Sex Differences in Immune Cell Infiltration and Hematuria in SCI-Induced Hemorrhagic Cystitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retrospective Study
2.2. Spinal Cord Contusion Injury
2.3. Urinalysis
2.4. Urine Analysis: Wright Stain Differential
2.5. Urine Analysis: Total Leukocyte Count
2.6. Tissue Processing for Histological Evaluation
2.7. Hematoxylin and Eosin Staining
2.8. Wright Staining
2.9. Masson’s Trichrome Staining
2.10. TUNEL Assay
2.11. Statistical Analysis
3. Results
3.1. Hemorrhagic Cystitis with Acute Vascular Injury and Neutrophils Infiltration after SCI
3.2. Sex Differences in Hemorrhagic Cystitis in the Acute Phase after Spinal Cord Injury
3.3. Apoptotic Umbrella Cells Early after SCI
3.4. Severe Pathological Changes Occur during the Chronic Phase after SCI
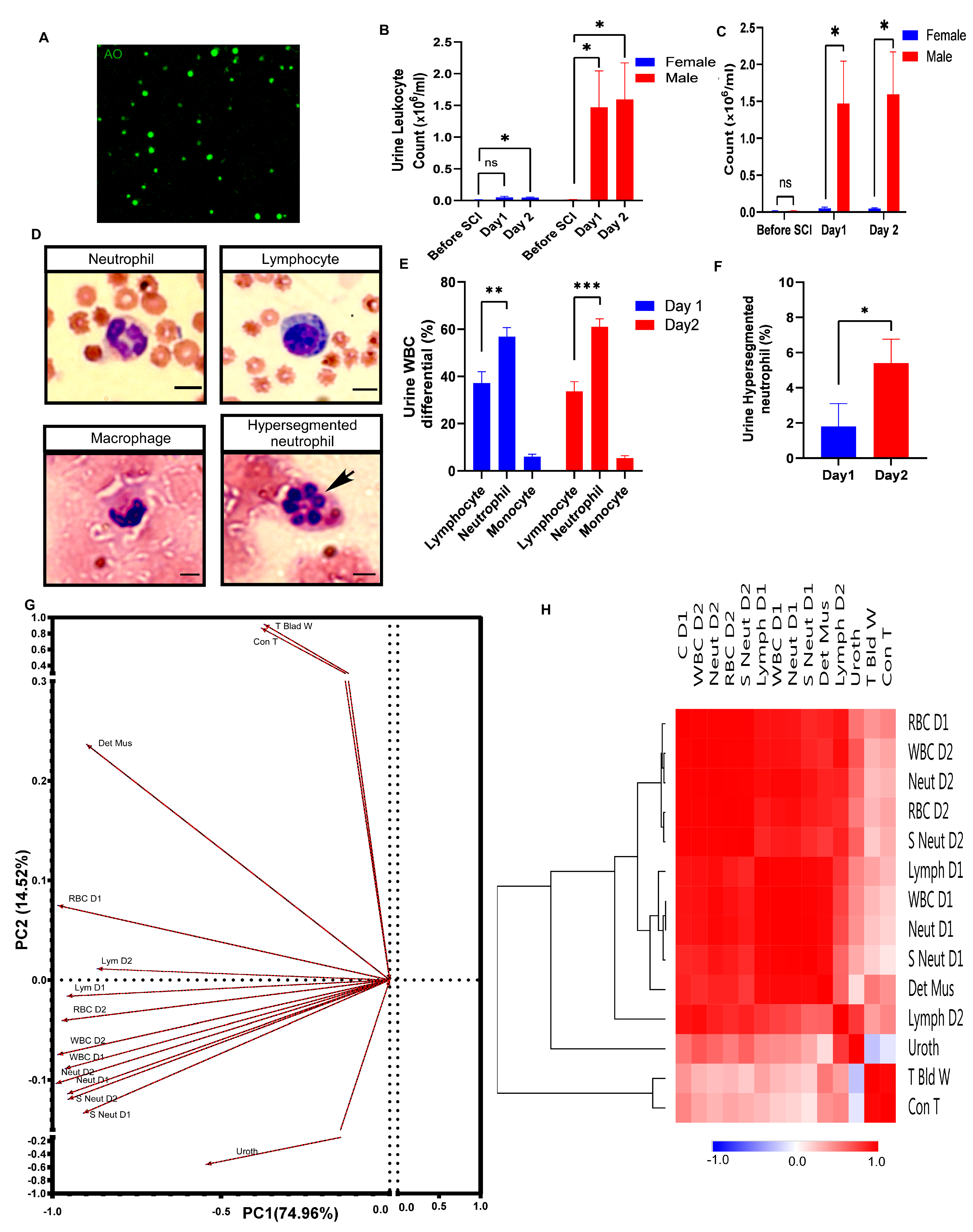
3.5. Robust Neutrophil Infiltration during Hemorrhagic Cystitis in Male Rats
3.6. Underlying Trends among the Variables in an Unbiased Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Groat, W.C.; Yoshimura, N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2006, 152, 59–84. [Google Scholar] [PubMed]
- Lavelle, J.; Meyers, S.; Ramage, R.; Bastacky, S.; Doty, D.; Apodaca, G.; Zeidel, M.L. Bladder permeability barrier: Recovery from selective injury of surface epithelial cells. Am. J. Physiol. Ren. Physiol. 2002, 283, F242–F253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronald, A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am. J. Med. 2002, 113 (Suppl. S1A), 14s–19s. [Google Scholar] [CrossRef] [PubMed]
- Subramonian, K.; Cartwright, R.A.; Harnden, P.; Harrison, S.C. Bladder cancer in patients with spinal cord injuries. BJU Int. 2004, 93, 739–743. [Google Scholar] [CrossRef]
- Apodaca, G.; Kiss, S.; Ruiz, W.; Meyers, S.; Zeidel, M.; Birder, L. Disruption of bladder epithelium barrier function after spinal cord injury. Am. J. Physiol. Ren. Physiol. 2003, 284, F966–F976. [Google Scholar] [CrossRef]
- Janzen, J.; Bersch, U.; Pietsch-Breitfeld, B.; Pressler, H.; Michel, D.; Bültmann, B. Urinary bladder biopsies in spinal cord injured patients. Spinal Cord 2001, 39, 568–570. [Google Scholar] [CrossRef] [Green Version]
- Herrera, J.J.; Haywood-Watson, R.J., II; Grill, R.J. Acute and chronic deficits in the urinary bladder after spinal contusion injury in the adult rat. J. Neurotrauma 2010, 27, 423–431. [Google Scholar] [CrossRef]
- Kullmann, F.A.; Clayton, D.R.; Ruiz, W.G.; Wolf-Johnston, A.; Gauthier, C.; Kanai, A.; Birder, L.A.; Apodaca, G. Urothelial proliferation and regeneration after spinal cord injury. Am. J. Physiol. Ren. Physiol. 2017, 313, F85–F102. [Google Scholar] [CrossRef] [Green Version]
- van Velzen, D.; Krishnan, K.R.; Parsons, K.F.; Soni, B.M.; Howard, C.V.; Fraser, M.H.; Vaidyanathan, S. Vesical urothelium proliferation in spinal cord injured persons: An immunohistochemical study of PCNA and MIB.1 labelling. Paraplegia 1995, 33, 523–529. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Liu, H.-T.; Kuo, H.-C. Urothelial dysfunction and chronic inflammation in patients with spinal cord injuries at different levels and correlation with urodynamic findings. Neurourol. Urodyn. 2015, 34, 757–762. [Google Scholar] [CrossRef]
