An Overview of PRR- and NLR-Mediated Immunities: Conserved Signaling Components across the Plant Kingdom That Communicate Both Pathways
Abstract
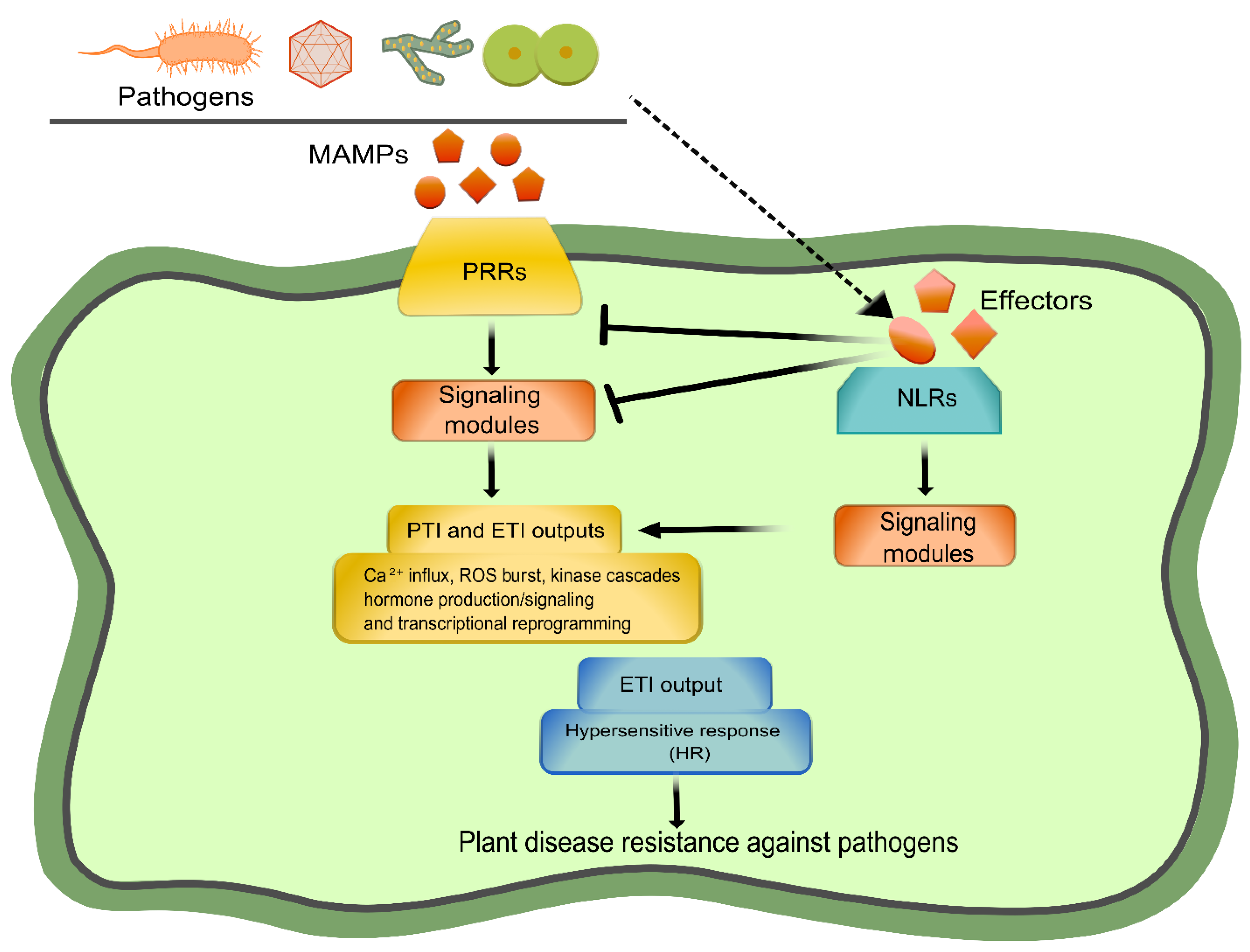
:1. Plant Immunity at a Glance
2. Deconvoluting the PRR-Mediated Immunity: Perception, Activation, and Early Signaling Mechanisms
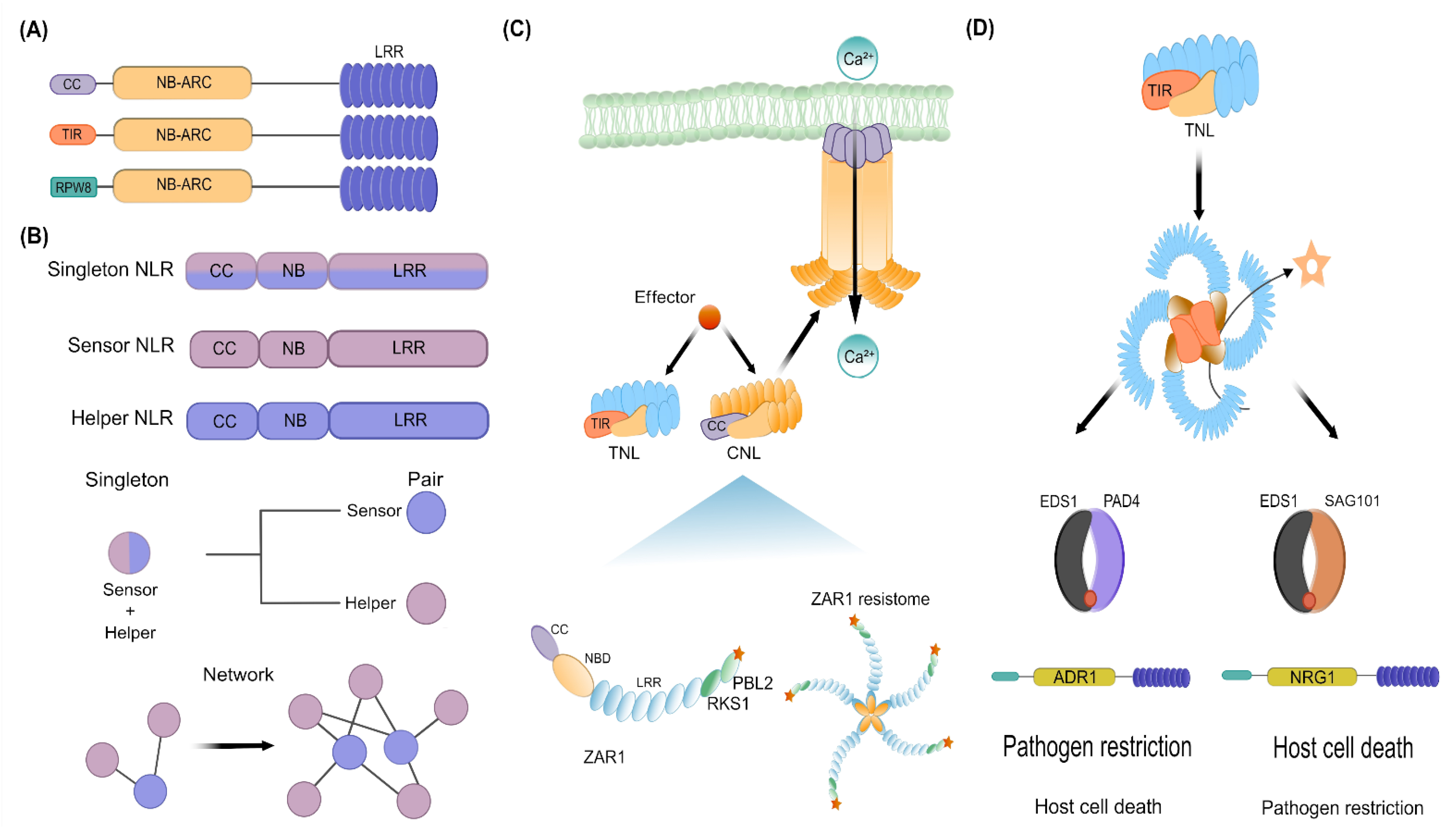
3. Deconvoluting the NLR-Mediated Immunity: Perception, Activation, and Early Signaling Mechanisms
4. PTI and ETI as One Integrated Pathway
4.1. Inherent Relations between PTI and ETI: Plant Immunity and Cell Death
4.2. EDS1-PAD4, a Convergence Hub between PRR- and NLR-Mediated Immune Responses
RNL Helpers Co-Evolved with EDS1/PAD4/SAG101
4.3. ROS and SA Are Common Outputs of PTI and ETI
4.3.1. RBOHD-Dependent ROS Production
4.3.2. SA at the Central Core of Metabolic Defense Strategies
4.3.3. Alternative Routes Provide Resilience of Plant Immunity
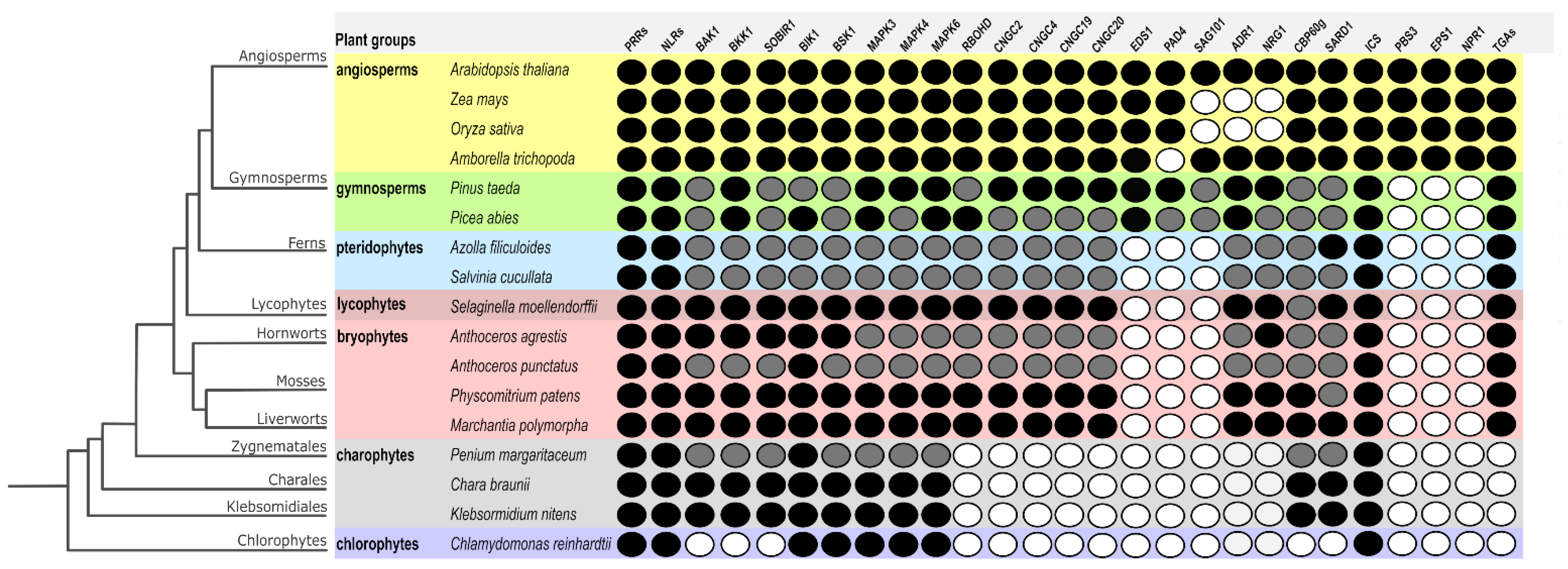
5. Plant Immunity in the Light of Evolution
5.1. A Snapshot of Canonical Components Shared between PTI and ETI, So Far
5.2. Ancient Host-Microbe Interactions
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, G.-Z. Origin and Evolution of the Plant Immune System. New Phytol. 2019, 222, 70–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gougherty, A.V.; Davies, T.J. Towards a Phylogenetic Ecology of Plant Pests and Pathogens. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200359. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.; de Vries, J.; von Dahlen, J.K.; Gould, S.B.; Archibald, J.M.; Rose, L.E.; Slamovits, C.H. On Plant Defense Signaling Networks and Early Land Plant Evolution. Commun. Integr. Biol. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tsuda, K. Evolutionary Footprint of Plant Immunity. Curr. Opin. Plant Biol. 2022, 67, 102209. [Google Scholar] [CrossRef] [PubMed]
- Cesari, S. Multiple Strategies for Pathogen Perception by Plant Immune Receptors. New Phytol. 2018, 219, 17–24. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling Mechanisms in Pattern-Triggered Immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; van Wersch, R.; Zhang, Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol. Plant-Microbe Interact. MPMI 2018, 31, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.