Perilipin Isoforms and PGC-1α Are Regulated Differentially in Rat Heart during Pregnancy-Induced Physiological Cardiac Hypertrophy
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Handling
2.2. Quantification of PLINs and PGC-1α Genes
2.3. Western Blot Analysis and Stain-Free Technology Normalization
2.4. Triacylglycerol and Cholesterol Ester Measurement
2.5. Statistical Analysis
3. Results
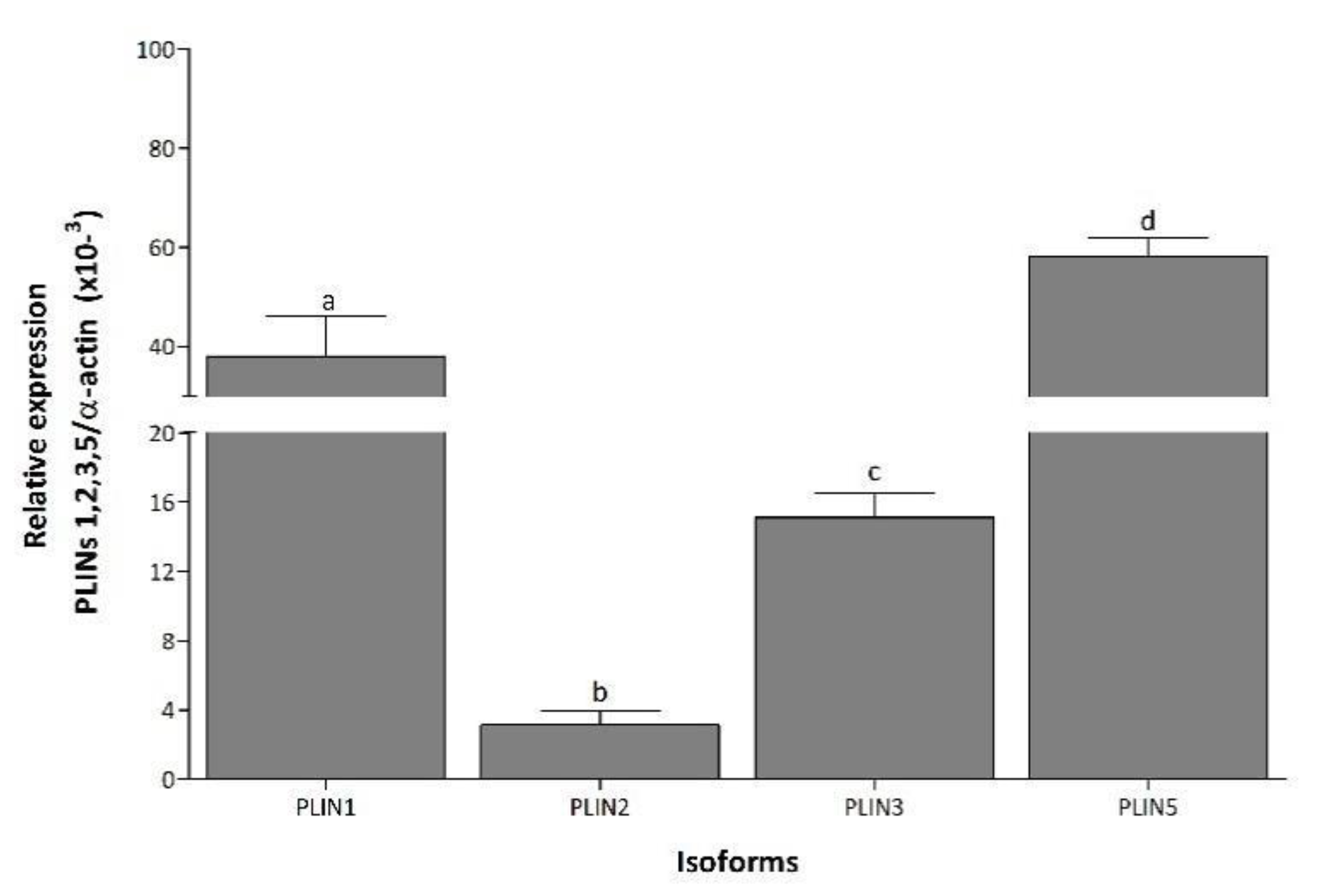
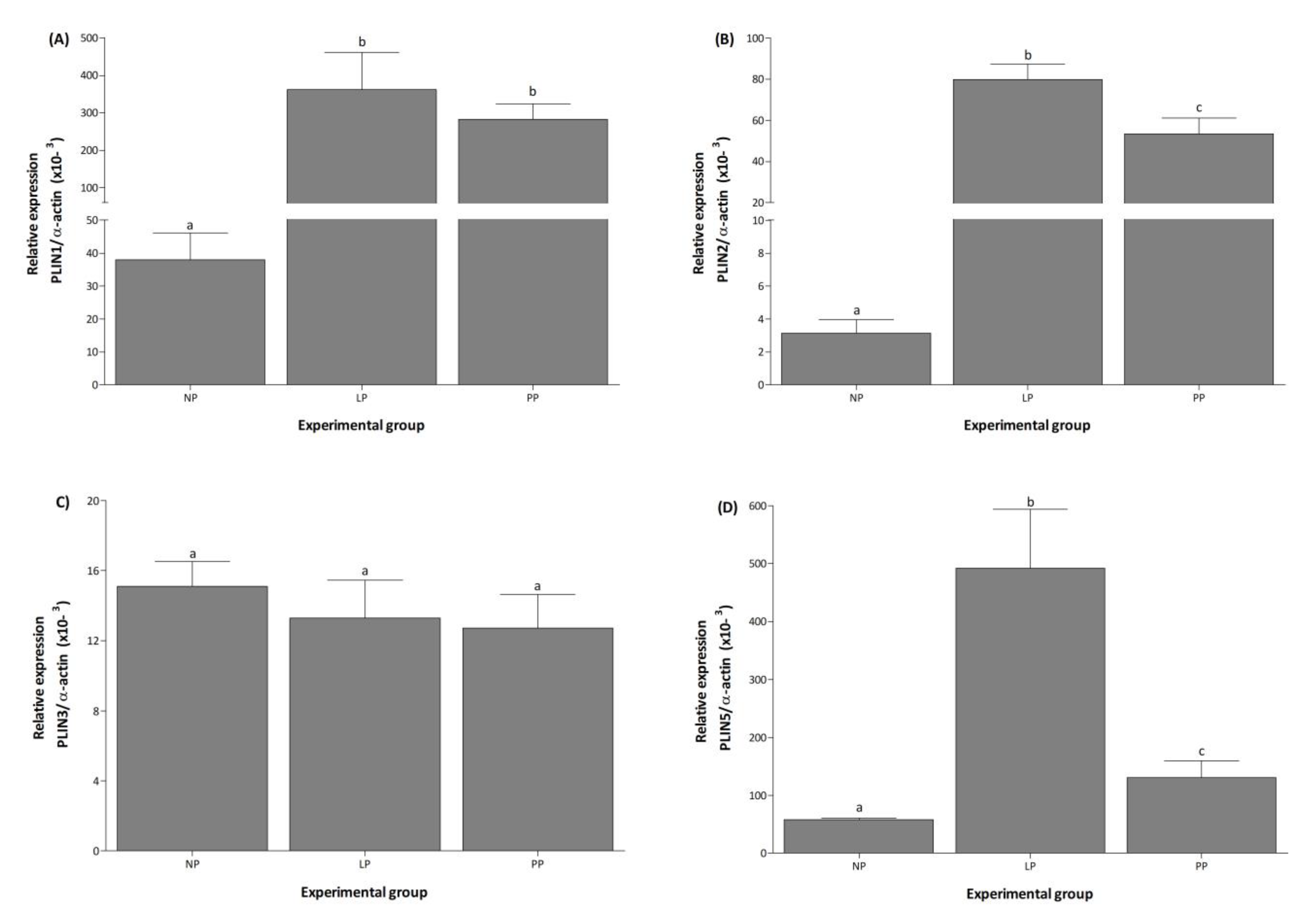
3.1. PLIN Isoforms Are Differentially Regulated during and after Pregnancy
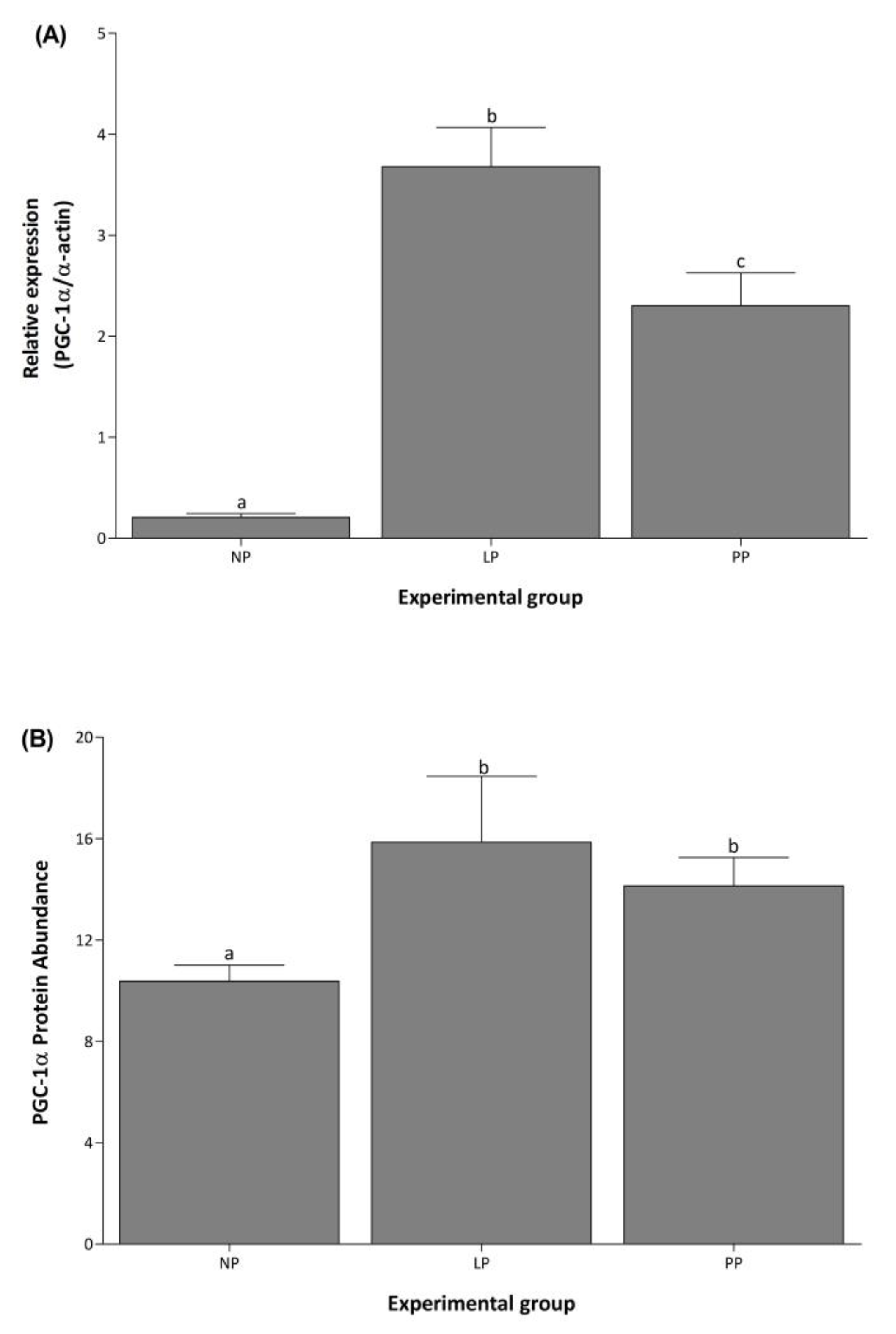
3.2. mRNA and Protein Expression of PGC-1α Increased during and after Pregnancy
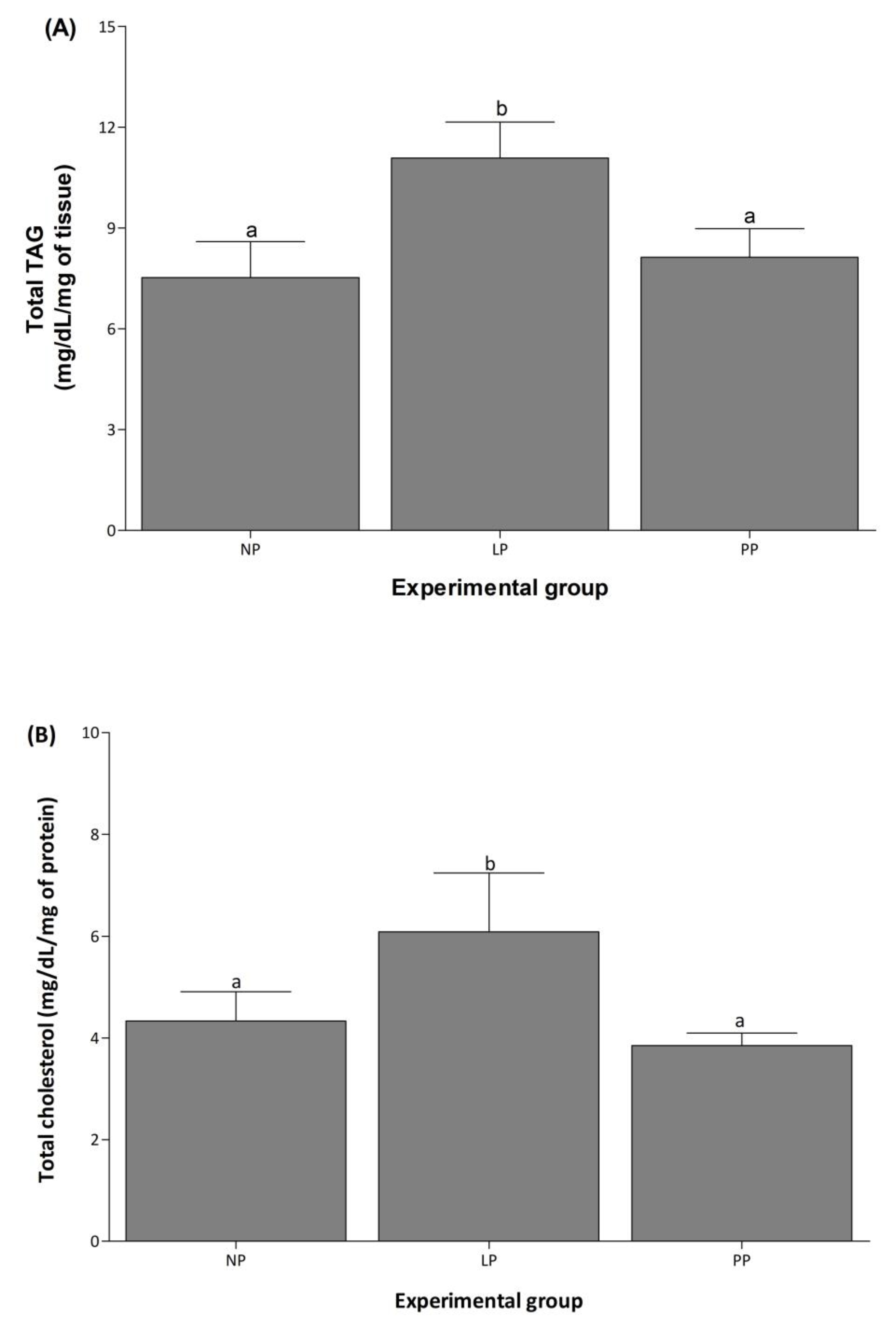
3.3. Cardiac Total TAG and Cholesterol Concentration Increased during Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beller, M.; Thiel, K.; Thul, P.J.; Jäckle, H. Lipid droplets: A dynamic organelle moves into focus. FEBS Lett. 2010, 584, 2176–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krahmer, N.; Farese, R.V.; Walther, T.C. Balancing the fat: Lipid droplets and human disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [CrossRef]
- Kimmel, A.R.; Brasaemle, D.L.; McAndrews-Hill, M.; Sztalryd, C.; Londos, C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 2010, 51, 468–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Gruia-Gray, J.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Londos, C.; Kimmel, A.R. The murine perilipin gene: The lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm. Genome 2001, 12, 741–749. [Google Scholar] [CrossRef]
