Evaluation of Full Thickness Wounds Following Application of a Visco-Liquid Hemostat in a Swine Model
Abstract
:1. Introduction
2. Materials and Methods
3. Results
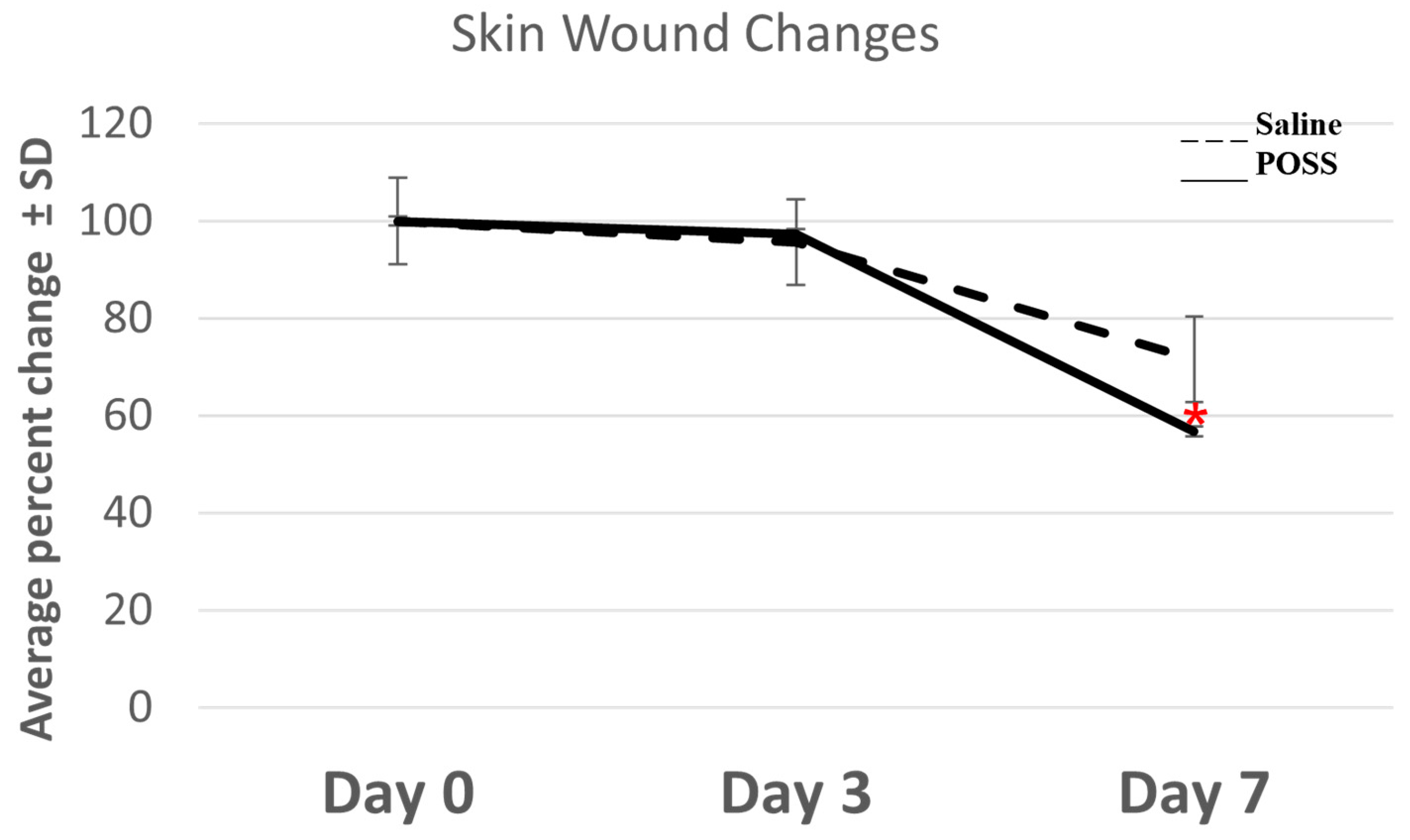
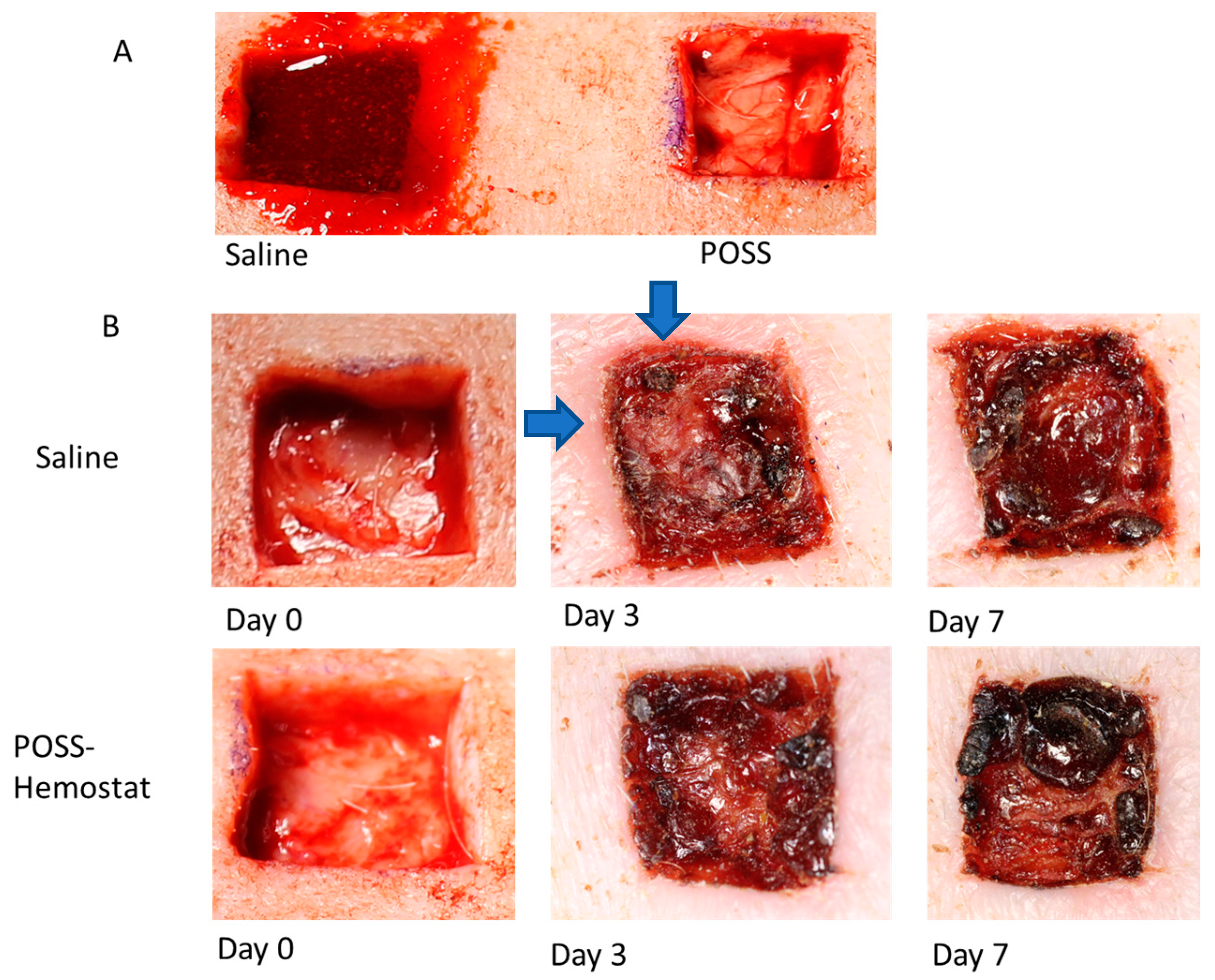