-H.; Coaker, G. Effector Triggered Immunity: NLR Immune Perception and Downstream Defense Responses. Arab. Book 2015, 13, e0183. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From Pathogen Perception to Robust Defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Lu, Y.; Tsuda, K. Intimate Association of PRR- and NLR-Mediated Signaling in Plant Immunity. Mol. Plant-Microbe Interact. 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of Pattern Recognition Receptor Signalling in Plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Shiu, S.-H.; Bleecker, A.B. Receptor-like Kinases from Arabidopsis Form a Monophyletic Gene Family Related to Animal Receptor Kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [Green Version]
- Shiu, S.H.; Bleecker, A.B. Expansion of the Receptor-like Kinase/Pelle Gene Family and Receptor-like Proteins in Arabidopsis. Plant Physiol. 2003, 132, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, P.A.; Shan, L.; He, P. Plant Cell Surface Molecular Cypher: Receptor-like Proteins and Their Roles in Immunity and Development. Plant Sci. 2018, 274, 242–251. [Google Scholar] [CrossRef]
- Steinbrenner, A.D. The Evolving Landscape of Cell Surface Pattern Recognition across Plant Immune Networks. Curr. Opin. Plant Biol. 2020, 56, 135–146. [Google Scholar] [CrossRef]
- Gong, Z.; Han, G. Flourishing in Water: The Early Evolution and Diversification of Plant Receptor-like Kinases. Plant J. 2021, 106, 174–184. [Google Scholar] [CrossRef]
- Fischer, I.; Diévart, A.; Droc, G.; Dufayard, J.-F.; Chantret, N. Evolutionary Dynamics of the Leucine-Rich Repeat Receptor-Like Kinase (LRR-RLK) Subfamily in Angiosperms. Plant Physiol. 2016, 170, 1595–1610. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.-L.; Du, L.; Huang, Y.; Gao, S.-M.; Yu, M. Origin and Diversification of Leucine-Rich Repeat Receptor-like Protein Kinase (LRR-RLK) Genes in Plants. BMC Evol. Biol. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- de Azevedo Manhães, A.M.E.; Ortiz-Morea, F.A.; He, P.; Shan, L. Plant Plasma Membrane-Resident Receptors: Surveillance for Infections and Coordination for Growth and Development. J. Integr. Plant Biol. 2021, 63, 79–101. [Google Scholar] [CrossRef]