- Miura, S.; Gan, J.W.; Brzostowski, J.; Parisi, M.J.; Schultz, C.J.; Londos, C.; Oliver, B.; Kimmel, A.R. Functional conservation for lipid storage droplet association among perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 2002, 277, 32253–32257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnusson, B.; Asp, L.; Boström, P.; Ruiz, M.; Stillemark-Billton, P.; Lindén, D.; Borén, J.; Olofsson, S.O. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arter. Thromb. Vasc. Biol. 2006, 26, 1566–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolins, N.E.; Skinner, J.R.; Schoenfish, M.J.; Tzekov, A.; Bensch, K.G.; Bickel, P.E. Adipocyte protein S3-12 coats nascent lipid droplets. J. Biol. Chem. 2003, 278, 37713–37721. [Google Scholar] [CrossRef] [Green Version]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Matsushita, S.; Motojima, K.; Hirose, F.; Osumi, T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 2006, 281, 14232–14240. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Chang, B.; Wu, X.; Li, L.; Sleeman, M.; Chan, L. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E770–E779. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Wang, W.; Liu, S.; Zhao, X.; Lyv, Y.; Du, C.; Su, X.; Geng, B.; Xu, G. Spontaneous hypertension occurs with adipose tissue dysfunction in perilipin-1 null mice. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 182–191. [Google Scholar] [CrossRef]
- Liu, S.; Geng, B.; Zou, L.; Wei, S.; Wang, W.; Deng, J.; Xu, C.; Zhao, X.; Lyu, Y.; Su, X.; et al. Development of hypertrophic cardiomyopathy in perilipin-1 nullmice with adipose tissue dysfunction. Cardiovasc. Res. 2015, 105, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Straub, B.K.; Gyoengyoesi, B.; Koenig, M.; Hashani, M.; Pawella, L.M.; Herpel, E.; Mueller, W.; Macher-Goeppinger, S.; Heid, H.; Schirmacher, P. Adipophilin/perilipin-2 as a lipid droplet-specific marker for metabolically active cells and diseases associated with metabolic dysregulation. Histopathology 2013, 62, 617–631. [Google Scholar] [CrossRef]