3.1. Evaluation of the Wound Beds over 7 Days
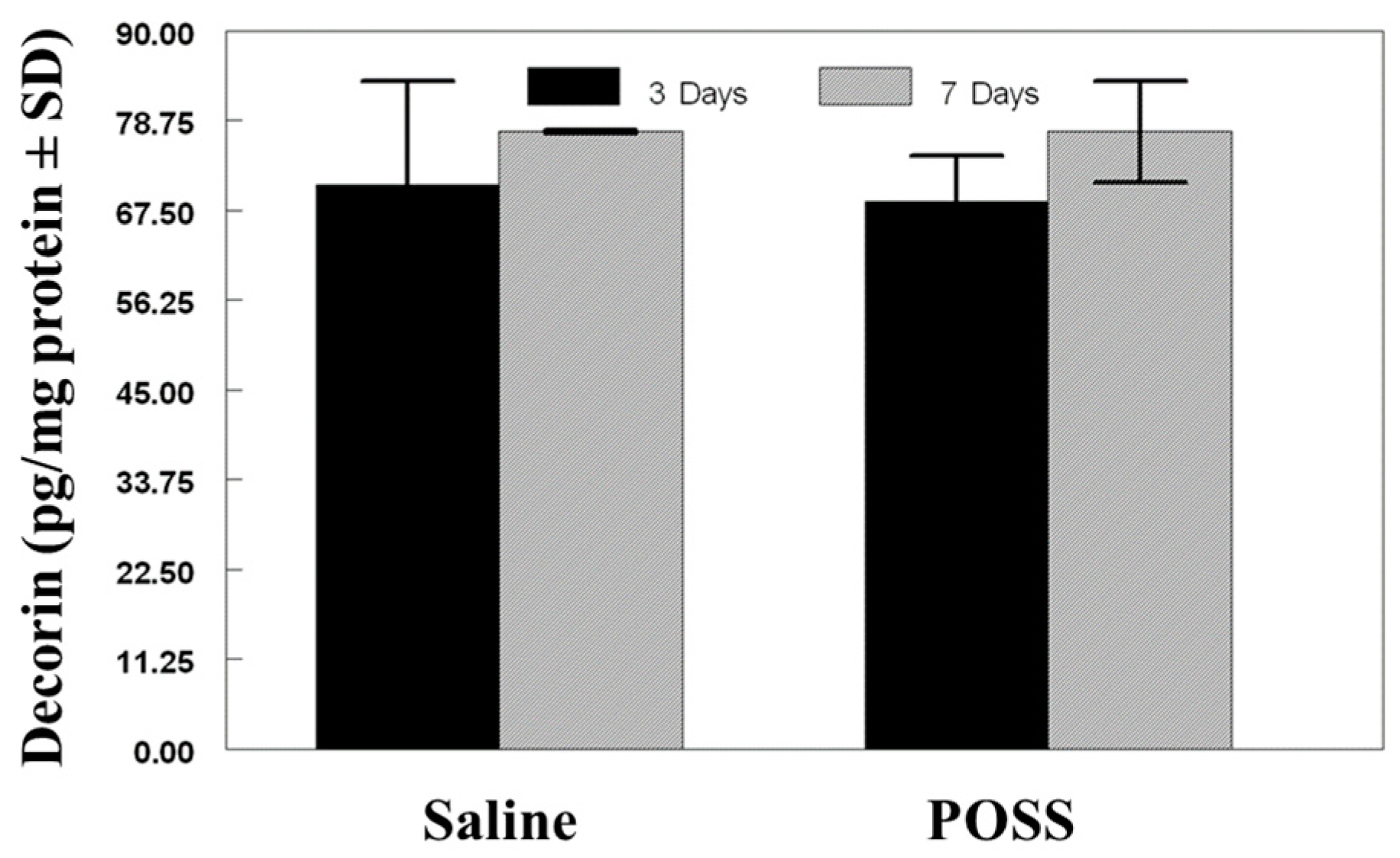
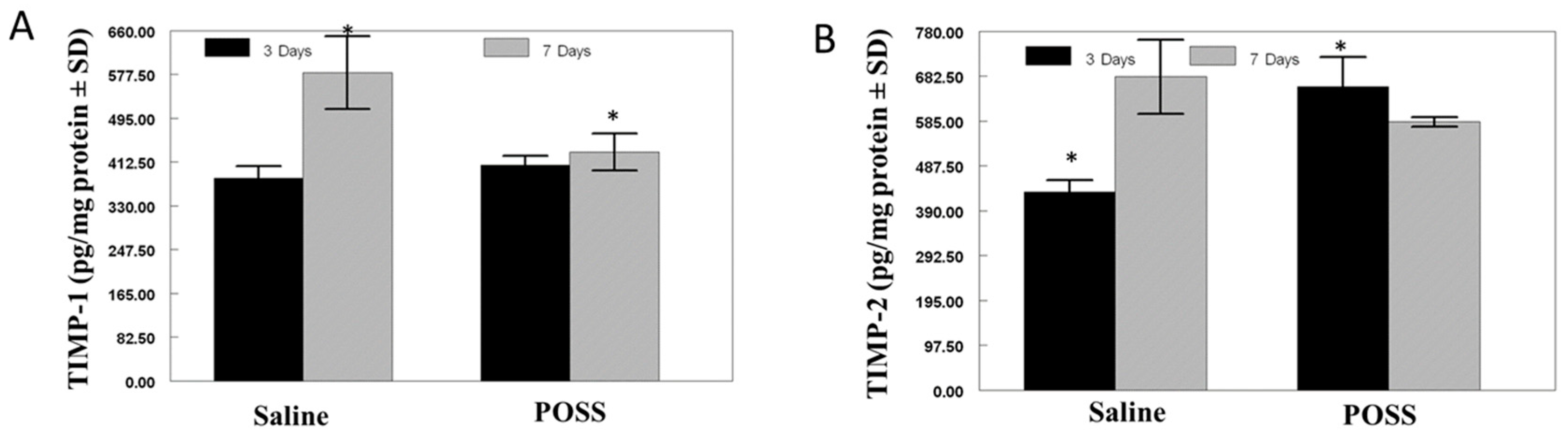
