Antioxidants from Hyeronima macrocarpa Berries Loaded on Nanocellulose: Thermal and Antioxidant Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of Bioactive Substances of Different Hyeronima macrocarpa Extracts
2.1.1. Effect of Extraction on the Content of Total Anthocyanins
2.1.2. Extraction Effect on the Polyphenol and Flavonoid Content
2.1.3. Effect of Extraction on Reducing Capacity and Free-Radical Scavenging
2.2. Nanocellulose from Banana Pseudo-Stems
2.2.1. Functional Group Analysis by FT-IR
2.2.2. X-Ray Diffraction (XRD)
2.2.3. Particle Sizes Using DLS
2.2.4. Nanocellulose Surface Properties
2.2.5. TEM Microscopy
2.3. Preparation of Hyeronima macrocarpa and Nanocellulose Powder
2.3.1. Thermogravimetric Analysis
2.3.2. Powder Microstructure
2.4. Antioxidants Released from the Powder
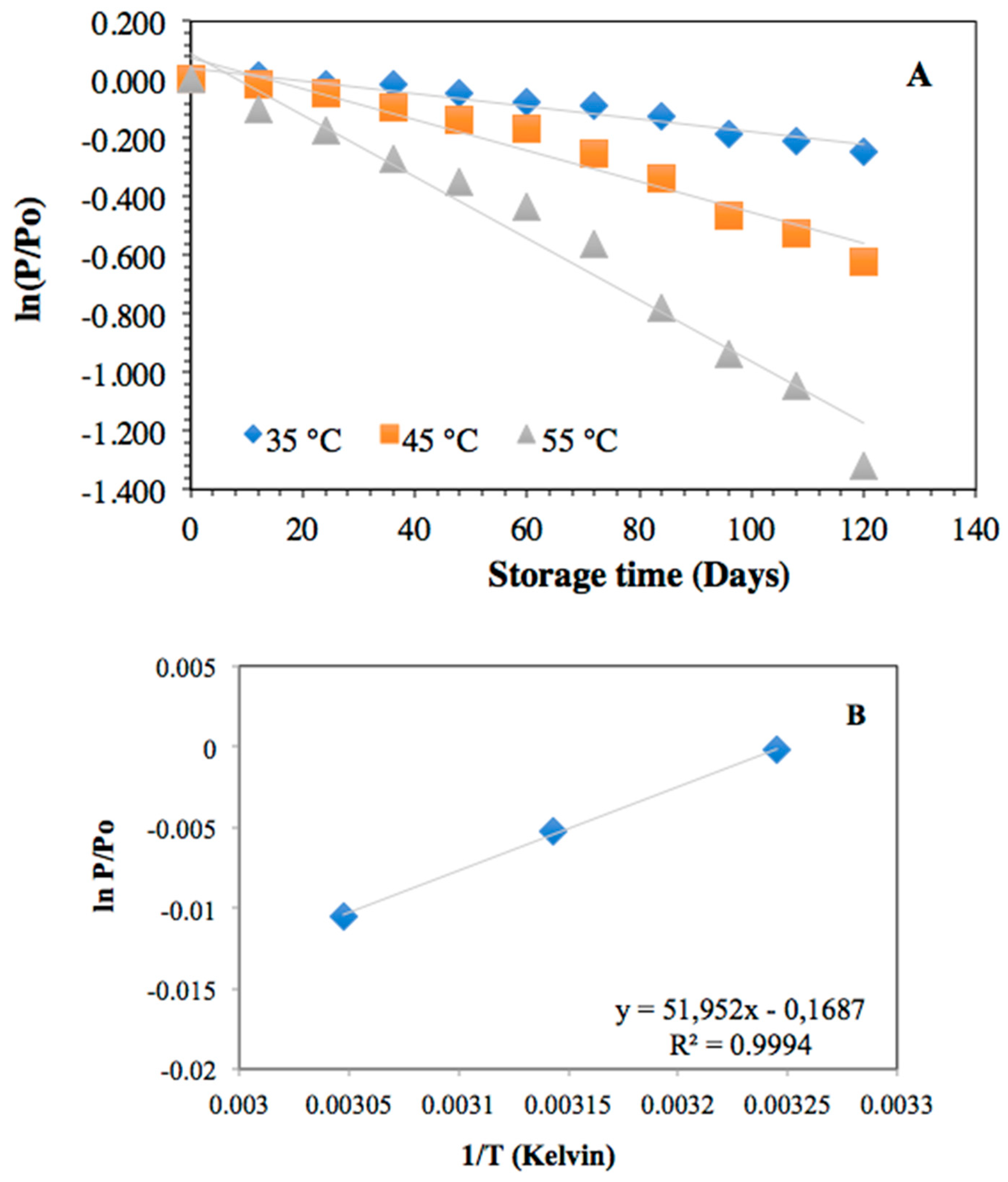
2.5. Powder Stability
2.5.1. Stability of Anthocyanins from H. macrocarpa and Nanocellulose Powder
2.5.2. Color Stability
2.5.3. Oxidative Stability Measured by FRAP
3. Materials and Methods
3.1. Vegetal Material
3.2. Extract Preparation
3.2.1. Extraction with HCl/Ethanol (1:99 v/v)
3.2.2. Extraction with HCl/Water (1:99 v/v)
3.2.3. Water at 70 °C
3.2.4. Aqueous Two-Phase Extraction (ATPE)
3.3. Analysis of Bioactive Compounds
3.3.1. Total Anthocyanin Content
3.3.2. Total Polyphenol Content (TPC)
3.3.3. Total Flavonoid Content
3.4. Free-Radical Scavenging and Reducing Capacity
3.4.1. ORAC Assay (Oxygen Radical Absorbance Capacity)
3.4.2. Superoxide Radical (O2−●)
3.4.3. Hydroxyl Radical (OH●)
3.4.4. Reducing Capacity by FRAP Method (Ferric Reducing Antioxidant Power)
3.5. Nanocellulose from Banana Pseudo-Stem Waste
3.5.1. FT-IR Spectroscopy
3.5.2. Particle Size Distribution Using DLS
3.5.3. SEM Microscopy
3.5.4. TEM Microscopy
3.5.5. Thermogravimetric Analysis (TGA)
3.5.6. Surface Area and Pore Volume
3.5.7. X-Ray Diffraction (XRD)
3.6. Loading of H. macrocarpa Bioactives onto Nanocellulose Using the Incipient Impregnation Method
Color Assay
3.7. Delivery of Bioactives
3.8. Stability Assay of H. macrocarpa and Nanocellulose Powder
3.8.1. Kinetic Studies of Degradation of H. macrocarpa and Nanocellulose Powder
3.8.2. Effect of Temperature on the H. macrocarpa Nanocellulose Powder Degradation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| a* | red/green value |
| b* | blue/yellow value |
| BET | Brunauer–Emmett–Teller theory |
| C3G | cyanidin-3-glucoside |
| DLS | dynamic light scattering |
| EDTA | ethylenediaminetetraacetic acid disodium salt dihydrate |