- Dievart, A.; Gottin, C.; Périn, C.; Ranwez, V.; Chantret, N. Origin and Diversity of Plant Receptor-Like Kinases. Annu. Rev. Plant Biol. 2020, 71, 131–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ghelder, C.; Parent, G.J.; Rigault, P.; Prunier, J.; Giguère, I.; Caron, S.; Sena, J.S.; Deslauriers, A.; Bousquet, J.; Esmenjaud, D.; et al. The Large Repertoire of Conifer NLR Resistance Genes Includes Drought Responsive and Highly Diversified RNLs. Sci. Rep. 2019, 9, 11614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaux, P.-M.; Schornack, S. Plant Evolution Driven by Symbiotic and Pathogenic Interactions. Science 2021, 371, eaba6605. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty Years of Resistance: Zig-Zag through the Plant Immune System. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Ma, X.; Xu, G.; He, P.; Shan, L. SERKing Coreceptors for Receptors. Trends Plant Sci. 2016, 21, 1017–1033. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Han, Z.; Wang, J.; Qu, L.-J.; Chai, J. SERK Family Receptor-like Kinases Function as Co-Receptors with PXY for Plant Vascular Development. Mol. Plant 2016, 9, 1406–1414. [Google Scholar] [CrossRef] [Green Version]
- Liebrand, T.W.H.; van den Burg, H.A.; Joosten, M.H.A.J. Two for All: Receptor-Associated Kinases SOBIR1 and BAK1. Trends Plant Sci. 2014, 19, 123–132. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Zhang, S.; Gu, T.; Steinmetz, G.; Yu, H.; Guo, G.; Liu, X.; Fan, S.; Wang, F.; et al. Structural Analysis of Receptor-like Kinase SOBIR1 Reveals Mechanisms That Regulate Its Phosphorylation-Dependent Activation. Plant Commun. 2022, 3, 100301. [Google Scholar] [CrossRef]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM Receptor Kinase, is Essential for Chitin Elicitor Signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y.; et al. The FLS2-Associated Kinase BIK1 Directly Phosphorylates the NADPH Oxidase RbohD to Control Plant Immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Ma, X.; Shan, L.; He, P. Big Roles of Small Kinases: The Complex Functions of Receptor-like Cytoplasmic Kinases in Plant Immunity and Development. J. Integr. Plant Biol. 2013, 55, 1188–1197. [Google Scholar] [CrossRef] [Green Version]