- Buers, I.; Hofnagel, O.; Ruebel, A.; Severs, N.J.; Robenek, H. Lipid droplet associated proteins: An emerging role in atherogenesis. Histol. Histopathol. 2011, 26, 631–642. [Google Scholar] [CrossRef]
- Pollak, N.M.; Schweiger, M.; Jaeger, D.; Kolb, D.; Kumari, M.; Schreiber, R.; Kolleritsch, S.; Markolin, P.; Grabner, G.F.; Heier, C.; et al. Haemmerle, cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J. Lipid Res. 2013, 54, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Osumi, T.; Kuramoto, K. Heart lipid droplets and lipid droplet-binding proteins: Biochemistry, physiology, and pathology. Exp. Cell Res. 2016, 340, 198–204. [Google Scholar] [CrossRef]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Rimbaud, S.; Sanchez, H.; Garnier, A.; Fortin, D.; Bigard, X.; Veksler, V.; Ventura-Clapier, R. Stimulus specific changes of energy metabolism in hypertrophied heart. J. Mol. Cell. Cardiol. 2009, 46, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.J.; Kelly, D.P. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail. Rev. 2002, 7, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Hill, B.G. Metabolic coordination of physiological and pathological cardiac remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.V.; Turnbull, P.C.; Macpherson, R.E.K.; Leblanc, P.J.; Ward, W.E.; Peters, S.J. Changes in mitochondrial perilipin 3 and perilipin 5 protein content in rat skeletal muscle following endurance training and acute stimulated contraction. Exp. Physiol. 