| FT-IR | Fourier-transform infrared spectroscopy |
| L* | lightness |
| NC | nanocellulose |
| ROS | reactive oxygen species |
| SEM | scanning electronic microscopy |
| TEM | transmission electronic microscopy |
| TGA | thermogravimetric analysis |
| TPTZ | 2,4,6-tris(2-pyridyl)-s-triazine |
| XRD | X-ray diffraction analysis |
References
- Tineo, D.; Bustamante, D.E.; Calderon, M.S.; Huaman, E. Exploring the diversity of andean berries from northern Peru based on molecular analyses. Heliyon 2022, 8, e08839. [Google Scholar] [CrossRef] [PubMed]
- Vega-Polo, P.; Cobo, M.M.; Argudo, A.; Gutierrez, B.; Rowntree, J.; Torres, M.D.L. Characterizing the genetic diversity of the Andean blueberry (Vaccinium floribundum Kunth.) across the Ecuadorian Highlands. PLoS ONE 2020, 15, e0243420. [Google Scholar] [CrossRef]
- Da Costa, I.R.; Forni-Martins, E.R. Karyotype analysis in South American species of Myrtaceae. Bot. J. Linn. Soc. 2007, 155, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R.; Gao, Y.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Licursi, M.; Straub, K.D. Antioxidant’berries, anthocyanins, resveratrol and rosmarinic acid oxidize hydrogen sulfide to polysulfides and thiosulfate: A novel mechanism underlying their biological actions. Free Radic. Biol. Med. 2021, 165, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Uddin, M.S. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Devi, K.P.; Tejada, S.; Farzaei, M.H. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT Food Sci. Technol. 2022, 153, 112527. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; de Souza-Mesquita, L.M.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Bai, W. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef] [PubMed]
- Charmongkolpradit, S.; Somboon, T.; Phatchana, R.; Sang-aroon, W.; Tanwanichkul, B. Influence of drying temperature on anthocyanin and moisture contents in purple waxy corn kernel using a tunnel dryer. Case Stud. Therm. Eng. 2021, 25, 100886. [Google Scholar] [CrossRef]
- Mazuco, R.A.; Cardoso, P.M.M.; Bindaco, É.S.; Scherer, R.; Castilho, R.O.; Faraco, A.A.G.; Endringer, D.C. Maltodextrin and gum Arabic-based microencapsulation methods for anthocyanin preservation in Juçara palm (Euterpe edulis Martius) fruit pulp. Plant Foods Hum. Nutr. 2018, 73, 209–215. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Kanha, N. Effects of processing conditions on powder properties of black glutinous rice (Oryza sativa L.) bran anthocyanins produced by spray drying and freeze drying. LWT Food Sci. Technol. 