Persistent Post COVID-19 Endothelial Dysfunction and Oxidative Stress in Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consent
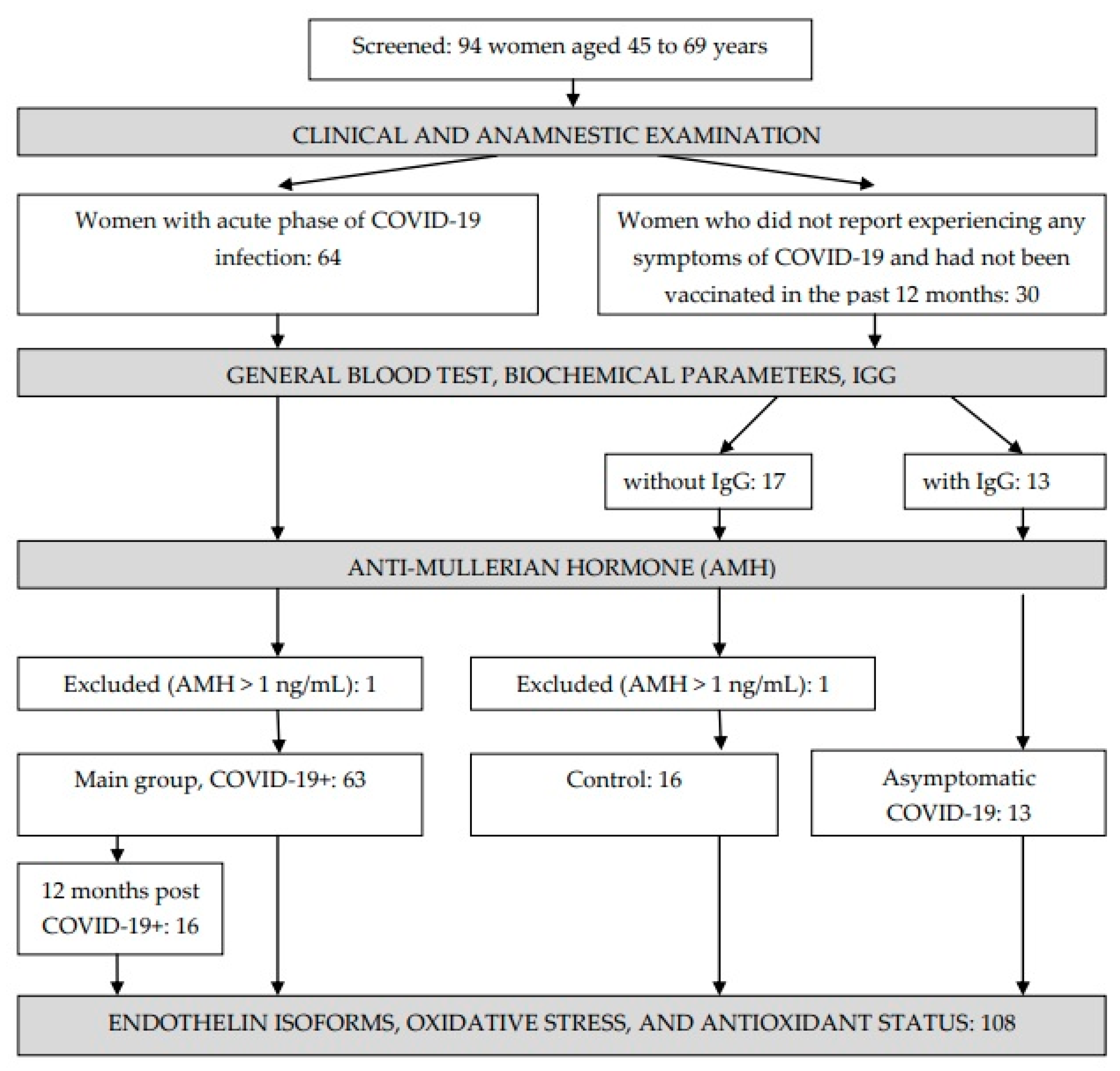
2.2. Subjects
2.3. Methods
2.3.1. Collection of Materials
2.3.2. General Blood Test, Biochemical and Hormonal Parameters, IgG
2.3.3. Endothelin Isoforms
2.3.4. Oxidative Damage Products
2.3.5. Antioxidant Status Parameters
2.3.6. Statistical Analysis
3. Results
3.1. Basic Characteristics of the Groups
3.2. Endothelin Isoforms, Oxidative Stress, and Antioxidant Status Parameters in the Control Group and in COVID-19 Patients
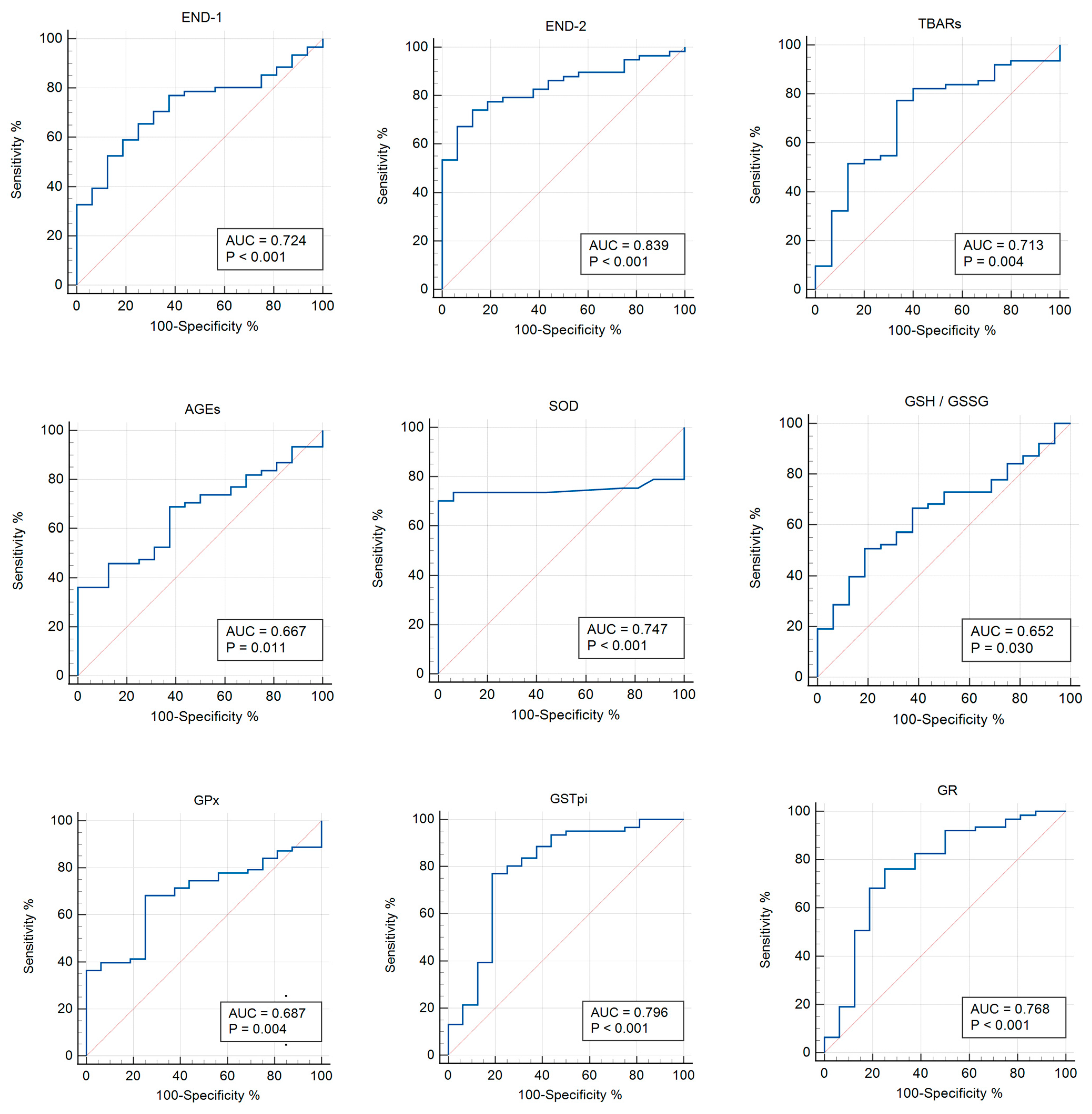
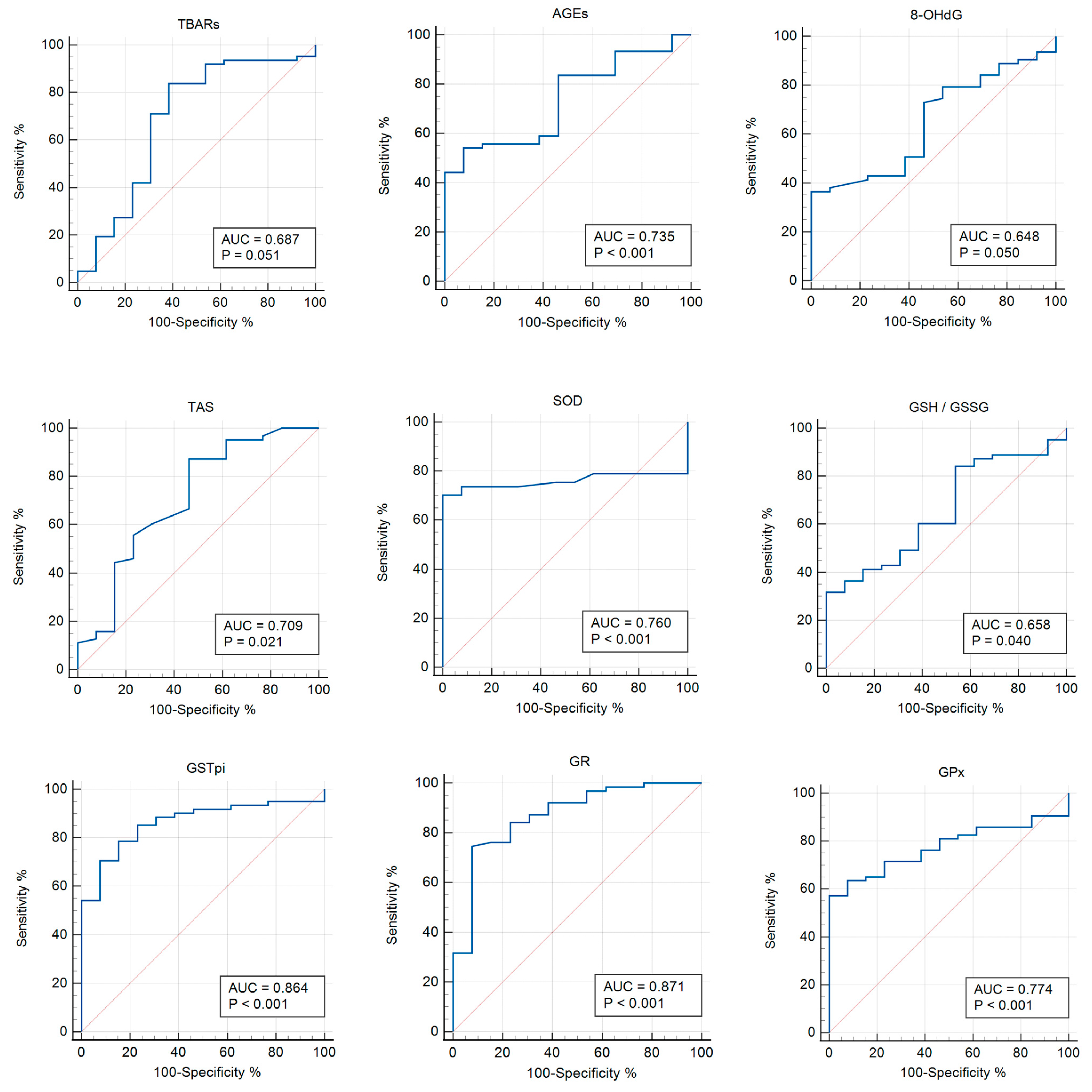
3.3. Receiver Operator Characteristic (ROC) Analysis
3.4. Correlation Analysis
3.5. Endothelin Isoforms, Oxidative Stress, and Antioxidant Status Parameters 12 Months Post COVID-19+
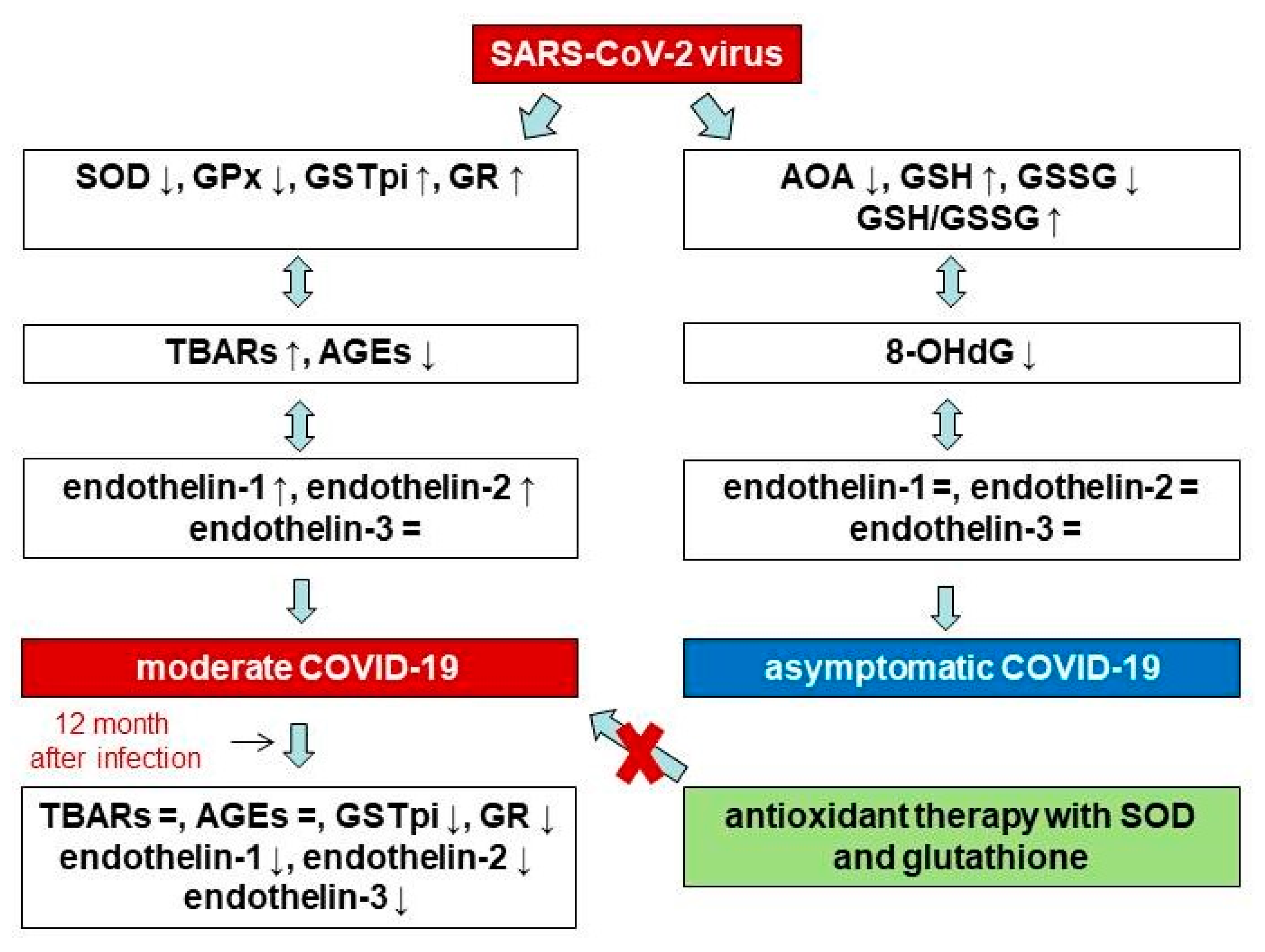
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2-deoxyguanosine |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
| AGEs | advanced glycation end products |
| ALT | alanine aminotransferase |
| AMH | anti-Mullerian hormone |
| AOD | antioxidant defense system |
| AOPP | advanced oxidation protein products |
| AST | aspartate aminotransferase |
| AUC | area under curve |
| BMI | body mass index |
| BRCA1 | breast cancer 1 |
| CI | confidence interval |
| CRP | C-reactive protein |
| DM2 | type 2 diabetes mellitus |
| DNA | deoxyribonucleic acid |
| EDTA-K3 | ethylenediaminetetraacetic acid |
| END | endothelin |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GSSG | oxidative glutathione |
| GSTpi | glutathione S-transferase |
| HT | hypertension |
| NADP | nicotinamide adenine dinucleotide phosphate |
| NADPH | nicotinamide adenine dinucleotide phosphate hydrogen |
| RAGE | receptor for advanced glycation end products |
| RNA | ribonucleic acid |
| ROC | receiver operator characteristic |
| SOD | superoxide dismutase |
| TAS | total antioxidant status |
| TBARs | thiobarbituric acid reactants |
References
- Prinelli, F.; Trevisan, C.; Noale, M.; Franchini, M.; Giacomelli, A.; Cori, L.; Jesuthasan, N.; Incalzi, R.A.; Maggi, S.; Adorni, F.; et al. Sex- and gender-related differences linked to SARS-CoV-2 infection among the participants in the web-based EPICOVID19 survey: The hormonal hypothesis. Maturitas 2022, 158, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative stress, inflammation, and mitochondrial and endothelial dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.A.; Gunawan, V.A.; Iragama, F.R.; Alfiansyah, R.; Hertanto, D.M.; Tjempakasari, A.; Thaha, M. Risk Factors and Clinical Characteristics of Acute Kidney Injury in Patients with COVID-19: A Systematic Review and Meta-Analysis. Pathophysiology 2023, 30, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Zinserling, V.A.; Semenova, N.Y.; Bikmurzina, A.E.; Kruglova, N.M.; Rybalchenko, O.V.; Markov, A.G. SARS-CoV-2-Induced