2015, 100, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Rowe, G.C.; Yang, S.; Li, J.; Damilano, F.; Chan, M.C.; Lu, W.; Jang, C.; Wada, S.; Morley, M.; et al. PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ. Res. 2017, 121, 1370–1378. [Google Scholar] [CrossRef]
- Gallardo-Montejano, V.I.; Saxena, G.; Kusminski, C.M.; Yang, C.; McAfee, J.L.; Hahner, L.; Hoch, K.; Dubinsky, W.; Narkar, V.A.; Bickel, P.E. Nuclear perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat. Commun. 2016, 7, 12723. [Google Scholar] [CrossRef] [Green Version]
- Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; Heier, J.L.; Mead, L.E.; Franklin, M.P.; Karanja, K.K.; Graham, M.J.; et al. Lipid droplet-derived monounsaturated fatty acids traffic via PLIN5 to allosterically activate SIRT1. Mol. Cell 2019, 77, 810–824.e8. [Google Scholar] [CrossRef]
- Soñanez-Organis, J.G.; Godoy-Lugo, J.A.; Hernández-Palomares, M.L.E.; Rodríguez-Martínez, D.; Rosas-Rodríguez, J.A.; González-Ochoa, G.; Virgen-Ortiz, A.; Ortiz, R.M. HIF-1α and PPARγ during physiological cardiac hypertrophy induced by pregnancy: Transcriptional activities and effects on target genes. Gene 2016, 591, 376–381. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Rosas-Rodríguez, J.A.; Soñanez-Organis, J.G.; Godoy-Lugo, J.A.; Espinoza-Salazar, J.A.; López-Jacobo, C.J.; Stephens-Camacho, N.A.; González-Ochoa, G. Betaine aldehyde dehydrogenase expression during physiological cardiac hypertrophy induced by pregnancy. Biochem. Biophys. Res. Commun. 2017, 490, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef] [PubMed]