2015, 64, 405–411. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.T. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Mei, C.; Cai, L.; Nadda, A.; VanLe, Q.; Xia, C. Advanced nanocellulose-based gas barrier materials: Present status and prospects. Chemosphere 2022, 286, 131891. [Google Scholar] [CrossRef]
- Luna-Cabrera, G.C.; Delgado-Vargas, I.A.; Burgos-Ordóñez, L.C. Árboles Conocimiento Local en el Corregimiento de Morasurco, Pasto-Nariño; Universidad de Nariño: Pasto, Colombia, 2022; pp. 18–106. [Google Scholar]
- Santacruz, L.; Carriazo, J.G.; Almanza, O.; Osorio, C. Anthocyanin composition of wild Colombian fruits and antioxidant capacity measurement by electron paramagnetic resonance spectroscopy. J. Agric. Food Chem. 2012, 60, 1397–1404. [Google Scholar] [CrossRef]
- Heffels, P.; Weber, F.; Schieber, A. Influence of accelerated solvent extraction and ultrasound-assisted extraction on the anthocyanin profile of different Vaccinium species in the context of statistical models for authentication. J. Agric. Food Chem. 2015, 63, 7532–7538. [Google Scholar] [CrossRef]
- Nekoukar, Z.; Zakariaei, Z.; Taghizadeh, F.; Musavi, F.; Banimostafavi, E.S.; Sharifpour, A.; Safanavaei, S. Methanol poisoning as a new world challenge: A review. Ann. Med. Surg. 2021, 66, 102445. [Google Scholar] [CrossRef]
- Kafkas, E.Y. Comparison of Fruit Quality Characteristics of Berries. Agri. Sci. 2021, 12, 907–915. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Fabien, A.; Lefebvre, G.; Calvignac, B.; Legout, P.; Badens, E.; Crampon, C. Interfacial tension of ethanol, water, and their mixtures in high pressure carbon dioxide: Measurements and modeling. J. Colloid Interface Sci. 2022, 613, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Sang, J.; Dang, K.K.; Ma, Q.; Li, B.; Huang, Y.Y.; & Li, C.Q. Partition Behaviors of Different Polar Anthocyanins in Aqueous Two-Phase Systems and Extraction of Anthocyanins from Nitraria tangutorun Bobr. and Lycium ruthenicum Murr. Food Anal. Methods 2018, 11, 980–991. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Cissé, M.; Bohuon, P.; Sambe, F.; Kane, C.; Sakho, M.; Dornier, M. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: Experimental kinetics and modeling. J. Food Eng. 2012, 109, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Xu, Q.; Shen, Y.; Wang, H.; Zhang, N.; Xu, S.; Zhang, L. Application of response surface methodology to optimise extraction of flavonoids from Fructus sophorae. Food Chem. 2013, 138, 2122–2129. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, J.; Ciampi, L.; Castilla, A.; Medel, F.; Schalchli, H.; Hormazabal, E.; Alberdi, M. Antioxidant capacity, anthocyanins, and total phenols of wild and cultivated berries in Chile. Chil. J. Agr. Res. 2010, 70, 537–544. [Google Scholar] [CrossRef]
- Mira, L.; Tereza-Fernandez, M.; Santos, M.; Rocha, R.; Helena-Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Ghoora, M.D.; Haldipur, A.C.; Srividya, N. Comparative evaluation of phytochemical content, antioxidant capacities and overall antioxidant potential of select culinary microgreens. J. Agric. Food Res. 2020, 2, 100046. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Klein, E.; Marković, J.M.D.; Milenković, D. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 2018, 246, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Marković, J.M.D.; Pejin, B.; Milenković, D.; Amić, D.; Begović, N.; Mojović, M.; Marković, Z.S. Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: The energy requirements calculations as a prediction of the possible antiradical mechanisms. Food Chem. 2017, 218, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, M.Y.; Lee, K.G. Categorization of fruits according to their content of polyphenols and vitamin C, antiradical activity, and quality parameters. J. Food Process. Pres. 2018, 42, e13421. [Google Scholar] [CrossRef]
- Kostka, T.; Ostberg-Potthoff, J.J.; Stärke, J.; Guigas, C.; Matsugo, S.; Mirčeski, V.; Esatbeyoglu, T. Bioactive phenolic compounds from Lingonberry (Vaccinium vitis-idaea L.): Extraction, chemical characterization, fractionation and cellular antioxidant activity. Antioxidants 2022, 11, 467. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. Mater. Chem. Phys. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Peng, H.; Wang, N.; Hu, Z.; Yu, Z.; Liu, Y.; Zhang, J.; Ruan, R. Physicochemical characterization of hemicelluloses from bamboo (Phyllostachys pubescens Mazel) stem. Ind. Crops Prod. 2012, 37, 41–50. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Farag, M.A.; Shao, P. Functionalized cellulose nanocrystal embedded into citrus pectin coating improves its barrier, antioxidant properties and potential application in food. Food Chem. 2022, 134079, in press. [Google Scholar] [CrossRef]