Pathology—Relevance to COVID-19 Pathophysiology. Pathophysiology 2022, 29, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Ryabkova, V.A.; Rubinskiy, A.V.; Marchenko, V.N.; Trofimov, V.I.; Churilov, L.P. Similar Patterns of Dysautonomia in Myalgic Encephalomyelitis/Chronic Fatigue and Post-COVID-19 Syndromes. Pathophysiology 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Vyrupaeva, E.V.; Semenova, N.V.; Rychkova, L.V.; Petrova, A.G.; Darenskaya, M.A.; Kolesnikov, S.I.; Sambyalova, A.Y.; Kolesnikova, L.I. Assessment of the general condition and quality of life of women of post-reproductive age after asymptomatic COVID-19 and 12 months after moderate COVID-19. Acta Biomed. Sci. 2022, 7, 77–85. [Google Scholar] [CrossRef]
- Semenova, N.V.; Kolesnikov, S.I.; Vyrupaeva, E.V.; Sholokhov, L.F.; Rychkova, L.V.; Petrova, A.G.; Akhmedzyanova, M.R.; Darenskaya, M.A.; Kolesnikova, L.I. Thyroid status and TNF-alpha in post-reproductive women with COVID-19 and 12 months after the disease. Acta Biomed. Sci. 2023, 8, 33–42. [Google Scholar] [CrossRef]
- Khimji, A.K.; Rockey, D.C. Endothelin–biology and disease. Cell. Signal. 2010, 22, 1615–1625. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Niemi, M.S.; Aljowaie, R.M.; Almutairi, S.M.; Alexiou, A.; Batiha, G.E. The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in COVID-19. Inflammation 2022, 45, 1651–1667. [Google Scholar] [CrossRef]
- Abraham, G.R.; Kuc, R.E.; Althage, M.; Greasley, P.J.; Ambery, P.; Maguire, J.J.; Wilkinson, I.B.; Hoole, S.P.; Cheriyan, J.; Davenport, A.P. Endothelin-1 is increased in the plasma of patients hospitalised with COVID-19. J. Mol. Cell. Cardiol. 2022, 167, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, A.C.; Lipinski, A.L.; Vasconcellos, F.T.; Marqueze, L.F.; Cunha, E.B.; Campos, A.C.; Oliveira, C.F.; Amaral, A.N.; Baena, C.P.; Telles, J.P.; et al. Susceptibility of the patients infected with SARS-CoV-2 to oxidative stress and possible interplay with severity of the disease. Free Radic. Biol. Med. 2021, 165, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Karkhanei, B.; Talebi Ghane, E.; Mehri, F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021, 42, 100897. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sánchez-de Prada, L.; Gorgojo, Ó.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox Biol. 2021, 6, 102181. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Lage, S.L.; Amaral, E.P.; Hilligan, K.L.; Laidlaw, E.; Rupert, A.; Namasivayan, S.; Rocco, J.; Galindo, F.; Kellogg, A.; Kumar, P.; et al. Persistent oxidative stress and inflammasome activation in CD14highCD16− monocytes from COVID-19 patients. Front. Immunol. 2022, 12, 799558. [Google Scholar] [CrossRef]