- Chan, W.-M.; Mohammed, Y.; Lee, I.; Pearse, D.D. Effect of gender on recovery after spinal cord injury. Transl. Stroke Res. 2013, 4, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Theis, T.; Kumar, S.; Wei, E.; Nguyen, J.; Glynos, V.; Paranjape, N.; Askarifirouzjaei, H.; Khajouienejad, L.; Berthiaume, F.; Young, W.; et al. Myristoylated alanine-rich C-kinase substrate effector domain peptide improves sex-specific recovery and axonal regrowth after spinal cord injury. FASEB J. 2020, 34, 12677–12690. [Google Scholar] [CrossRef] [PubMed]
- Lilley, E.; Andrews, M.R.; Bradbury, E.J.; Elliott, H.; Hawkins, P.; Ichiyama, R.M.; Keeley, J.; Michael-Titus, A.T.; Moon, L.D.F.; Pluchino, S.; et al. Refining rodent models of spinal cord injury. Exp. Neurol. 2020, 328, 113273. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; MacLean, S.M.; Stromberg, A.J.; Whelan, J.P.; Bailey, W.M.; Gensel, J.C.; Wilson, M.E. Considerations for Studying Sex as a Biological Variable in Spinal Cord Injury. Front. Neurol. 2020, 11, 802. [Google Scholar] [CrossRef]
- Ferrero, S.L.; Brady, T.D.; Dugan, V.P.; Armstrong, J.E.; Hubscher, C.H.; Johnson, R.D. Effects of lateral funiculus sparing, spinal lesion level, and gender on recovery of bladder voiding reflexes and hematuria in rats. J. Neurotrauma 2015, 32, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Voskuhl, R. Sex differences in autoimmune diseases. Biol. Sex Differ. 2011, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Dotson, A.L.; Offner, H. Sex differences in the immune response to experimental stroke: Implications for translational research. J. Neurosci. Res. 2017, 95, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Taoka, Y.; Okajima, K.; Uchiba, M.; Murakami, K.; Kushimoto, S.; Johno, M.; Naruo, M.; Okabe, H.; Takatsuki, K. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997, 79, 1177–1182. [Google Scholar] [CrossRef]
- Neirinckx, V.; Coste, C.; Franzen, R.; Gothot, A.; Rogister, B.; Wislet, S. Neutrophil contribution to spinal cord injury and repair. J. Neuroinflamm. 2014, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Brennan, F.H.; Jogia, T.; Gillespie, E.R.; Blomster, L.V.; Li, X.X.; Nowlan, B.; Williams, G.M.; Jacobson, E.; Osborne, G.W.; Meunier, F.A.; et al. Complement receptor C3aR1 controls neutrophil mobilization following spinal cord injury through physiological antagonism of CXCR2. JCI Insight 2019, 4, e98254. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Rosen, S.; Weinstein, P.; van Rooijen, N.; Noble-Haeusslein, L.J. Prevention of both neutrophil and monocyte recruitment promotes recovery after spinal cord injury. J. Neurotrauma 2011, 28, 1893–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sas, A.R.; Carbajal, K.S.; Jerome, A.D.; Menon, R.; Yoon, C.; Kalinski, A.L.; Giger, R.J.; Segal, B.M. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020, 21, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Doran, S.J.; Ritzel, R.M.; Glaser, E.P.; Henry, R.J.; Faden, A.I.; Loane, D.J. Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells. J. Neurotrauma 2019, 36, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Madalli, S.; Beyrau, M.; Whiteford, J.; Duchene, J.; Nandhra, I.S.; Patel, N.S.; Motwani, M.P.; Gilroy, D.W.; Thiemermann, C.; Nourshargh, S.; et al. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol. Sex Differ. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, W. Spinal cord contusion models. Prog. Brain Res. 2002, 137, 231–255. [Google Scholar]