3.2. Biochemical Analysis of the Wound Bed
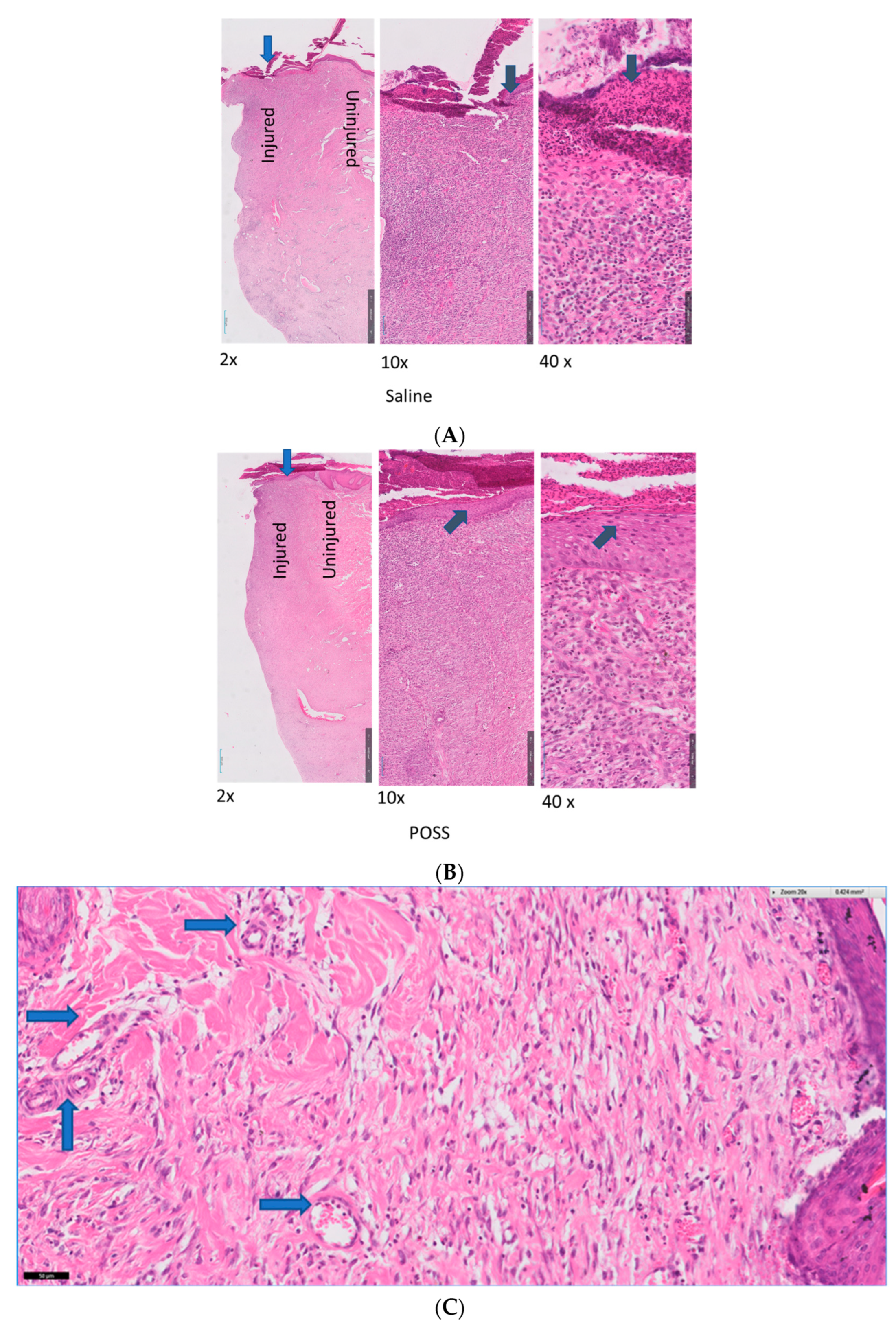
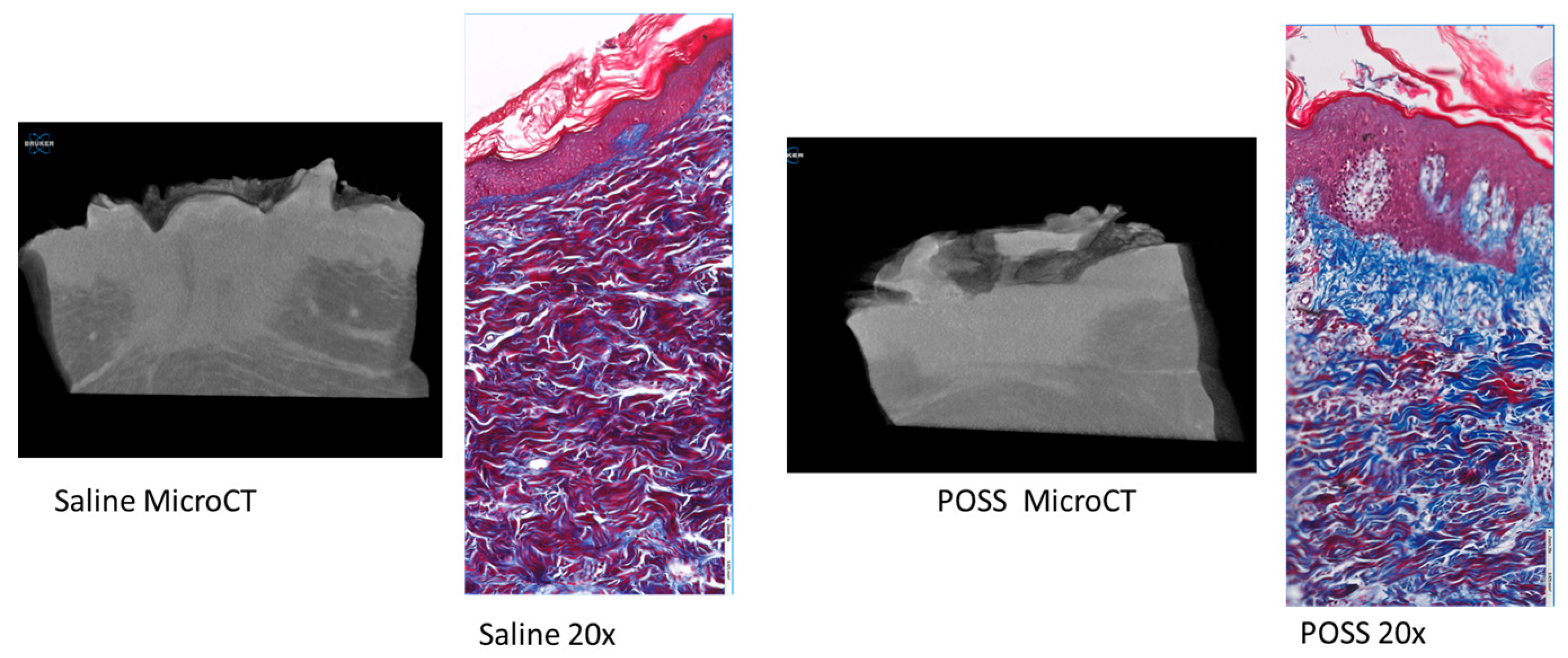