- Salim, M.R.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Rong, N.; Chen, C.; Ouyang, K.; Zhang, K.; Wang, X.; Xu, Z. Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep. Purif. Technol. 2021, 274, 119120. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Wei, L.; Agarwal, U.P.; Hirth, K.C.; Matuana, L.M.; Sabo, R.C.; Stark, N.M. Chemical modification of nanocellulose with canola oil fatty acid methyl ester. Carbohydr. Polym. 2017, 169, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanick, B. Preparation and characterization of nanocellulose fibre incorporated poly methyl methacrylic acid based superabsorbent gel. Mater. Today Proc. 2022, 60, 1994–2000. [Google Scholar] [CrossRef]
- Chieng, B.W.; Lee, S.H.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Isolation and characterization of cellulose nanocrystals from oil palm mesocarp fiber. Polymers 2017, 9, 355. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Christma, F.A.; Toyin, A.J.; Gopinath, S.C.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, M.; Wang, Z.; Wang, S.; Fu, J. Tunable-quaternary (N, S, O, P)-doped porous carbon microspheres with ultramicropores for CO2 capture. Appl. Surf. Sci. 2020, 507, 145130. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Pérez, M.O.; Valero-Romero, M.J.; Hernández, S.; Nieto, J.L.; Rodríguez-Mirasol, J.; Cordero, T. Lignocellulosic-derived mesoporous materials: An answer to manufacturing non-expensive catalysts useful for the biorefinery processes. Catal. Today 2012, 195, 155–161. [Google Scholar] [CrossRef]
- Lupaşcu, T.; Petuhov, O.; Ţîmbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B12 and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095. [Google Scholar] [CrossRef] [PubMed]
- Kadry, G. Comparison between gelatin/carboxymethyl cellulose and gelatin/carboxymethyl nanocellulose in tramadol drug loaded capsule. Heliyon 2019, 5, e02404. [Google Scholar] [CrossRef] [PubMed]
- El-Hadi, A.M. Increase the elongation at break of poly (lactic acid) composites for use in food packaging films. Sci. Rep. 2017, 7, 46767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzate-Arbeláez, A.F.; Dorta, E.; López-Alarcón, C.; Cortés, F.B.; Rojano, B.A. Immobilization of Andean berry (Vaccinium meridionale) polyphenols on nanocellulose isolated from banana residues: A natural food additive with antioxidant properties. Food Chem. 2019, 294, 503–517. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT Food Sci. Technol. 