- Horváth-Szalai, Z.; Jakabfi-Csepregi, R.; Szirmay, B.; Ragán, D.; Simon, G.; Kovács-Ábrahám, Z.; Szabó, P.; Sipos, D.; Péterfalvi, Á.; Miseta, A.; et al. Serum total antioxidant capacity (TAC) and TAC/Lymphocyte ratio as promising predictive markers in COVID-19. Int. J. Mol. Sci. 2023, 24, 12935. [Google Scholar] [CrossRef]
- Circu, M.; Aw, T.Y. Glutathione and modulation of cell apoptosis. Biochim. Biophys. Acta 2012, 1823, 1767–1777. [Google Scholar] [CrossRef]
- Wu, B.; Dong, D. Human cytosolic glutathione transferases: Structure, function, and drug discovery. Trends Pharmacol. Sci. 2012, 33, 656–668. [Google Scholar] [CrossRef]
- Dong, S.C.; Sha, H.H.; Xu, X.Y.; Hu, T.M.; Lou, R.; Li, H.; Wu, J.Z.; Dan, C.; Feng, J. Glutathione S-transferase π: Potential role in antitumor therapy. Drug Des. Devel. Ther. 2018, 12, 3535–3547. [Google Scholar] [CrossRef]
- Scire, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. Biofactors 2019, 45, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Shumaev, K.B.; Tikhaze, A.K.; Kurganov, B.I. Influence of dicarbonyls on kinetic characteristics of glutathione peroxidase. Dokl. Biochem. Biophys. 2017, 475, 287–290. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into manganese superoxide dismutase and human diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, E.V.; Ivanov, A.V.; Karpov, V.O.; Vasil’evich Aleksandrin, V.; Dygai, A.M.; Kruglova, M.P.; Kostiuchenko, G.I.; Kazakov, S.P.; Kubatiev, A.A. Plasma S-Adenosylmethionine is associated with lung injury in COVID-19. Dis. Markers 2021, 2021, 7686374. [Google Scholar] [CrossRef]
- Kryukov, E.V.; Ivanov, A.V.; Karpov, V.O.; Vasil’evich Alexandrin, V.; Dygai, A.M.; Kruglova, M.P.; Kostiuchenko, G.I.; Kazakov, S.P.; Kubatiev, A.A. Association of low molecular weight plasma aminothiols with the severity of coronavirus disease 2019. Oxid. Med. Cell. Longev. 2021, 2021, 9221693. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.L.; Peng, D.H.; Chen, W.; Hu, H.N.; Tang, P.; Liu, Y.Y.; Luo, Y.; Yao, T. Evaluation of serum hepatic enzyme activities in different COVID-19 phenotypes. J. Med. Virol. 2021, 93, 2365–2373. [Google Scholar] [CrossRef]
- Wang, L.; Ahn, Y.J.; Asmis, R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020, 31, 101410. [Google Scholar] [CrossRef]
- Fu, Z.D.; Csanaky, I.L.; Klaassen, C.D. Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab. Dispos. 2021, 40, 1216–1225. [Google Scholar] [CrossRef]
- Semenova, N.V.; Rychkova, L.V.; Darenskaya, M.A.; Kolesnikov, S.I.; Nikitina, O.A.; Petrova, A.G.; Vyrupaeva, E.V.; Kolesnikova, L.I. Superoxide dismutase activity in male and female patients of different age with moderate COVID-19. Bull. Exp. Biol. Med. 2022, 173, 51–53. [Google Scholar] [CrossRef]