- Nose, F.; Yamaguchi, T.; Kato, R.; Aiuchi, T.; Obama, T.; Hara, S.; Yamamoto, M.; Itabe, H. Crucial role of perilipin-3 (TIP47) in formation of lipid droplets and PGE2 production in HL-60-derived neutrophils. PLoS ONE 2013, 8, e71542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Sohn, J.H.; Han, J.S.; Park, Y.J.; Jeon, Y.G.; Ji, Y.; Dalen, K.T.; Sztalryd, C.; Kimmel, A.R.; Kim, J.B. Perilipin 3 deficiency stimulates thermogenic beige adipocytes through PPARα activation. Diabetes 2018, 67, 791–804. [Google Scholar] [CrossRef]
- Covington, J.D.; Noland, R.C.; Hebert, R.C.; Masinter, B.S.; Smith, S.R.; Rustan, A.C.; Ravussin, E.; Bajpeyi, S. Perilipin 3 differentially regulates skeletal muscle lipid oxidation in active, sedentary, and type 2 diabetic males. J. Clin. Endocrinol. Metab. 2015, 100, 3683–3692. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sreenivasan, U.; Gong, D.W.; O’Connell, K.A.; Dabkowski, E.R.; Hecker, P.A.; Ionica, N.; Konig, M.; Mahurkar, A.; Sun, Y.; et al. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J. Lipid Res. 2013, 54, 953–965. [Google Scholar] [CrossRef] [Green Version]
- Trent, C.M.; Yu, S.; Hu, Y.; Skoller, N.; Huggins, L.A.; Homma, S.; Goldberg, I.J. Lipoprotein lipase activity is required for cardiac lipid droplet production. J. Lipid Res. 2014, 55, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Hashani, M.; Witzel, H.R.; Pawella, L.M.; Lehmann-Koch, J.; Schumacher, J.; Mechtersheimer, G.; Schnölzer, M.; Schirmacher, P.; Roth, W.; Straub, B.K. Widespread expression of perilipin 5 in normal human tissues and in diseases is restricted to distinct lipid droplet subpopulations. Cell Tissue Res. 2018, 374, 121–136. [Google Scholar] [CrossRef]
