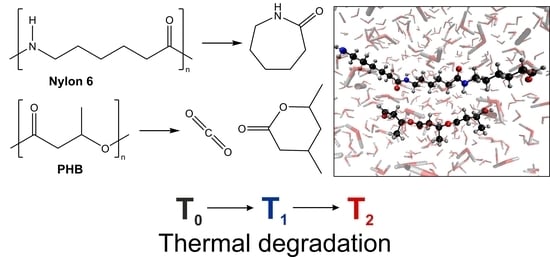
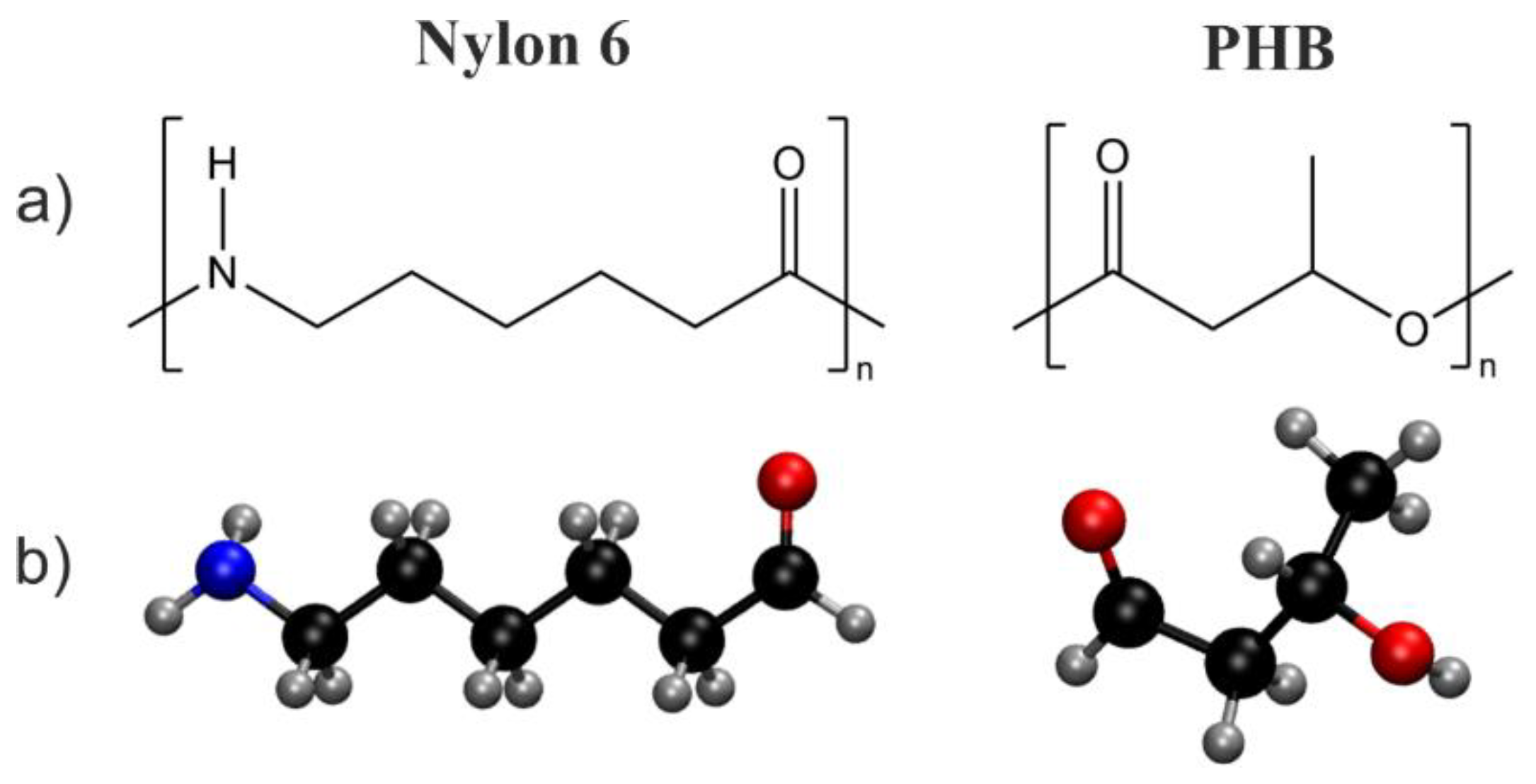
Theoretical Study on the Thermal Degradation Process of Nylon 6 and Polyhydroxybutyrate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Computational Details Effect
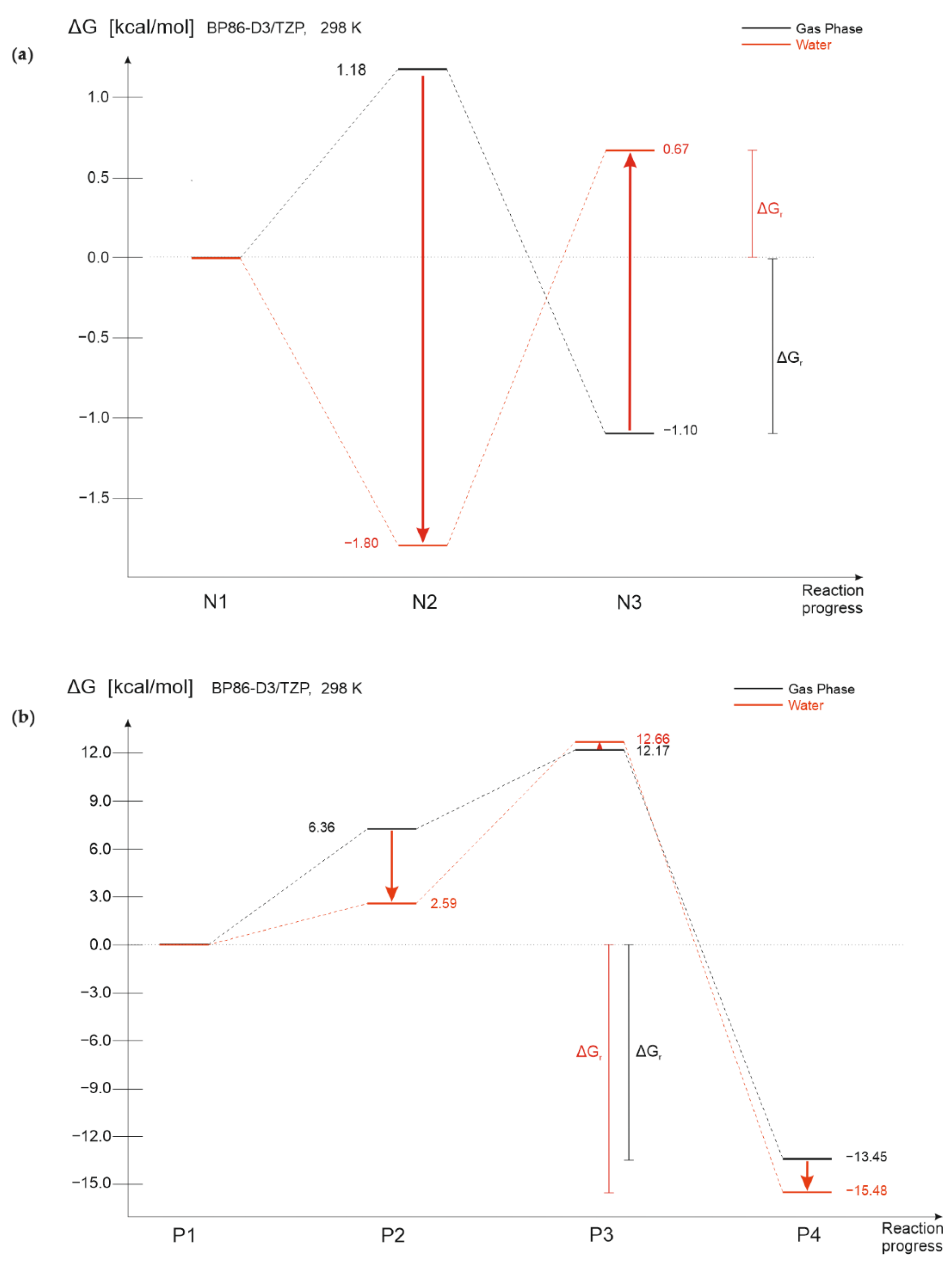
3.2. Solvation Effect
3.3. Temperature Effect
3.4. Solvation and Temperature Effect
3.5. Enthalpy and Entropy Contributions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vagholkar, P. Nylon (Chemistry, Properties and Uses). Int. J. Sci. Res. 2016, 5, 349–351. [Google Scholar]
- Sewidan, M. Nylon-6 (polyamide 6), Historical background, Properties and Limitations. Master’s Thesis, Mansoura University, Mansoura, Egypt, 2020. [Google Scholar]
- Brela, M.Z.; Wójcik, M.J.; Boczar, M.; Onishi, E.; Sato, H.; Nakajima, T.; Ozaki, Y. Study of hydrogen bond dynamics in Nylon 6 crystals using IR spectroscopy and molecular dynamics focusing on the differences between α and γ crystal forms. Int. J. Quantum Chem. 2018, 118, e25595. [Google Scholar] [CrossRef]
- Hoshina, H.; Kanemura, T.; Ruggiero, M.T. Exploring the Dynamics of Bound Water in Nylon Polymers with Terahertz Spectroscopy. J. Phys. Chem. B 2020, 124, 422–429. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ohnishi, E.; Sato, H.; Hoshina, H.; Ishikawa, D.; Ozaki, Y. Low-Frequency Vibrational Modes of Nylon 6 Studied by Using Infrared and Raman Spectroscopies and Density Functional Theory Calculations. J. Phys. Chem. B 2019, 123, 5368–5376. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, Y.; Yasunaga, M.; Sato, H.; Fukuda, R.; Ehara, M.; Ozaki, Y. Rydberg and π−π* Transitions in Film Surfaces of Various Kinds of Nylons Studied by Attenuated Total Reflection Far-Ultraviolet Spectroscopy and Quantum Chemical Calculations: Peak Shifts in the Spectra and Their Relation to Nylon Structure and Hydrogen Bondings. J. Phys. Chem. B 2014, 118, 11855–11861. [Google Scholar] [PubMed]