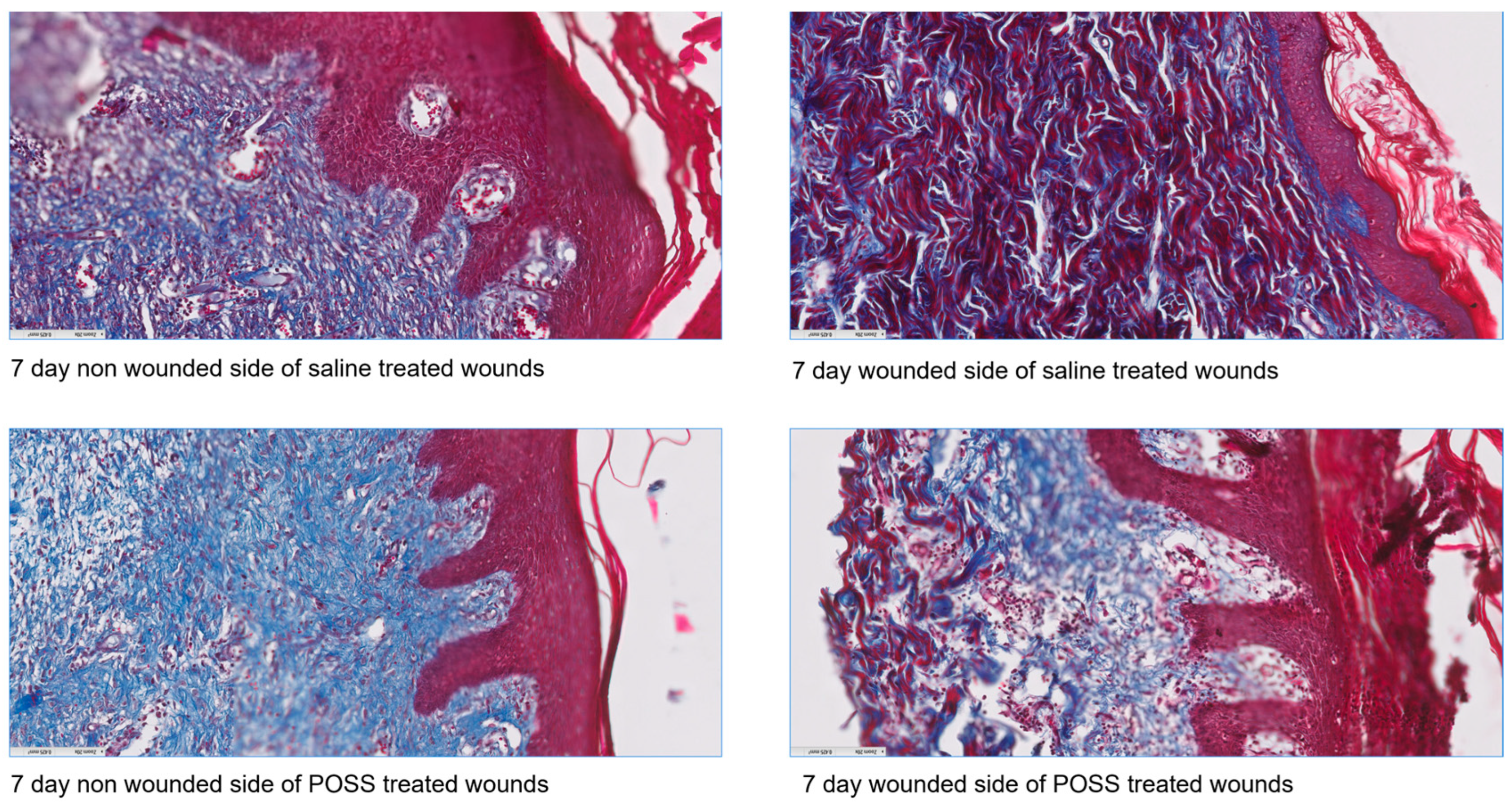
3.3. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Labib, A.; Winters, R. Complex Wound Management. [Updated 2023 July 4]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576385/ (accessed on 15 August 2024).