- Dalen, K.T.; Dahl, T.; Holter, E.; Arntsen, B.; Londos, C.; Sztalryd, C.; Nebb, H.I. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 210–227. [Google Scholar] [CrossRef]
- Kuramoto, K.; Sakai, F.; Yoshinori, N.; Nakamura, T.Y.; Wakabayashi, S.; Kojidani, T.; Haraguchi, T.; Hirose, F.; Osumi, T. Deficiency of a lipid droplet protein, perilipin 5, suppresses myocardial lipid accumulation, thereby preventing type 1 diabetes-induced heart malfunction. Mol. Cell. Biol. 2014, 34, 2721–2731. [Google Scholar] [CrossRef]
- Kuramoto, K.; Okamura, T.; Yamaguchi, T.; Nakamura, T.Y.; Wakabayashi, S.; Morinaga, H.; Nomura, M.; Yanase, T.; Otsu, K.; Usuda, N.; et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J. Biol. Chem. 2012, 287, 23852–23863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimmel, A.R.; Sztalryd, C. Perilipin 5, a lipid droplet protein adapted to mitochondrial energy utilization. Curr. Opin. Lipidol. 2014, 25, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgen-Ortiz, A.; Limón-Miranda, S.; Salazar-Enríquez, D.G.; Melnikov, V.; Sánchez-Pastor, E.A.; Castro-Rodríguez, E.M. Matrix metalloproteinases system and types of fibrosis in rat heart during late pregnancy and postpartum. Medicina 2019, 55, 199. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.; Yeung, F.; Leinwand, L.A. Calcineurin activity is required for cardiac remodelling in pregnancy. Cardiovasc. Res. 2013, 100, 402–410. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Nucleotide Sequences (5′-3′) | GenBank AccessionNumber | Product Size (Base Pairs) |
|---|---|---|---|
| PLIN1F1 PLIN1R2 | GAGTCACAACCCCACGATGT CGAGAGAGGAAAGAGTCGAC | NM_001308145.1 | 119 |
| PLIN2F1 PLIN2R2 | CAGTACTTGCCGCTCACTCA TCAGATGGACAGTGGAGTGG | NM_001007144.1 | 214 |
