, Yang K, Li Y, Rong J, Jia D, Jia Z, Naito K, Yu X, Zhang Q. A Pyridazine-Containing Phthalonitrile Resin for Heat-Resistant and Flame-Retardant Polymer Materials. Polymers. 2022; 14(19):4144. https://doi.org/10.3390/polym14194144
Chicago/Turabian StyleWu, Minjie, Kaixiong Yang, Yuanyuan Li, Jianxin Rong, Dianqiu Jia, Zhiyi Jia, Kimiyoshi Naito, Xiaoyan Yu, and Qingxin Zhang. 2022. "A Pyridazine-Containing Phthalonitrile Resin for Heat-Resistant and Flame-Retardant Polymer Materials" Polymers 14, no. 19: 4144. https://doi.org/10.3390/polym14194144
APA StyleWu, M., Yang, K., Li, Y., Rong, J., Jia, D., Jia, Z., Naito, K., Yu, X., & Zhang, Q. (2022). A Pyridazine-Containing Phthalonitrile Resin for Heat-Resistant and Flame-Retardant Polymer Materials. Polymers, 14(19), 4144. https://doi.org/10.3390/polym14194144