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Attinger, C.E.; Janis, J.E.; Steinberg, J.; Schwartz, J.; Al-Attar, A.; Couch, K. Clinical approach to wounds: Débridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg. 2006, 117 (Suppl. 7), 72S–109S. [Google Scholar] [CrossRef]
- Morin, R.J.; Tomaselli, N.L. Interactive dressings and topical agents. Clin. Plast. Surg. 2007, 34, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Poston, J. The use of silicone gel sheeting in the management of hypertrophic and keloid scars. J. Wound Care 2000, 9, 10–16. [Google Scholar] [CrossRef]
- O’Brien, L.; Pandit, A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006, 1, CD003826. [Google Scholar]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef]
- Philbeck, T.E.; Whittington, K.T.; Millsap, M.H.; Briones, R.B.; Wight, D.G.; Schroeder, W.J. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manag. 1999, 45, 41–50. [Google Scholar]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef]
- Kehlet, S.N.; Bager, C.L.; Willumsen, N.; Dasgupta, B.; Brodmerkel, C.; Curran, M.; Brix, S.; Leeming, D.J.; Karsdal, M.A. Cathepsin-S degraded decorin are elevated in fibrotic lung disorders-development and biological validation of a new serum biomarker. BMC Pulm. Med. 2017, 17, 110. [Google Scholar] [CrossRef]
- Frank, J.; Born, K.; Barker, J.H.; Marzi, I. In Vivo Effect of Tumor Necrosis Factor Alpha on Wound Angiogenesis and Epithelialization. Eur. J. Trauma 2003, 29, 208–219. [Google Scholar] [CrossRef]
- Schultz, G.S.; Barillo, D.J.; Mozingo, D.W.; Chin, G.A. Wound Bed Advisory Board Members. Wound bed preparation and a brief history of TIME. Int. Wound J. 2004, 1, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Harries, R.L.; Bosanquet, D.C.; Harding, K.G. Wound bed preparation: TIME for an update. Int. Wound J. 2016, 13 (Suppl. 3), 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, D.T.; Vermeulen, H.; Goossens, A.; Kelner, R.B.; Schreuder, S.M.; Lubbers, M.J. Occlusive vs gauze dressings for local wound care in surgical patients: A randomized clinical trial. Arch. Surg. 2008, 143, 950–955. [Google Scholar] [CrossRef]
- Huth, S.; Huth, L.; Marquardt, Y.; Cheremkhina, M.; Heise, R.; Baron, J.M. MMP-3 plays a major role in calcium pantothenate-promoted wound healing after fractional ablative laser treatment. Lasers Med. Sci. 2022, 37, 887–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002, 4, 157. [Google Scholar] [CrossRef]
- Marturano, A.; Hendrickx, M.L.V.; Falcinelli, E.; Sebastiano, M.; Guglielmini, G.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S.; Declerck, P.J.; Gresele, P. Development of anti-matrix metalloproteinase-2 (MMP-2) nanobodies as potential therapeutic and diagnostic tools. Nanomedicine 2020, 24, 102103. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mobashery, S.; Chang, M. Roles of Matrix Metalloproteinases in Cutaneous Wound Healing; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Mäkelä, M.; Larjava, H.; Pirilä, E.; Maisi, P.; Salo, T.; Sorsa, T.; Uitto, V. Matrix Metalloproteinase 2 (Gelatinase A) Is Related to Migration of Keratinocytes. Exp. Cell Res. 1999, 251, 67–78. [Google Scholar] [CrossRef]
- Martins, V.L.; Caley, M.; O’Toole, E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013, 351, 255. Available online: https://link.gale.com/apps/doc/A334946380/AONE?u=anon~56b44961&sid=googleScholar&xid=7a5841c6 (accessed on 29 April 2024). [CrossRef]
- Toriseva, M.; Laato, M.; Carpén, O.; Ruohonen, S.T.; Savontaus, E.; Inada, M.; Krane, S.M.; Kähäri, V.M. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS ONE 2012, 7, e42596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannelli, G.; Falk-Marzillier, J.; Schiraldi, O.; Stetler-Stevenson, W.G.; Quaranta, V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 1997, 277, 225–228. [Google Scholar] [CrossRef]