- Bi, G.; Zhou, Z.; Wang, W.; Li, L.; Rao, S.; Wu, Y.; Zhang, X.; Menke, F.L.H.; Chen, S.; Zhou, J.-M. Receptor-Like Cytoplasmic Kinases Directly Link Diverse Pattern Recognition Receptors to the Activation of Mitogen-Activated Protein Kinase Cascades in Arabidopsis. Plant Cell 2018, 30, 1543–1561. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Zhou, Z.; Miao, P.; Bi, G.; Hu, M.; Wu, Y.; Feng, F.; Zhang, X.; Zhou, J.-M. Roles of Receptor-Like Cytoplasmic Kinase VII Members in Pattern-Triggered Immune Signaling. Plant Physiol. 2018, 177, 1679–1690. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Bi, Y.; Youn, J.-H.; Kim, S.-H.; Kim, J.-G.; Xu, N.Y.; Shrestha, R.; Burlingame, A.L.; Xu, S.-L.; Mudgett, M.B.; et al. Deconvoluting Signals Downstream of Growth and Immune Receptor Kinases by Phosphocodes of the BSU1 Family Phosphatases. Nat. Plants 2022, 8, 646–655. [Google Scholar] [CrossRef]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.G.; Boller, T.; Felix, G. Perception of the Bacterial PAMP EF-Tu by the Receptor EFR Restricts Agrobacterium-Mediated Transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef]
- Suarez-Rodriguez, M.C.; Adams-Phillips, L.; Liu, Y.; Wang, H.; Su, S.-H.; Jester, P.J.; Zhang, S.; Bent, A.F.; Krysan, P.J. MEKK1 Is Required for Flg22-Induced MPK4 Activation in Arabidopsis Plants. Plant Physiol. 2007, 143, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Nitta, Y.; Zhang, Q.; Wu, D.; Tian, H.; Lee, J.S.; Zhang, Y. Antagonistic Interactions between Two MAP Kinase Cascades in Plant Development and Immune Signaling. EMBO Rep. 2018, 19, e45324. [Google Scholar] [CrossRef]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.-H.; Sheen, J. Differential Innate Immune Signalling via Ca2+ Sensor Protein Kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, S. Phosphorylation of 1-Aminocyclopropane-1-Carboxylic Acid Synthase by MPK6, a Stress-Responsive Mitogen-Activated Protein Kinase, Induces Ethylene Biosynthesis in Arabidopsis. Plant Cell 2004, 16, 3386–3399. [Google Scholar] [CrossRef]