2017, 84, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Lv, Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lee, Y.J.; Yoon, W.B. Effect of moisture content on the grinding process and powder properties in food: A review. Processes 2018, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Wang, X.; Han, J.; Yu, L.; Chen, J.; Wu, Q.; Jiang, J. Effects of nanocellulose on sodium alginate/polyacrylamide hydrogel: Mechanical properties and adsorption-desorption capacities. Carbohydr. Polym. 2019, 206, 289–301. [Google Scholar] [CrossRef]
- Kara, Ş.; Erçelebi, E.A. Thermal degradation kinetics of anthocyanins and visual colour of Urmu mulberry (Morus nigra L.). J. Food Eng. 2013, 116, 541–547. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef]
- Hardinasinta, G.; Salengke, S.; Juaedi, M.; Mursalim, M. Ohmic heating characteristics and degradation kinetics of anthocyanin in mulberry juice. In IOP Conference Series: Earth and Environmental Science; Zhou, J., Zhang, R., Eds.; IOP Publishing: London, UK, 2019; Volume 355, p. 012094. [Google Scholar]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J. Food Sci. 2010, 75, C173–C176. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Finten, G.; Agüero, M.V.; Jagus, R.J.; Niranjan, K. High hydrostatic pressure blanching of baby spinach (Spinacia oleracea L.). LWT Food Sci. Technol. 2016, 73, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Lee, J.; Park, G.; Chang, Y.H. Nutraceuticals and antioxidant properties of Lonicera japonica Thunb. as affected by heating time. Int. J. Food Prop. 2019, 22, 630–645. [Google Scholar] [CrossRef] [Green Version]
- Amalia, F.; Afnani, G.N. Extraction and stability test of anthocyanin from buni fruits (Antidesma bunius L) as an alternative natural and safe food colorants. J. Food Pharm. Sci. 2013, 1, 49–53. [Google Scholar]
- Zapata, I.C.; Alzate, A.F.; Zapata, K.; Arias, J.P.; Puertas, M.A.; Rojano, B. Effect of pH, temperature and time of extraction on the antioxidant properties of Vaccinium meridionale Swartz. J. Berry Res. 2019, 9, 39–49. [Google Scholar] [CrossRef]
- Hua, Z.; Yuesheng, D.; Ge, X.; Menglu, L.; Liya, D.; LiJia, A.; Zhilong, X. Extraction and purification of anthocyanins from the fruit residues of Vaccinium uliginosum Linn. J. Chromatogr. Sep. Tech. 2013, 4, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Papoti, V.T.; Xystouris, S.; Papagianni, G.; Tsimidou, M.Z. Total flavonoid content assessment via aluminum [AL (III)] complexation reactions. What we really measure? Ital. J. Food Sci. 2011, 23, 252–259. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT Food Sci. Technol. 2021, 139, 110590. [Google Scholar] [CrossRef]