- Arabnejad, S.; Manzhos, S. Defects in alpha and gamma crystalline nylon6: A computational study. AIP Adv. 2015, 5, 107123. [Google Scholar] [CrossRef]
- Quarti, C.; Milani, A.; Civalleri, B.; Orlando, R.; Castiglioni, C. Ab Initio Calculation of the Crystalline Structure and IR Spectrum of Polymers: Nylon 6 Polymorphs. J. Phys. Chem. B 2012, 116, 8299–8311. [Google Scholar] [CrossRef] [PubMed]
- Milani, A. Unpolarized and Polarized Raman Spectroscopy of Nylon-6 Polymorphs: A Quantum Chemical Approach. J. Phys. Chem. B 2015, 119, 3868–3874. [Google Scholar] [CrossRef] [PubMed]
- Kök, F.N.; Hasirci, V. Polyhydroxybutyrate and its copolymers: Applications in the medical field. In Tissue Engineering and Novel Delivery Systems; Taylor & Francis: Abingdon-on-Thames, UK, 2004; pp. 543–561. [Google Scholar]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Wang, H.; Tashiro, K. Reinvestigation of Crystal Structure and Intermolecular Interactions of Biodegradable Poly(3-Hydroxybutyrate) α-Form and the Prediction of Its Mechanical Property. Macromolecules 2016, 49, 581–594. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. The Influence of the Industrial Processing on the Degradation of Poly(hidroxybutyrate)—PHB. Mater. Res. 2013, 16, 327–332. [Google Scholar] [CrossRef]
- Beć, K.B.; Morisawa, Y.; Kobashi, K.; Grabska, J.; Tanabe, I.; Tanimura, E.; Sato, H.; Wójcik, M.J.; Ozaki, Y. Rydberg transitions as a probe for structural changes and phase transition at polymer surfaces: An ATR-FUV-DUV and quantum chemical study of poly(3-hydroxybutyrate) and its nanocomposite with graphene. Phys. Chem. Chem. Phys. 2018, 20, 8859. [Google Scholar] [CrossRef] [PubMed]