- Hisin, P.J.; Hilf, R. Fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Brichagina, A.S.; Semenova, N.V.; Kolesnikova, L.I. Age-related menopause and carbonyl stress. Adv. Gerontol. 2022, 12, 456–462. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, M.A.; Zacarias-Flores, M.; Arronte-Rosales, A.; Correa-Muno, E.; Mendoza-Nunez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, L.; Semenova, N.; Madaeva, I.; Suturina, L.; Solodova, E.; Grebenkina, L.; Darenskaya, M. Antioxidant status in peri- and postmenopausal women. Maturitas 2015, 81, 83–87. [Google Scholar] [CrossRef]
- Semenova, N.V.; Brichagina, A.S.; Madaeva, I.M.; Kolesnikova, L.I. Enzymatic component of the glutathione system in russian and buryat women depends on the menopausal phase. J. Evol. Biochem. Physiol. 2022, 58, 971–978. [Google Scholar] [CrossRef]
- Baeza, I.; Fdez-Tresguerres, J.; Ariznavarreta, C.; De la Fuente, M. Effects of growth hormone, melatonin, oestrogens and phytoestrogens on the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio and lipid peroxidation in aged ovariectomized rats. Biogerontology 2010, 11, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Y.; Lv, Z.D.; Wang, K.; Qian, L.; Song, X.X.; Li, X.F.; Shen, H.X. Estradiol alleviates intervertebral disc degeneration through modulating the antioxidant enzymes and inhibiting autophagy in the model of menopause rats. Oxid. Med. Cell Longev. 2018, 2018, 7890291. [Google Scholar] [CrossRef]
- Lim, J.Y.; Oh, E.; Kim, Y.; Jung, W.W.; Kim, H.S.; Lee, J.; Sul, D. Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus. Acta Virol. 2014, 58, 253–260. [Google Scholar] [CrossRef]
- Liu, M.; Chen, F.; Liu, T.; Chen, F.; Liu, S.; Yang, J. The role of oxidative stress in influenza virus infection. Microbes Infect. 2017, 19, 580–586. [Google Scholar] [CrossRef]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Yegiazaryan, A.; Abnousian, A.; Alexander, L.J.; Badaoui, A.; Flaig, B.; Sheren, N.; Aghazarian, A.; Alsaigh, D.; Amin, A.; Mundra, A.; et al. Recent developments in the understanding of immunity, pathogenesis and management of COVID-19. Int. J. Mol. Sci. 2022, 23, 9297. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Jimenez, L.A.; Torres, M.; Forman, H.J. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox. Signal. 2005, 7, 42–59. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Sharafati-Chaleshtori, R.; Shirzad, H.; Rafieian-Kopaei, M.; Soltani, A. Melatonin and human mitochondrial diseases. J. Res. Med. Sci. 2017, 22, 2. [Google Scholar]
- Camp, O.G.; Bai, D.; Gonullu, D.C.; Nayak, N.; Abu-Soud, H.M. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem. 2021, 223, 111546. [Google Scholar] [CrossRef] [PubMed]