- Abrams, P.; Agarwal, M.; Drake, M.; El-Masri, W.; Fulford, S.; Reid, S.; Singh, G.; Tophill, P. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 2008, 101, 989–994. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C.; Anderson, D.K.; Faden, A.I.; Gruner, J.A.; Holford, T.R.; Hsu, C.Y.; Noble, L.J.; Nockels, R.; et al. MASCIS evaluation of open field locomotor scores: Effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 1996, 13, 343–359. [Google Scholar] [CrossRef]
- Askarifirouzjaei, H.; Khajoueinejad, L.; Farrokhi, A.S.; Tahoori, M.-T.; Fazeli, M.; Tiraihi, T.; Pourfathollah, A.A. Implications of immunotherapy with high-dose glatiramer acetate in acute phase of spinal cord injury in rats. Immunopharmacol. Immunotoxicol. 2019, 41, 150–162. [Google Scholar] [CrossRef]
- Khajoueinejad, L.; Askarifirouzjaei, H.; Namazi, F.; Mohammadi, A.; Pourfathollah, A.A.; Rajaian, H.; Fazeli, M. Immunomodulatory effects of Calcitriol in acute spinal cord injury in rats. Int. Immunopharmacol. 2019, 74, 105726. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Ko, I.-G.; Kim, S.-E.; Lee, S.-M.; Shin, M.-S.; Kim, C.-J.; Kim, S.-H.; Jin, J.-J.; Kim, K.-H. Oral mucosa stem cells alleviates spinal cord injury-induced neurogenic bladder symptoms in rats. J. Biomed. Sci. 2014, 21, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Ding, X.-Y.; Li, X.-H.; Gong, M.-J.; An, J.-Q.; Lai, J.-H.; Huang, S.-L. Cellular Inflammatory Response of the Spleen After Acute Spinal Cord Injury in Rat. Inflammation 2019, 42, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Janzen, J.; Vuong, P.N.; Bersch, U.; Michel, D.; Zaech, G.A. Bladder tissue biopsies in spinal cord injured patients: Histopathologic aspects of 61 cases. Neurourol. Urodyn. 1998, 17, 525–530. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Changes in afferent activity after spinal cord injury. Neurourol. Urodyn. 2010, 29, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groat, W.C.; Yoshimura, N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp. Neurol. 2012, 235, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Tesser Poloni, J.A.; Bosan, I.B.; Garigali, G.; Fogazzi, G.B. Urinary red blood cells: Not only glomerular or nonglomerular. Nephron Clin. Pract. 2012, 120, c36–c41, Discussion c41. [Google Scholar] [CrossRef]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of vascular disruption and blood–spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Birder, L.A. More than just a barrier: Urothelium as a drug target for urinary bladder pain. Am. J. Physiol. Ren. Physiol. 2005, 289, F489–F495. [Google Scholar] [CrossRef]
- Acharya, P.; Beckel, J.; Ruiz, W.G.; Wang, E.; Rojas, R.; Birder, L.; Apodaca, G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol. Ren. Physiol. 2004, 287, F305–F318. [Google Scholar] [CrossRef]
- Matarrese, P.; Colasanti, T.; Ascione, B.; Margutti, P.; Franconi, F.; Alessandri, C.; Conti, F.; Riccieri, V.; Rosano, G.; Ortona, E.; et al. Gender disparity in susceptibility to oxidative stress and apoptosis induced by autoantibodies specific to RLIP76 in vascular cells. Antioxid. Redox Signal. 2011, 15, 2825–2836. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Fang, Z.; Liu, M.; Wang, Z.; Li, L.; Ming, S.; Lu, C.; Dong, H.; Zhang, W.; Wang, Q.; et al. Testosterone induces renal tubular epithelial cell death through the HIF-1α/BNIP3 pathway. J. Transl. Med. 2019, 17, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shunmugavel, A.; Khan, M.; Chou, P.C.T.; Dhindsa, R.K.; Martin, M.M.; Copay, A.G.; Subach, B.R.; Schuler, T.C.; Bilgen, M.; Orak, J.K.; et al. Simvastatin protects bladder and renal functions following spinal cord injury in rats. J. Inflamm. 2010, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, M.N.; Bray, L.A.; de Groat, W.C. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J. Auton. Nerv. Syst. 1995, 54, 215–224. [Google Scholar] [CrossRef]