- Phongtamrug, S.; Tashiro, K. X-ray Crystal Structure Analysis of Poly(3-hydroxybutyrate) β-Form and the Proposition of a Mechanism of the Stress-Induced α-to-β Phase Transition. Macromolecules 2019, 52, 2995–3009. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morisawa, Y.; Sato, H.; Hoshina, H.; Ozaki, Y. Quantum Mechanical Interpretation of Intermolecular Vibrational Modes of Crystalline Poly-(R)-3-Hydroxybutyrate Observed in Low- Frequency Raman and Terahertz Spectra. J. Phys. Chem. B 2013, 117, 2180–2187. [Google Scholar] [CrossRef]
- Vohlidal, J. Polymer degradation: A short review. CTI 2021, 3, 213–220. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948. [Google Scholar] [CrossRef]
- Lehrle, R.S.; Parsons, I.W.; Rollinson, M. Kinetics and Mechanisms of the Thermal Degradation of Nylon 6. In Ageing Studies and Lifetime Extension of Materials; Mallinson, L.G., Ed.; Springer: Boston, MA, USA, 2001; pp. 87–96. [Google Scholar]
- Shahryar, P.; Siddaramaiah; Maziar, A.M.; Akheel, S.A. Thermal degradation kinetics of nylon6/GF/crysnano nanoclay nanocomposities by TGA. Chem. Ind. Chem. Eng. Q. 2011, 17, 141–151. [Google Scholar]
- Kopinke, F.-D.; Remmler, M.; Mackenzie, K. Thermal decomposition of biodegradable polyesters—I: Poly(β-hydroxybutyric acid). Polym. Degrad. Stab. 1996, 52, 25–38. [Google Scholar] [CrossRef]
- Velde, G.T.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; Velde, G.T.; Baerends, E.J. Towards an order-N DFT method. Theor. Chem. Acc. 1998, 99, 391. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ziegler, T.; Atkins, A.J.; Autschbach, J.; Baseggio, O.