- Sen, A. Deficient synthesis of melatonin in COVID-19 can impair the resistance of coronavirus patients to mucormycosis. Med. Hypotheses 2021, 158, 110722. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine storm-definition, causes, and implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Szydełko, A.; Gostomska-Pampuch, K.; Kuzan, A.; Pietkiewicz, J.; Krzystek-Korpacka, M.; Gamian, A. Effect of advanced glycation end-products in a wide range of medical problems including COVID-19. Adv. Med. Sci. 2024, 69, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.; Pillat, M.M. CD147 as a target for COVID-19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020, 16, 434–440. [Google Scholar] [CrossRef]
- Salehi, M.; Amiri, S.; Ilghari, D.; Hasham, L.F.A.; Piri, H. The remarkable roles of the receptor for advanced glycation end products (RAGE) and its soluble isoforms in COVID-19: The importance of RAGE pathway in the lung injuries. Ind. J. Clin. Biochem. 2023, 38, 159–171. [Google Scholar] [CrossRef]
- Waraich, R.S.; Sohail, F.A.; Khan, G.; Durr-E-Shahwar, S.; Khan, B.; Rafi, S.; Nasir, S. Enhanced expression of RAGE AXIS is associated with severity of COVID-19 in patients with comorbidities. Metab. Syndr. Relat. Disord. 2023, 21, 141–147. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Yokota, T.; Garcia, G., Jr.; Palermo, A.; Wang, Y.; Farrell, C.; Wang, Y.C.; Wu, R.; Zhou, Z.; et al. Metabolic reprogramming and epigenetic changes of vital organs in SARS-CoV-2-induced systemic toxicity. JCI Insight 2021, 6, e145027. [Google Scholar] [CrossRef]
- Wang, R.; Hao, W.; Pan, L.; Boldogh, I.; Ba, X. The roles of base excision repair enzyme OGG1 in gene expression. Cell Mol. Life Sci. 2018, 75, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Basi, Z.; Turkoglu, V. In vitro effect of oxidized and reduced glutathione peptides on angiotensin converting enzyme purified from human plasma. J. Chromatogr. B 2019, 1104, 190–195. [Google Scholar] [CrossRef]
- Acworth, I.N.; McCabe, D.R.; Maher, T.J. The analysis of free radicals, their reaction products, and antioxidants. In Oxidants, Antioxidants and Free Radicals; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–77. [Google Scholar]
- Hori, A.; Mizoue, T.; Kasai, H. Body iron store as a predictor of oxidative DNA damage in healthy men and women. Cancer Sci. 2010, 101, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, J.C. DNA-beschadiging en veroudering [DNA damage and aging]. Ned. Tijdschr. Geneeskd. 2003, 147, 2578–2581. [Google Scholar]
- Nour Eldin, E.E.M.; El-Readi, M.Z.; Nour Eldein, M.M. 8-Hydroxy-2’-deoxyguanosine as a discriminatory biomarker for early detection of breast cancer. Clin. Breast Cancer 2019, 19, 385–393. [Google Scholar] [CrossRef]
- Esmaeili-Nadimi, A.; Imanparast, F.; Alizadeh, S.; Vatannejad, A.; Mohaghegh, P.; Seyedmehdi, S.M.; La Vecchia, C.; Jamali, Z. Total antioxidant capacity and total oxidant status and disease severity in a cohort study of COVID-19 patients. Clin. Lab. 2023, 69. [Google Scholar] [CrossRef]
- Aykac, K.; Ozsurekci, Y.; Yayla, B.C.C.; Gurlevik, S.L.; Oygar, P.D.; Bolu, N.B.; Tasar, M.A.; Erdinc, F.S.; Ertem, G.T.; Neselioglu, S.; et al. Oxidant and antioxidant balance in patients with COVID-19. Pediatr. Pulmonol. 2021, 56, 2803–2810. [Google Scholar] [CrossRef]