- Chen, H.Y. Why the reactive oxygen species of the Fenton reaction switches from oxoiron (IV) species to hydroxyl radical in phosphate buffer solutions? A computational rationale. ACS Omega 2019, 4, 14105–14113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.H.; Kuang, X.M.; Scott, J.A.; Rocha, G.O.; Paulson, S.E. Terephthalate probe for hydroxyl radicals: Yield of 2-hydroxyterephthalic acid and transition metal interference. Anal. Lett. 2018, 51, 2488–2497. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Viloria, P.R.; Marfisi, S.; Rondón, P.O.; Rojas, B. Obtención de celulosa microcristalina a partir de desechos agrícolas del cambur (Musa sapientum). Síntesis de celulosa microcristalina. Rev. Iberoam. Polímer. 2014, 15, 286–300. [Google Scholar]
- Fahma, F.; Hori, N.; Iwata, T.; Takemura, A. PVA nanocomposites reinforced with cellulose nanofibers from oil palm empty fruit bunches (OPEFBs). Emir. J. Food Agr. 2017, 29, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Lefatshe, K.; Muiva, C.M.; Kebaabetswe, L.P. Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanced photocatalytic and antibacterial activity. Carbohydr. Polym. 2017, 164, 301–308. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Fu, S.Y.; Zheng, L.M.; Zhan, H.Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly (vinyl alcohol) nanocomposite films. Express Polym. Lett. 2012, 6, 794–804. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Mohan, D.J.; Kang, I.A.; Doh, G.H.; Lee, S.; Han, S.O. Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading. Fiber Polym. 2009, 10, 77–82. [Google Scholar] [CrossRef]
- Ferraz, N.; Leschinskaya, A.; Toomadj, F.; Fellström, B.; Strømme, M.; Mihranyan, A. Membrane characterization and solute diffusion in porous composite nanocellulose membranes for hemodialysis. Cellulose 2013, 20, 2959–2970. [Google Scholar] [CrossRef]
- Piedrahita, A.M.; Peñaloza, J.; Cogollo, Á.; Rojano, B.A. Kinetic study of the oxidative degradation of choibá oil (Dipteryx oleifera Benth.) with addition of rosemary extract (Rosmarinus officinalis L.). Food Nutr. Sci. 2015, 6, 466–479. [Google Scholar]
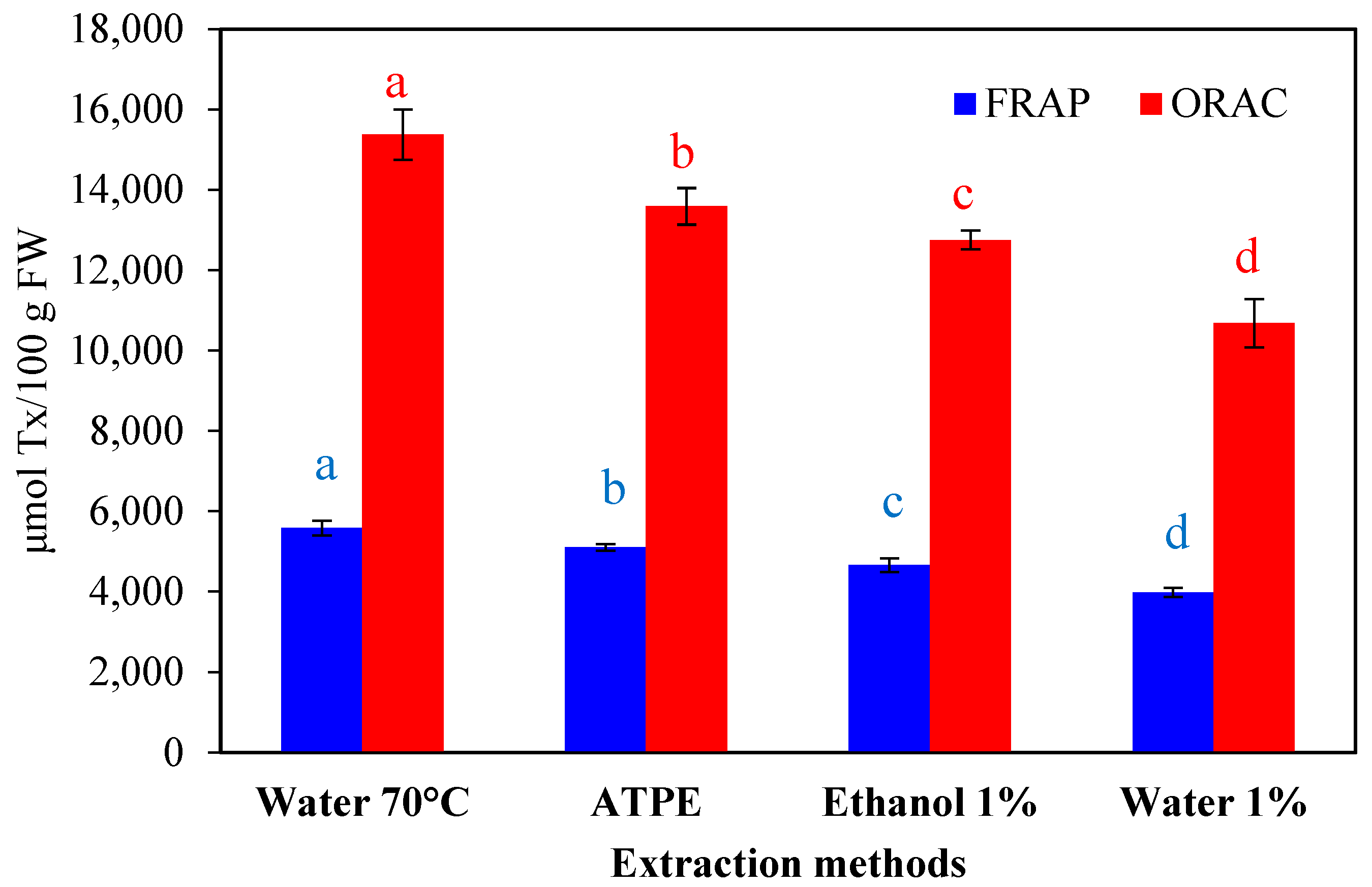
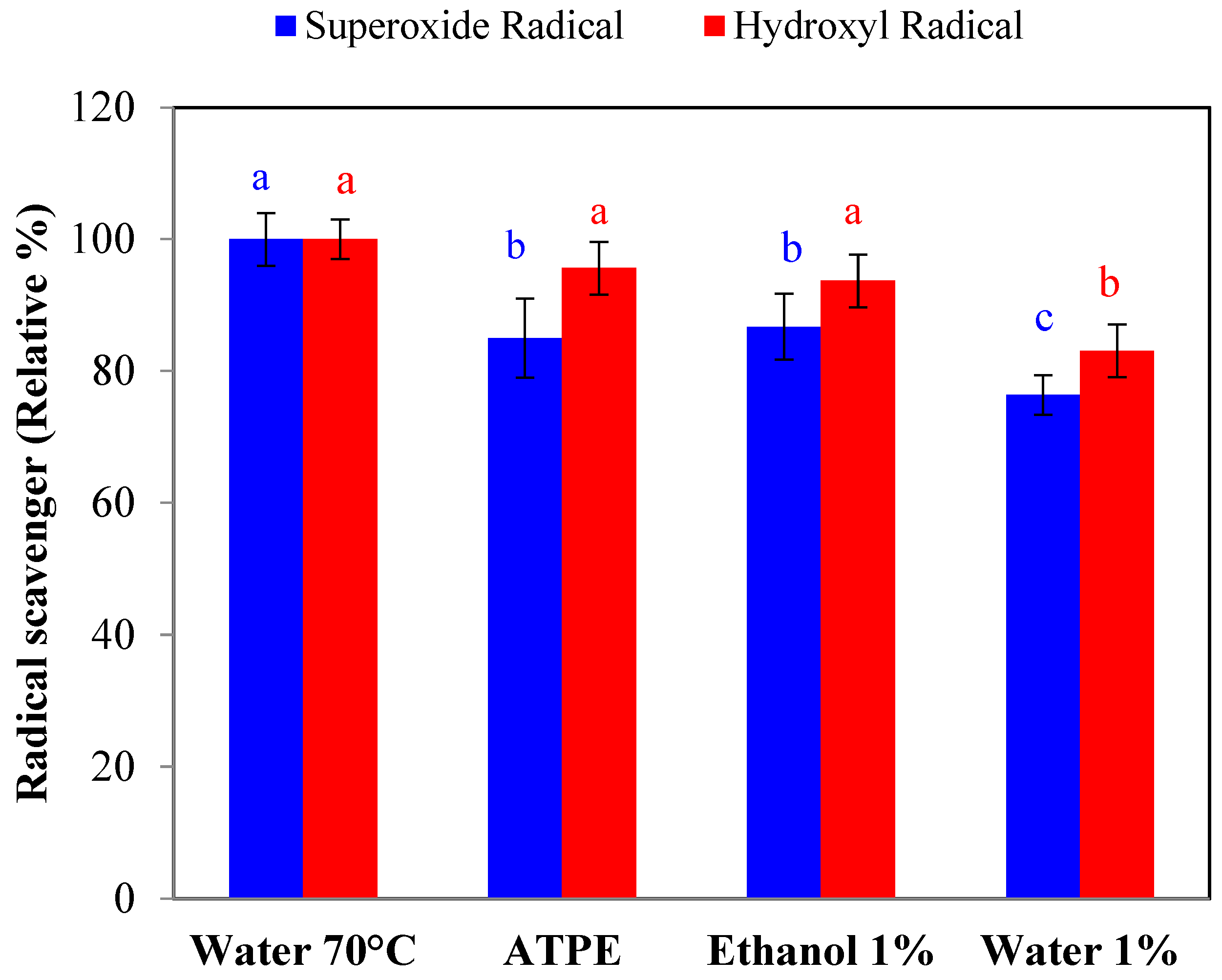
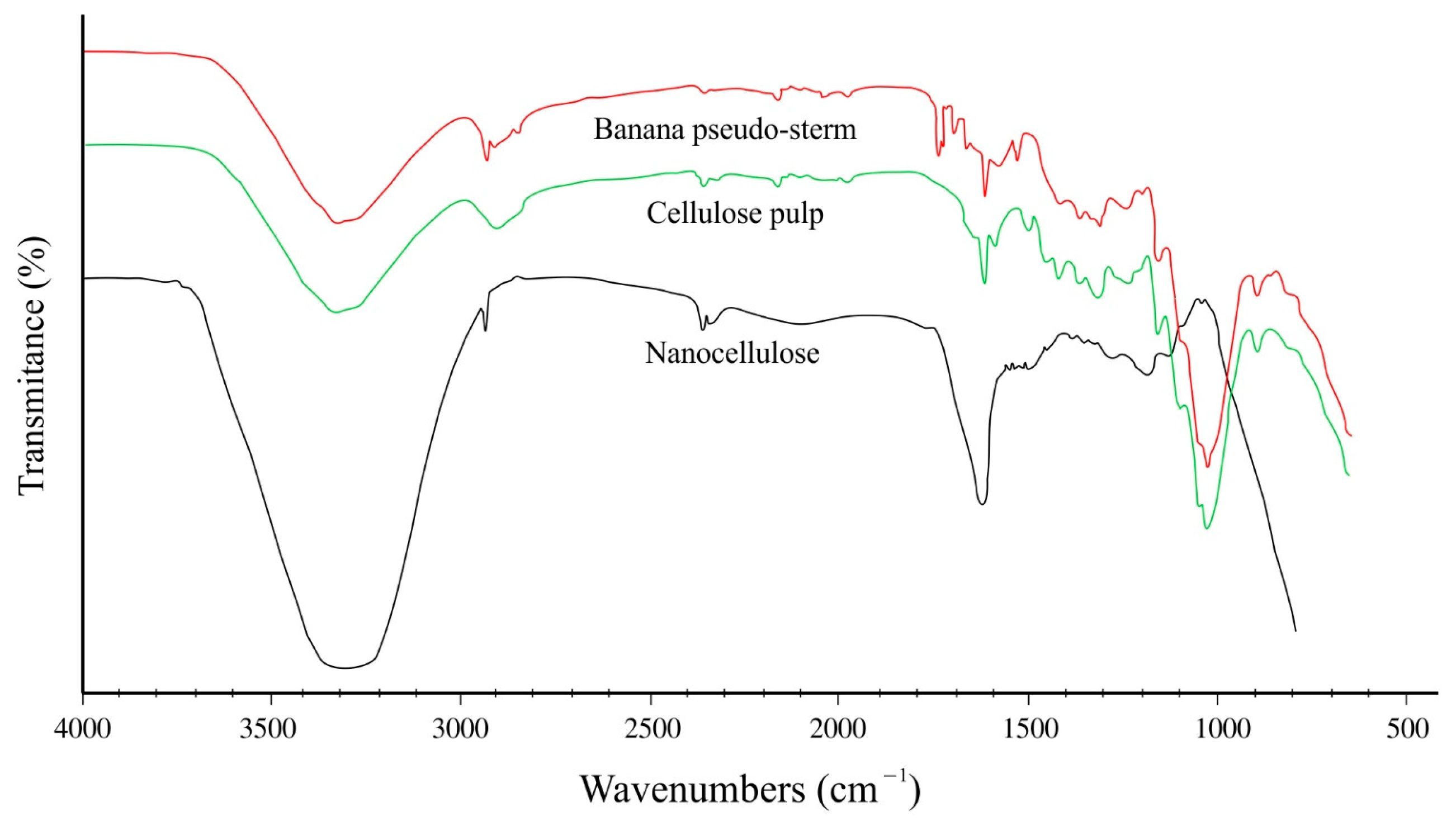


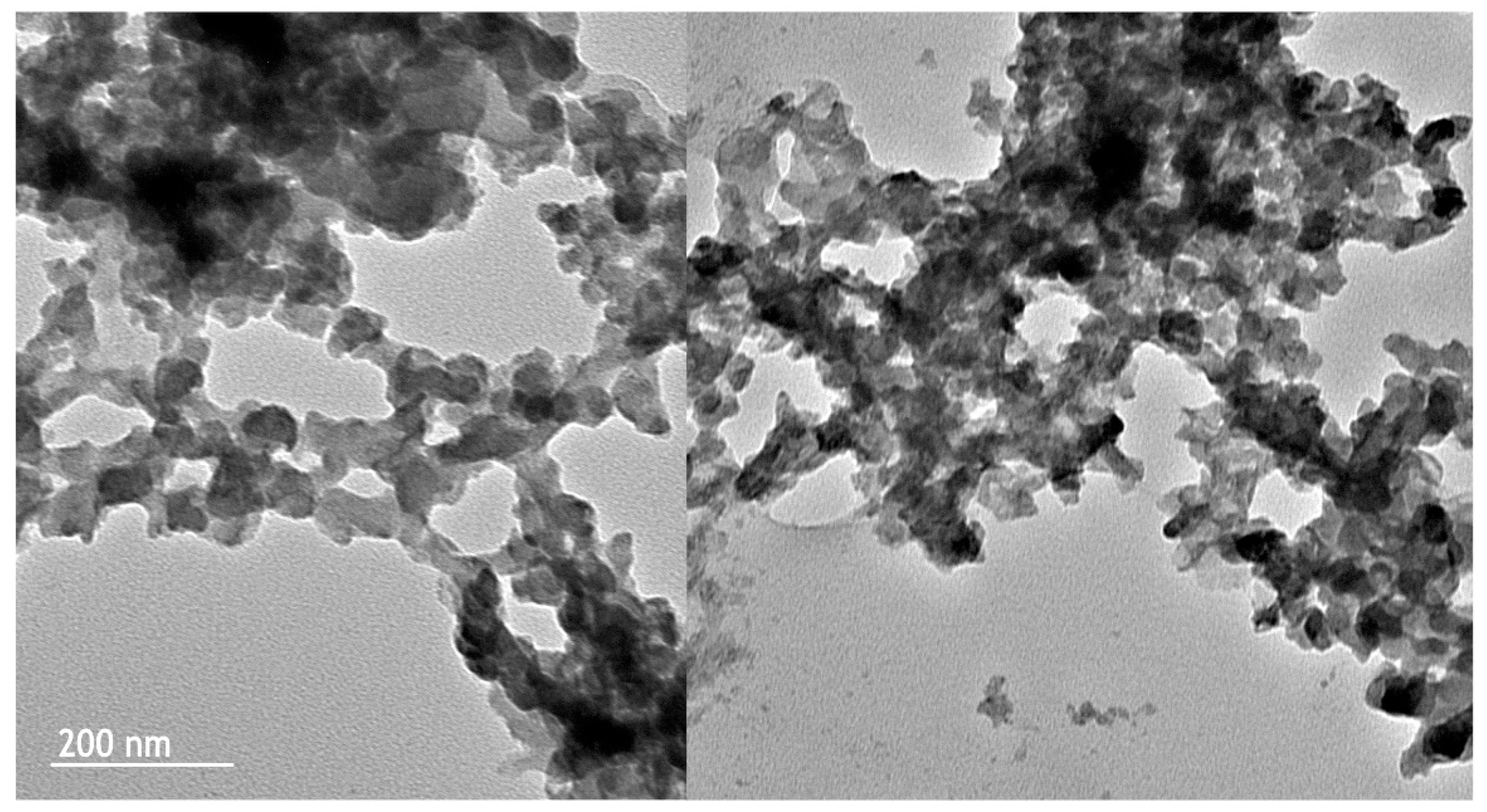


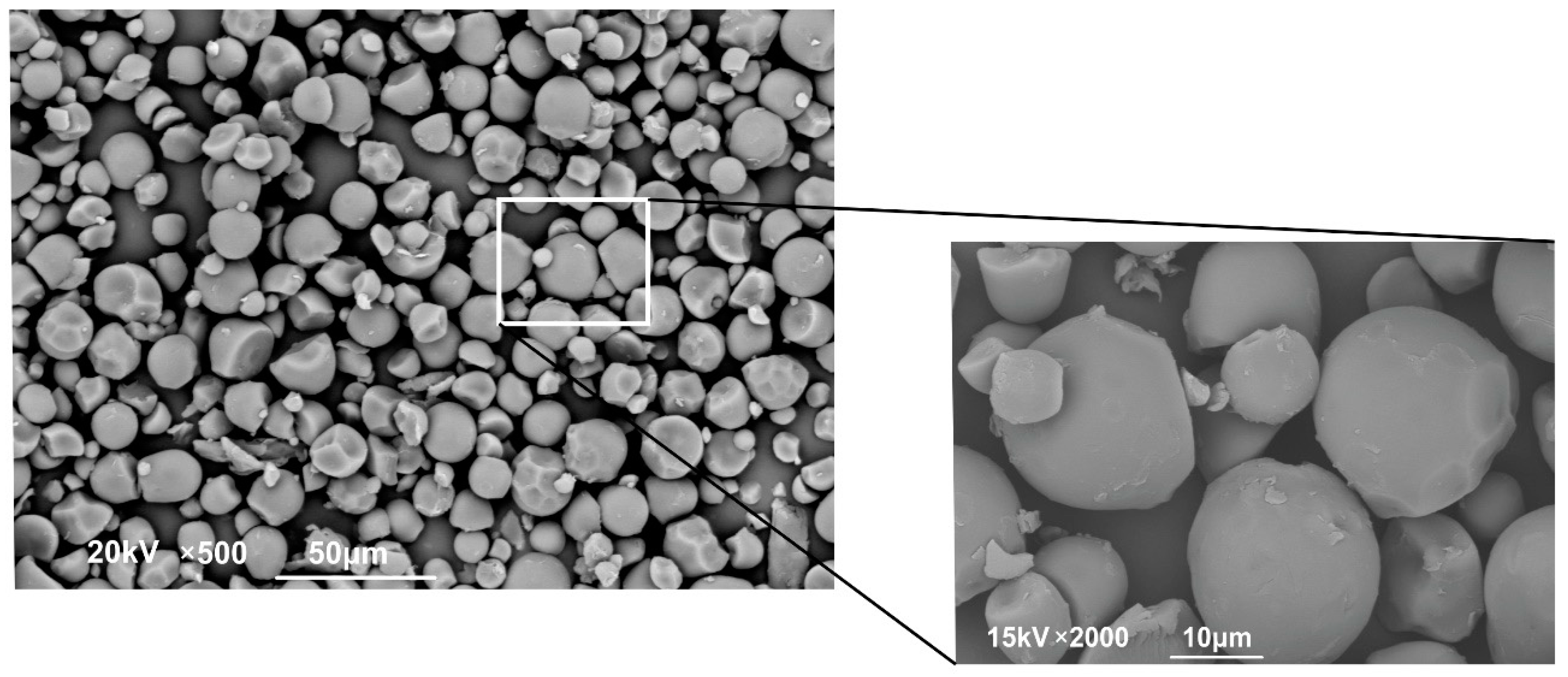


| Extraction Methods | Total Anthocyanins (mg C3G */100 g FW) | Total Polyphenols (mg GAE **/100 g FW) | Total Flavonoids (mg Catechin Eq./100 g FW) |