- Li, H.; Nahm, N.; Borchert, A.; Wong, P.; Atiemo, H. Contemporary Treatment of Detrusor Sphincter Dyssynergia: A Systematic Review. Curr. Bladder Dysfunct. Rep. 2018, 13, 206–214. [Google Scholar] [CrossRef]
- Burns, A.S.; Rivas, D.A.; Ditunno, J.F. The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine 2001, 26, S129–S136. [Google Scholar] [CrossRef]
- Hung, C.-S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Hess, M.J.; Zhan, E.H.; Foo, D.K.; Yalla, S.V. Bladder cancer in patients with spinal cord injury. J. Spinal Cord Med. 2003, 26, 335–338. [Google Scholar] [CrossRef]
- Pannek, J.; Bersch, U.L.F.; Moulin, P. Urological rehabilitation of spinal cord injury patients. ArgoSpine News J. 2007, 16, 26–31. [Google Scholar] [CrossRef]
- Hamann, K.; Nehrt, G.; Ouyang, H.; Duerstock, B.; Shi, R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J. Neurochem. 2008, 104, 708–718. [Google Scholar] [CrossRef]
- Hamann, K.; Durkes, A.; Ouyang, H.; Uchida, K.; Pond, A.; Shi, R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J. Neurochem. 2008, 107, 712–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraiser, L.; Kehrer, J.P. Murine strain differences in metabolism and bladder toxicity of cyclophosphamide. Toxicology 1992, 75, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Hader, J.E.; Marzella, L.; Myers, R.A.; Jacobs, S.C.; Naslund, M.J. Hyperbaric oxygen treatment for experimental cyclophosphamide-induced hemorrhagic cystitis. J. Urol. 1993, 149, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, S.; Sakura, N.; Namera, A.; Yashiki, M. Monitoring of urinary acrolein concentration in patients receiving cyclophosphamide and ifosphamide. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 806, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.P.; Ershler, W.B.; Newman, R.A.; Fagan, M.A. Chronobiologic fluctuation of cyclophosphamide induced urinary bladder damage in mice. Chronobiologia 1983, 10, 301–306. [Google Scholar] [PubMed]
- Cannon, J.; Linke, C.A.; Cos, L.R. Cyclophosphamide-associated carcinoma of urothelium: Modalities for prevention. Urology 1991, 38, 413–416. [Google Scholar] [CrossRef]
- Al-Rawithi, S.; El-Yazigi, A.; Ernst, P.; Al-Fiar, F.; Nicholls, P.J. Urinary excretion and pharmacokinetics of acrolein and its parent drug cyclophosphamide in bone marrow transplant patients. Bone Marrow Transplant. 1998, 22, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Batista, C.K.; Brito, G.A.; Souza, M.L.; Leitão, B.T.; Cunha, F.Q.; Ribeiro, R.A. A model of hemorrhagic cystitis induced with acrolein in mice. Braz. J. Med. Biol. Res. 2006, 39, 1475–1481. [Google Scholar] [CrossRef]