| PLIN3F2 PLIN3R2 | CTCAGTGTCTGGTGCAAAGG CTAGTTCTGTGTCTGTCAGG | XM_236783.6 | 233 |
| Plin4F1 PLIN4R1 | CCGAGTACTCTCTGCCAACC AATCTGCCACCTTGCATCCC | XM_006244373.3 | 218 |
| PLIN5F2 PLIN5R2 | CCAAGCCATGGACACTGTGC GCCGATAGGGATCCCAGACG | NM_001134637.1 | 210 |
| PGC-1αF1 PGC-1αR1 | TTCTTCCACAGATTCAAGCC CATTACTGAAGTTGCCATCC | AB025784.1 | 210 |
| aActinF1 aActinR1 | ATGTGTGACGACGAGGAGACC CTACATAGGAGTCTTTCTGCC | X80130.1 | 169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Rodríguez, J.A.; Virgen-Ortíz, A.; Ruiz, E.A.; Ortiz, R.M.; Soñanez-Organis, J.G. Perilipin Isoforms and PGC-1α Are Regulated Differentially in Rat Heart during Pregnancy-Induced Physiological Cardiac Hypertrophy. Medicina 2022, 58, 1433. https://doi.org/10.3390/medicina58101433
Rosas-Rodríguez JA, Virgen-Ortíz A, Ruiz EA, Ortiz RM, Soñanez-Organis JG. Perilipin Isoforms and PGC-1α Are Regulated Differentially in Rat Heart during Pregnancy-Induced Physiological Cardiac Hypertrophy. Medicina. 2022; 58(10):1433. https://doi.org/10.3390/medicina58101433
Chicago/Turabian StyleRosas-Rodríguez, Jesús A., Adolfo Virgen-Ortíz, Enrico A. Ruiz, Rudy M. Ortiz, and José G. Soñanez-Organis. 2022. "Perilipin Isoforms and PGC-1α Are Regulated Differentially in Rat Heart during Pregnancy-Induced Physiological Cardiac Hypertrophy" Medicina 58, no. 10: 1433. https://doi.org/10.3390/medicina58101433
APA StyleRosas-Rodríguez, J. A., Virgen-Ortíz, A., Ruiz, E. A., Ortiz, R. M., & Soñanez-Organis, J. G. (2022). Perilipin Isoforms and PGC-1α Are Regulated Differentially in Rat Heart during Pregnancy-Induced Physiological Cardiac Hypertrophy. Medicina, 58(10), 1433. https://doi.org/10.3390/medicina58101433