- Çakırca, G.; Damar Çakırca, T.; Üstünel, M.; Torun, A.; Koyuncu, İ. Thiol level and total oxidant/antioxidant status in patients with COVID-19 infection. Ir. J. Med. Sci. 2022, 191, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Semenova, N.V.; Vyrupaeva, E.V.; Kolesnikov, S.I.; Sholokhov, L.F.; Rychkova, L.V.; Petrova, A.G.; Akhmedzyanova, M.R.; Darenskaya, M.A.; Kolesnikova, L.I. Neuroendocrine changes among 45–60 years old women with COVID-19 and 12 months after the disease. Adv. Gerontol. 2023, 36, 477–483. [Google Scholar] [PubMed]
- Hofmann, H.; Önder, A.; Becker, J.; Gröger, M.; Müller, M.M.; Zink, F.; Stein, B.; Radermacher, P.; Waller, C. Markers of oxidative stress during post-COVID-19 fatigue: A hypothesis-generating, exploratory pilot study on hospital employees. Front. Med. 2023, 10, 1305009. [Google Scholar] [CrossRef] [PubMed]
- Stufano, A.; Isgrò, C.; Palese, L.L.; Caretta, P.; De Maria, L.; Lovreglio, P.; Sardanelli, A.M. Oxidative damage and post-COVID syndrome: A cross-sectional study in a cohort of Italian workers. Int. J. Mol. Sci. 2023, 24, 7445. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Wedgwood, S.; Black, S.M. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am. J. Physiol. 2005, 288, L480–L487. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. How mental stress affects endothelial function. Pflugers. Arch. 2011, 462, 779–794. [Google Scholar] [CrossRef]



| Parameters | Control | Asymptomatic COVID-19 | COVID-19+ | P(ANOVA) | Pχ2 |
|---|---|---|---|---|---|
| n = 16 | n = 13 | n = 63 | |||
| Age, years | 57 ± 6.24 | 54 ± 7.75 | 58 ± 6.4 | 0.175 | - |
| BMI, kg/m2 | 27.04 ± 3.69 | 28.63 ± 4.96 | 30.03 ± 5.96 | 0.143 | - |
| Systolic blood pressure, mmHg | 127.6 ± 11.27 | 126.15 ± 16.6 | 111.38 ± 9.23 | 0.015 | - |
| Diastolic blood pressure, mmHg | 78.7 ± 8.34 | 77.69 ± 5.63 | 66.74 ± 4.39 | 0.203 | - |
| Heart rate, bpm | 69.5 ± 3.58 | 71.6 ± 4.87 | 93.7 ± 4.12 | 0.011 | - |
| Type 2 diabetes mellitus, % | - | - | 15.9 | - | 0.076 |
| Hypertension, % | 25 | 38.5 | 66.7 | - | 0.005 |
| Kidney disease, % | - | 15.4 | 4.76 | - | 0.176 |
| Thyroid disease, % | 12.5 | 7.69 | 9.52 | - | 0.904 |
| Erythrocytes, 1012/L | 4.4 (4.27; 4.58) | 4.73 (4.46; 4.81) | 4.58 (4.2; 4.87) | 0.081 | - |
| Eosinophils, 109/L | 0.1 (0.08; 0.15) | 0.09 (0.06; 0.12) | 0 (0; 0.1) *, ^ | 0.001 | - |
| Thrombocytes, 109/L | 251 (217; 280) | 278 (226.25; 316) | 220 (171; 269) ^ | 0.083 | - |
| Hemoglobin, g/L | 135 (128; 144) | 141.5 (138; 145.5) | 134 (125; 143) ^ | 0.045 | - |
| CRP, mg/L | 4.25 (2.7; 8.8) | 2.2 (1; 6.3) | 12 (6.8; 13.5) *, ^ | 0.0006 | - |
| ALT, U/L | 23.2 (19.05; 29.4) | 21.35 (18; 29.5) | 30 (20; 46.8) | 0.141 | - |
| AST, U/L | 27.7 (27.4; 31.1) | 27.1 (24.3; 32.7) | 36.1 (27; 46) | 0.104 | - |