- Han, L.; Li, G.-J.; Yang, K.-Y.; Mao, G.; Wang, R.; Liu, Y.; Zhang, S. Mitogen-Activated Protein Kinase 3 and 6 Regulate Botrytis Cinerea-Induced Ethylene Production in Arabidopsis. Plant J. 2010, 64, 114–127. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, J.; Shan, L.; Meng, X. Plant Cell Surface Receptor-Mediated Signaling—A Common Theme amid Diversity. J. Cell Sci. 2018, 131, jcs209353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kärkönen, A.; Kuchitsu, K. Reactive Oxygen Species in Cell Wall Metabolism and Development in Plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A Calmodulin-Gated Calcium Channel Links Pathogen Patterns to Plant Immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, A.; Ren, Y.; Wu, F.; Wang, G.; Xu, Y.; Lei, C.; Zhu, S.; Pan, T.; et al. A Cyclic Nucleotide-Gated Channel Mediates Cytoplasmic Calcium Elevation and Disease Resistance in Rice. Cell Res. 2019, 29, 820–831. [Google Scholar] [CrossRef]
- Xu, G.; Moeder, W.; Yoshioka, K.; Shan, L. A Tale of Many Families: Calcium Channels in Plant Immunity. Plant Cell 2022, 34, 1551–1567. [Google Scholar] [CrossRef]
- Boudsocq, M.; Sheen, J. CDPKs in Immune and Stress Signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.-P.; Schulze, W.X.; Romeis, T. Calcium-Dependent Protein Kinase/NADPH Oxidase Activation Circuit Is Required for Rapid Defense Signal Propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [Green Version]
- Marcec, M.J.; Tanaka, K. Crosstalk between Calcium and ROS Signaling during Flg22-Triggered Immune Response in Arabidopsis Leaves. Plants 2022, 11, 14. [Google Scholar] [CrossRef]
- Albert, I.; Böhm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23–SOBIR1–BAK1 Complex Mediates NLP-Triggered Immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef]
- Böhm, H.; Albert, I.; Oome, S.; Raaymakers, T.M.; van den Ackerveken, G.; Nürnberger, T. A Conserved Peptide Pattern from a Widespread Microbial Virulence Factor Triggers Pattern-Induced Immunity in Arabidopsis. PLoS Pathog. 2014, 10, e1004491. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.-L.; Zhang, L.; Pruitt, R.; Zaidem, M.; Brugman, R.; Ma, X.; Krol, E.; Perraki, A.; Kilian, J.; Grossmann, G.; et al. Comparing Arabidopsis Receptor Kinase and Receptor Protein-Mediated Immune Signaling Reveals BIK1-Dependent Differences. New Phytol. 2019, 221, 2080–2095. [Google Scholar] [CrossRef]