|---|---|---|---|
| Ethanol 1% | 1317.39 ± 17.18 a | 859.47 ± 12.03 a | 173.76 ± 6.43 a |
| Water 1% | 969.42 ± 6.71 b | 810.01 ± 9.62 b | 133.29 ± 9.23 b |
| ATPE | 824.38 ± 12.74 c | 861.48 ± 18.02 a | 198.34 ± 11.64 c |
| Water 70 °C | 724.32 ± 9.56 d | 929.85 ± 12.97 c | 209.90 ± 4.78 c |
| Parameter | Value |
|---|---|
| BET specific surface area measured with N2 | 48.3 ± 5.1 m2/g |
| Ultramicroporous area measured with CO2 | 6.2 ± 0.4 m2/g |
| Particle diameter measured using DLS (90%) | 87.3–108.7 nm |
| Particle size measured using TEM | 87−124 nm |
| Mean pore diameter | 3.8 ± 0.2 nm |
| Total pore volume | 0.28 cm3/g |
| Parameter | Temp. | k × 103 (days−1) | R2 | Ea (kJ/mol−1) | t1/2 (days) |
|---|---|---|---|---|---|
| Total anthocyanins | 35 °C | 2.20 ± 0.13 a | 0.9413 | 65.76 ± 4.34 (R2: 0.9736) | 315 ± 16 a |
| 45 °C | 5.30 ± 0.38 b | 0.9495 | 131 ± 7 b | ||
| 55 °C | 10.50 ± 0.97 c | 0.9662 | 66 ± 4 c | ||
| FRAP | 35 °C | 0.40 ± 0.02 a | 0.9737 | 46.44 ± 2.71 (R2: 0.9707) | 1733 ± 104 a |
| 45 °C | 1.00 ± 0.08 b | 0.9829 | 693 ± 32 b | ||
| 55 °C | 1.30 ± 0.07 c | 0.9061 | 533 ± 17 c |
| Storage Time (days) | 35 °C | 45 °C | 55 °C |
|---|---|---|---|
| 12 | 0.72 ± 0.01 | 1.00 ± 0.04 | 3.60 ± 0.02 |
| 24 | 1.73 ± 0.02 | 1.96 ± 0.01 | 6.62 ± 0.05 |
| 36 | 2.66 ± 0.02 | 3.69 ± 0.08 | 11.03 * ± 0.03 |
| 48 | 3.37 ± 0.04 | 5.54 * ± 0.05 | 14.23 * ± 0.07 |
| 60 | 4.28 ± 0.03 | 7.26 * ± 0.03 | 17.04 * ± 0.02 |
| 72 | 4.75 ± 0.05 | 8.19 * ± 0.06 | 19.40 ** ± 0.07 |
| 84 | 6.32 * ± 0.04 | 10.01 * ± 0.04 | 20.20 ** ± 0.11 |
| 96 | 9.14 * ± 0.06 | 11.05 * ± 0.08 | 21.36 ** ± 0.11 |
| 108 | 11.43 * ± 0.03 | 13.89 * ± 0.11 | 22.45 ** ± 0.13 |
| 120 | 11.31 * ± 0.11 | 14.73 * ± 0.09 | 23.38 ** ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzate-Arbelaez, A.F.; Cortés, F.B.; Rojano, B.A. Antioxidants from Hyeronima macrocarpa Berries Loaded on Nanocellulose: Thermal and Antioxidant Stability. Molecules 2022, 27, 6661. https://doi.org/10.3390/molecules27196661
Alzate-Arbelaez AF, Cortés FB, Rojano BA. Antioxidants from Hyeronima macrocarpa Berries Loaded on Nanocellulose: Thermal and Antioxidant Stability. Molecules. 2022; 27(19):6661. https://doi.org/10.3390/molecules27196661
Chicago/Turabian StyleAlzate-Arbelaez, Andrés Felipe, Farid B. Cortés, and Benjamín A. Rojano. 2022. "Antioxidants from Hyeronima macrocarpa Berries Loaded on Nanocellulose: Thermal and Antioxidant Stability" Molecules 27, no. 19: 6661. https://doi.org/10.3390/molecules27196661