| Glucose, mM/L | 5.08 (4.22; 5.46) | 4.97 (4.48; 5.37) | 7.23 (5.9; 9.11) | 0.000 | - |
| Parameters | Control | Asymptomatic COVID-19 | COVID-19+ | P(ANOVA) |
|---|---|---|---|---|
| n = 16 | n = 13 | n = 63 | ||
| END-1, pg/mL | 408.89 (377.68; 431.55) | 445.57 (406.77; 455.88) | 449.62 (414.53; 496.45) * | 0.047 |
| END-2, pg/mL | 479.67 (423.99; 520.80) | 557.22 (463.85; 711.01) | 673.69 (536.16; 843.8) * | 0.001 |
| END-3, pg/mL | 434.79 (380.13; 470.31) | 440.61 (422.26; 449.12) | 443.46 (406.66; 489.59) | 0.33 |
| TBARs, μmol/L | 0.65 (0.35; 1.17) | 0.47 (0.27; 1.33) | 1.28 (0.82; 1.8) *, ^ | 0.175 |
| AOPP, nmol/L | 3.62 (3.27; 4.15) | 3.76 (3.48; 4.15) | 3.76 (2.38; 4.62) | 0.61 |
| AGEs, ng/mL | 3813.52 (2633.73; 4588.35) | 4691.18 (2932; 6072.88) | 2755.78 (2318.88; 3990.91) *, ^ | 0.287 |
| 8-OHdG, ng/mL | 1.40 (0.62; 1.74) | 0.55 (0.46; 1.06) * | 0.92 (0.54; 1.68) | 0.09 |
| TAS, U/L | 1.48 (1.29; 1.55) | 1.27 (1.11; 1.44) * | 1.45 (1.34; 1.6) ^ | 0.007 |
| SOD, U/L | 1.58 (1.55; 1.58) | 1.59 (1.57; 1.62) | 1.24 (0.92; 1.59) *, ^ | 0.001 |
| GSH, mmol/L | 2.02 (1.7; 2.52) | 2.45 (2.26; 3.08) * | 2.35 (2.07; 2.73) | 0.046 |
| GSSG, mmol/L | 2.1 (1.82; 2.37) | 1.84 (1.62; 1.95) * | 1.87 (1.62; 2.34) | 0.072 |
| GSH/GSSG | 0.93 (0.85; 1.22) | 1.44 (1.16; 1.78) * | 1.26 (0.89; 1.51) | 0.016 |
| GPx, U/L | 2126 (1820.5; 2412.5) | 2377 (2056; 2558) | 1804 (1321; 2162) *, ^ | 0.002 |
| GSTpi, ng/mL | 5.01 (3.67; 10.59) | 6.02 (4.94; 7.85) | 14.15(11.52; 18.2) *, ^ | 0.000 |
| GR, U/L | 79.3 (70.75; 86.65) | 73.3 (63.3; 79.6) | 101.4 (86.1; 115.4) *, ^ | 0.00001 |
| Parameter | AUC | p-Value | Cut-Off Point | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 8-OHdG | 0.763 | 0.006 | ≤1.203 | 0.557–0.906 | 100 | 53.85 |
| TAS | 0.714 | 0.048 | ≤1.46 | 0.517–0.865 | 84.62 | 68.75 |
| GSH | 0.714 | 0.030 | >1.89 | 0.517–0.865 | 92.31 | 50.00 |
| GSSG | 0.712 | 0.031 | ≤2.08 | 0.514–0.863 | 84.62 | 56.25 |
| GSH/GSSG | 0.837 | <0.0001 | >0.947 | 0.653–0.947 | 100 | 56.25 |
| Parameter | AUC | p-Value | Cut-Off Point | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| END-1 | 0.724 | <0.001 | >428.57 | 0.611–0.820 | 65.6 | 75 |
| END-2 | 0.839 | <0.010 | >549.54 | 0.736–0.915 | 74.1 | 87.5 |
| TBARs | 0.713 | 0.004 | >0.759 | 0.598–0.810 | 77.4 | 66.7 |
| AGEs | 0.667 | 0.011 | ≤2488.56 | 0.550–0.770 | 36.1 | 100 |
| SOD | 0.747 | <0.001 | ≤1.48 | 0.636–0.858 | 70.2 | 100 |
| GSH/GSSG | 0.652 | 0.030 | >1.23 | 0.515–0.789 | 50.8 | 81.2 |
| GPx | 0.687 | 0.004 | ≤1931 | 0.561–0.812 | 68.3 | 75 |
| GSTpi | 0.796 | <0.001 | >11.32 | 0.649–0.943 | 77 | 81.2 |
| GR | 0.768 | <0.001 | >84.9 | 0.620–0.916 | 76.2 | 75 |
| Parameter | AUC | p-Value | Cut-Off Point | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| TBARs | 0.687 | 0.051 | >0.553 | 0.570–0.789 | 83.87 | 61.54 |