- Matz, E.L.; Hsieh, M.H. Review of Advances in Uroprotective Agents for Cyclophosphamide- and Ifosfamide-induced Hemorrhagic Cystitis. Urology 2017, 100, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Hughes, F.M., Jr.; Corn, A.G.; Nimmich, A.R.; Pratt-Thomas, J.D.; Purves, J.T. Cyclophosphamide Induces an Early Wave of Acrolein-Independent Apoptosis in the Urothelium. Adv. Biosci. Biotechnol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Hermant, B.; Bibert, S.; Concord, E.; Dublet, B.; Weidenhaupt, M.; Vernet, T.; Gulino-Debrac, D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J. Biol. Chem. 2003, 278, 14002–14012. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.V.; Cepinskas, G.; Savickiene, J.; Sandig, M.; Kvietys, P.R. Neutrophils induce sequential focal changes in endothelial adherens junction components: Role of elastase. Microcirculation 2003, 10, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Kettritz, R.; Falk, R.J.; Jennette, J.C.; Gaido, M.L. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am. J. Pathol. 1996, 149, 1617–1626. [Google Scholar] [PubMed]
- Kumar, H.; Choi, H.; Jo, M.-J.; Joshi, H.P.; Muttigi, M.; Bonanomi, D.; Kim, S.B.; Ban, E.; Kim, A.; Lee, S.-H.; et al. Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury. Acta Neuropathol. Commun. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharff, A.Z.; Rousseau, M.; Mariano, L.L.; Canton, T.; Consiglio, C.R.; Albert, M.L.; Fontes, M.; Duffy, D.; Ingersoll, M.A. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight 2019, 4, e122998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tak, T.; Wijten, P.; Heeres, M.; Pickkers, P.; Scholten, A.; Heck, A.J.R.; Vrisekoop, N.; Leenen, L.P.; Borghans, J.A.M.; Tesselaar, K.; et al. Human CD62L(dim) neutrophils identified as a separate subset by proteome profiling and in vivo pulse-chase labeling. Blood 2017, 129, 3476–3485. [Google Scholar] [CrossRef] [Green Version]
- Hellebrekers, P.; Vrisekoop, N.; Koenderman, L. Neutrophil phenotypes in health and disease. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12943. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.-W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T.; et al. Mature CD10+ and immature CD10− neutrophils present in G-CSF–treated donors display opposite effects on T cells. Blood 2017, 129, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.G.; Cassino, C.; Babitz, L.; Meola, T.; Berman, R.; Lipkin, M., Jr.; Freedman, M. Hypersegmented neutrophils and vitamin B12 deficiency: Hypersegmentation in B12 deficiency. Acta Haematol. 1989, 81, 186–191. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, P.J. Disordered control of the urinary bladder after human spinal cord injury: What are the problems? Prog. Brain Res. 2006, 152, 51–57. [Google Scholar] [PubMed]
- Monteiro, S.; Pinho, A.G.; Macieira, M.; Serre-Miranda, C.; Cibrão, J.R.; Lima, R.; Soares-Cunha, C.; Vasconcelos, N.L.; Lentilhas-Graça, J.; Duarte-Silva, S.; et al. Splenic sympathetic signaling contributes to acute neutrophil infiltration of the injured spinal cord. J. Neuroinflamm. 2020, 17, 282. [Google Scholar] [CrossRef]
- Tomay, F.; Wells, K.; Duong, L.; Tsu, J.W.; Dye, D.E.; Radley-Crabb, H.G.; Grounds, M.D.; Shavlakadze, T.; Metharom, P.; Nelson, D.J.; et al. Aged neutrophils accumulate in lymphoid tissues from healthy elderly mice and infiltrate T- and B-cell zones. Immunol. Cell Biol. 2018, 96, 831–840. [Google Scholar] [CrossRef]