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; et al. ADF2019, SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands. Available online: http://www.scm.com (accessed on 25 May 2019).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 54104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Jensen, F. Introduction to Computational Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 426–439. [Google Scholar]









| Nylon 6 | PHB | |
|---|---|---|
| Solvation effect | ΔG ↓ intermediate structure ΔG ↑ final product reversed process profile | ΔG ↓ intermediate structure (P2) ΔG ↑ intermediate structure (P3) ΔG ↓ final product |
| Temperature effect | ΔG ↑ intermediate structure ΔG~ final product | ΔG~ intermediate structures ΔG~ final product |
| Solvation and temperature effect | ΔG ↓ intermediate structure ΔG ↓ final product reversed process profile | ΔG ↓ intermediate structure (P2) ΔG ↑ intermediate structure (P3) ΔG ↓ final product |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didovets, Y.; Brela, M.Z. Theoretical Study on the Thermal Degradation Process of Nylon 6 and Polyhydroxybutyrate. Physchem 2022, 2, 334-346. https://doi.org/10.3390/physchem2040024
Didovets Y, Brela MZ. Theoretical Study on the Thermal Degradation Process of Nylon 6 and Polyhydroxybutyrate. Physchem. 2022; 2(4):334-346. https://doi.org/10.3390/physchem2040024
Chicago/Turabian StyleDidovets, Yuliia, and Mateusz Z. Brela. 2022. "Theoretical Study on the Thermal Degradation Process of Nylon 6 and Polyhydroxybutyrate" Physchem 2, no. 4: 334-346. https://doi.org/10.3390/physchem2040024
APA StyleDidovets, Y., & Brela, M. Z. (2022). Theoretical Study on the Thermal Degradation Process of Nylon 6 and Polyhydroxybutyrate. Physchem, 2(4), 334-346. https://doi.org/10.3390/physchem2040024