| AGEs | 0.735 | 0.0004 | ≤2799.07 | 0.620–0.831 | 54.10 | 92.31 |
| 8-OHdG | 0.648 | 0.050 | >1.203 | 0.530–0.754 | 36.51 | 100 |
| TAS | 0.709 | 0.020 | >1.27 | 0.593–0.807 | 87.30 | 53.85 |
| SOD | 0.760 | <0.0001 | ≤1.48 | 0.643–0.854 | 70.18 | 100 |
| GSH/GSSG | 0.658 | 0.039 | ≤0.941 | 0.540–0.763 | 31.75 | 100 |
| GPx | 0.774 | <0.0001 | ≤1833 | 0.664–0.862 | 57.14 | 100 |
| GSTpi | 0.864 | <0.001 | >10.37 | 0.764–0.932 | 78.69 | 84.62 |
| GR | 0.871 | <0.0001 | >86.1 | 0.775–0.937 | 74.60 | 92.31 |
| Control | Asymptomatic COVID-19 | COVID-19 | ||||
|---|---|---|---|---|---|---|
| Correlation | r | p | r | p | r | p |
| GSH/GSH/GSSG | 0.82 | 0.000 | 0.64 | 0.019 | 0.61 | 0.00000 |
| GSSG/GPx | 0.66 | 0.005 | ||||
| GSH/AGEs | 0.61 | 0.013 | −0.38 | 0.002 | ||
| GSH/END-2 | −0.58 | 0.018 | −0.68 | 0.011 | ||
| GSSG/GSH/GSSG | −0.57 | 0.022 | −0.73 | 0.00000 | ||
| GSSG/8-OHdG | 0.56 | 0.045 | ||||
| GSH/GSSG/AGEs | 0.63 | 0.009 | −0.37 | 0.003 | ||
| GPx/END-3 | 0.52 | 0.040 | 0.35 | 0.006 | ||
| GSTpi/AOPP | −0.68 | 0.003 | ||||
| AGEs/AOPP | 0.58 | 0.018 | ||||
| AGEs/END-2 | −0.60 | 0.013 | 0.74 | 0.004 | ||
| END-1/END-3 | 0.68 | 0.004 | 0.55 | 0.049 | 0.67 | 0.00000 |
| GSH/TBARs | −0.59 | 0.032 | ||||
| GSH/GSSG/END-2 | −0.64 | 0.017 | ||||
| GSTpi/END-2 | 0.63 | 0.021 | ||||
| SOD/AOPP | 0.64 | 0.019 | ||||
| TAS/8-OHdG | 0.63 | 0.020 | ||||
| TBARs/END-2 | 0.60 | 0.029 | ||||
| AGEs/END-1 | 0.65 | 0.016 | ||||
| AOPP/END-3 | 0.63 | 0.021 | ||||
| GSSG/END-2 | 0.30 | 0.023 | ||||
| GSH/GSSG/END-3 | 0.31 | 0.017 | ||||
| GPx/8-OHdG | 0.32 | 0.009 | ||||
| GPx/END-1 | 0.42 | 0.0006 | ||||
| GSTpi/8-OHdG | −0.31 | 0.015 | ||||
| 8-OHdG/END-1 | 0.38 | 0.002 | ||||
| 8-OHdG/END-3 | 0.32 | 0.014 | ||||
| AGEs/END-3 | −0.30 | 0.022 | ||||
| GPx/SOD | 0.33 | 0.010 | ||||
| SOD/8-OHdG | 0.36 | 0.005 | ||||
| SOD/AGEs | −0.32 | 0.013 | ||||
| SOD/END-1 | 0.35 | 0.006 | ||||
| SOD/END-3 | 0.30 | 0.024 | ||||
| TAS/AOPP | 0.36 | 0.003 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, N.; Vyrupaeva, E.; Kolesnikov, S.; Darenskaya, M.; Nikitina, O.; Rychkova, L.; Kolesnikova, L. Persistent Post COVID-19 Endothelial Dysfunction and Oxidative Stress in Women. Pathophysiology 2024, 31, 436-457. https://doi.org/10.3390/pathophysiology31030033
Semenova N, Vyrupaeva E, Kolesnikov S, Darenskaya M, Nikitina O, Rychkova L, Kolesnikova L. Persistent Post COVID-19 Endothelial Dysfunction and Oxidative Stress in Women. Pathophysiology. 2024; 31(3):436-457. https://doi.org/10.3390/pathophysiology31030033
Chicago/Turabian StyleSemenova, Natalya, Ekaterina Vyrupaeva, Sergey Kolesnikov, Marina Darenskaya, Olga Nikitina, Lyubov Rychkova, and Liubov Kolesnikova. 2024. "Persistent Post COVID-19 Endothelial Dysfunction and Oxidative Stress in Women" Pathophysiology 31, no. 3: 436-457. https://doi.org/10.3390/pathophysiology31030033