- Kawano, Y.; Fukui, C.; Shinohara, M.; Wakahashi, K.; Ishii, S.; Suzuki, T.; Sato, M.; Asada, N.; Kawano, H.; Minagawa, K.; et al. G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood 2017, 129, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Leliefeld, P.H.C.; Pillay, J.; Vrisekoop, N.; Heeres, M.; Tak, T.; Kox, M.; Rooijakkers, S.H.M.; Kuijpers, T.W.; Pickkers, P.; Leenen, L.P.H.; et al. Differential antibacterial control by neutrophil subsets. Blood Adv. 2018, 2, 1344–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prüss, H.; Tedeschi, A.; Thiriot, A.; Lynch, L.; Loughhead, S.M.; Stutte, S.; Mazo, I.B.; A Kopp, M.; Brommer, B.; Blex, C.; et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017, 20, 1549–1559. [Google Scholar] [CrossRef]
- Failli, V.; Kopp, M.A.; Gericke, C.; Martus, P.; Klingbeil, S.; Brommer, B.; Laginha, I.; Chen, Y.; DeVivo, M.J.; Dirnagl, U.; et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 2012, 135, 3238–3250. [Google Scholar] [CrossRef] [Green Version]
- Erard, V.; Storer, B.; Corey, L.; Nollkamper, J.; Huang, M.-L.; Limaye, A.; Boeckh, M. BK virus infection in hematopoietic stem cell transplant recipients: Frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clin. Infect. Dis. 2004, 39, 1861–1865. [Google Scholar] [CrossRef] [Green Version]
- Hofland, C.A.; Eron, L.J.; Washecka, R.M. Hemorrhagic adenovirus cystitis after renal transplantation. Transplant. Proc. 2004, 36, 3025–3027. [Google Scholar] [CrossRef]
- Lee, K.D.; Chow, W.N.; Sato-Bigbee, C.; Graf, M.R.; Graham, R.S.; Colello, R.J.; Young, H.F.; Mathern, B.E. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J. Neurotrauma 2009, 26, 2335–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, T.; Estrada-Hernandez, T.; Paik, J.-H.; Wu, M.-T.; Venkataraman, K.; Brinkmann, V.; Claffey, K.; Hla, T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003, 278, 47281–47290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Yemisci, M.; Kim, H.-H.; Yung, L.M.; Shin, H.K.; Hwang, S.-K.; Guo, S.; Qin, T.; Alsharif, N.; Brinkmann, V.; et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 2011, 69, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deitch, E.A.; Ananthakrishnan, P.; Cohen, D.B.; Xu, D.Z.; Feketeova, E.; Hauser, C.J. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1456–H1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askarifirouzjaei, H.; Khajoueinejad, L.; Wei, E.; Cheruvu, S.; Ayala, C.; Chiang, N.; Theis, T.; Sun, D.; Fazeli, M.; Young, W. Sex Differences in Immune Cell Infiltration and Hematuria in SCI-Induced Hemorrhagic Cystitis. Pathophysiology 2023, 30, 275-295. https://doi.org/10.3390/pathophysiology30030023
Askarifirouzjaei H, Khajoueinejad L, Wei E, Cheruvu S, Ayala C, Chiang N, Theis T, Sun D, Fazeli M, Young W. Sex Differences in Immune Cell Infiltration and Hematuria in SCI-Induced Hemorrhagic Cystitis. Pathophysiology. 2023; 30(3):275-295. https://doi.org/10.3390/pathophysiology30030023
Chicago/Turabian StyleAskarifirouzjaei, Hadi, Leila Khajoueinejad, Elena Wei, Sruti Cheruvu, Carlos Ayala, Ning Chiang, Thomas Theis, Dongming Sun, Mehdi Fazeli, and Wise Young. 2023. "Sex Differences in Immune Cell Infiltration and Hematuria in SCI-Induced Hemorrhagic Cystitis" Pathophysiology 30, no. 3: 275-295. https://doi.org/10.3390/pathophysiology30030023
APA StyleAskarifirouzjaei, H., Khajoueinejad, L., Wei, E., Cheruvu, S., Ayala, C., Chiang, N., Theis, T., Sun, D., Fazeli, M., & Young, W. (2023). Sex Differences in Immune Cell Infiltration and Hematuria in SCI-Induced Hemorrhagic Cystitis. Pathophysiology, 30(3), 275-295. https://doi.org/10.3390/pathophysiology30030023