- Endo, K.; Takino, T.; Miyamori, H.; Kinsen, H.; Yoshizaki, T.; Furukawa, M.; Sato, H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003, 278, 40764–40770. [Google Scholar] [CrossRef]
- Vaalamo, M.; Leivo, T.; Saarialho-Kere, U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum. Pathol. 1999, 30, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Vaalamo, M.; Weckroth, M.; Puolakkainen, P.; Kere, J.; Saarinen, P.; Lauharanta, J.; Saarialho-Kere, U.K. Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br. J. Dermatol. 1996, 135, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sparks, H.D.; Mandla, S.; Vizely, K.; Rosin, N.; Radisic, M.; Biernaskie, J. Application of an instructive hydrogel accelerates re-epithelialization of xenografted human skin wounds. Sci. Rep. 2022, 12, 14233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebert, S.; Zang, L.; Ismail, N.; Otabil, M.; Fröhlich, A.; Egea, V.; Ács, S.; Hoeberg, M.; Berres, M.-L.; Weber, C.; et al. Tissue Inhibitor of Metalloproteinases-1 Interacts with CD74 to Promote AKT Signaling, Monocyte Recruitment Responses, and Vascular Smooth Muscle Cell Proliferation. Cells 2023, 12, 1899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, D.C.; Vogel, K.G. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix 1989, 9, 468. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Fisher, L.W.; MacDonald, E.D.; Jacobs, L., Jr.; Perlish, J.S.; Termine, J.D. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J. Struct. Biol. 1991, 106, 82. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.J.; Liu, Y.; Zhang, X.; Li, Y.Y.; Xu, W.S. Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns 2007, 33, 634. [Google Scholar] [CrossRef]
- Ding, Q.; Wei, Q.; Sheng, G.; Wang, S.; Jing, S.; Ma, T.; Zhang, R.; Wang, T.; Li, W.; Tang, X.; et al. The Preventive Effect of Decorin on Epidural Fibrosis and Epidural Adhesions After Laminectomy. Front. Pharmacol. 2021, 12, 774316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scott, I.U.; Flynn, H.W., Jr.; Murray, T.G.; Smiddy, W.E.; Davis, J.L.; Feuer, W.J. Outcomes of complex retinal detachment repair using 1000- vs 5000-centistoke silicone oil. Arch. Ophthalmol. 2005, 123, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Lorenc, Z.P. Synthetic Fillers for Facial Rejuvenation. Clin. Plast. Surg. 2016, 43, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Svensjö, T.; Pomahac, B.; Yao, F.; Slama, J.; Eriksson, E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast. Reconstr. Surg. 2000, 106, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, J.J.; Slama, J.; Preuss, S.; Perez, N.; Svensjö, T.; Visovatti, S.; Breuing, K.; Bartlett, R.; Pribaz, J.; Weiss, D.; et al. Wet wound healing. Plast. Reconstr. Surg. 2002, 110, 1680–1687. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucci, M.; Hildebrandt, D.; Lichtenhan, J.; Benghuzzi, H. Evaluation of Full Thickness Wounds Following Application of a Visco-Liquid Hemostat in a Swine Model. Pathophysiology 2024, 31, 458-470. https://doi.org/10.3390/pathophysiology31030034
Tucci M, Hildebrandt D, Lichtenhan J, Benghuzzi H. Evaluation of Full Thickness Wounds Following Application of a Visco-Liquid Hemostat in a Swine Model. Pathophysiology. 2024; 31(3):458-470. https://doi.org/10.3390/pathophysiology31030034
Chicago/Turabian StyleTucci, Michelle, Drew Hildebrandt, Joseph Lichtenhan, and Hamed Benghuzzi. 2024. "Evaluation of Full Thickness Wounds Following Application of a Visco-Liquid Hemostat in a Swine Model" Pathophysiology 31, no. 3: 458-470. https://doi.org/10.3390/pathophysiology31030034