- Dias, M.G.; Soleimani, F.; Monaghan, J. Activation and Turnover of the Plant Immune Signaling Kinase BIK1: A Fine Balance. Essays Biochem. 2022, 66, 207–218. [Google Scholar] [CrossRef]
- Willmann, R.; Lajunen, H.M.; Erbs, G.; Newman, M.-A.; Kolb, D.; Tsuda, K.; Katagiri, F.; Fliegmann, J.; Bono, J.-J.; Cullimore, J.V.; et al. Arabidopsis Lysin-Motif Proteins LYM1 LYM3 CERK1 Mediate Bacterial Peptidoglycan Sensing and Immunity to Bacterial Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 19824–19829. [Google Scholar] [CrossRef] [Green Version]
- Shinya, T.; Yamaguchi, K.; Desaki, Y.; Yamada, K.; Narisawa, T.; Kobayashi, Y.; Maeda, K.; Suzuki, M.; Tanimoto, T.; Takeda, J.; et al. Selective Regulation of the Chitin-Induced Defense Response by the Arabidopsis Receptor-like Cytoplasmic Kinase PBL27. Plant J. 2014, 79, 56–66. [Google Scholar] [CrossRef]
- Gong, B.-Q.; Guo, J.; Zhang, N.; Yao, X.; Wang, H.-B.; Li, J.-F. Cross-Microbial Protection via Priming a Conserved Immune Co-Receptor through Juxtamembrane Phosphorylation in Plants. Cell Host Microbe 2019, 26, 810–822. [Google Scholar] [CrossRef]
- Halter, T.; Imkampe, J.; Mazzotta, S.; Wierzba, M.; Postel, S.; Bücherl, C.; Kiefer, C.; Stahl, M.; Chinchilla, D.; Wang, X.; et al. The Leucine-Rich Repeat Receptor Kinase BIR2 is a Negative Regulator of BAK1 in Plant Immunity. Curr. Biol. 2014, 24, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Imkampe, J.; Halter, T.; Huang, S.; Schulze, S.; Mazzotta, S.; Schmidt, N.; Manstretta, R.; Postel, S.; Wierzba, M.; Yang, Y.; et al. The Arabidopsis Leucine-Rich Repeat Receptor Kinase BIR3 Negatively Regulates BAK1 Receptor Complex Formation and Stabilizes BAK1. Plant Cell 2017, 29, 2285–2303. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Wang, X.; Wang, D.; Xu, F.; Ding, X.; Zhang, Z.; Bi, D.; Cheng, Y.T.; Chen, S.; Li, X.; et al. Regulation of Cell Death and Innate Immunity by Two Receptor-like Kinases in Arabidopsis. Cell Host Microbe 2009, 6, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Ding, P.; Lian, K.; Wang, J.; Ma, M.; Li, L.; Li, L.; Li, M.; Zhang, X.; Chen, S.; et al. Arabidopsis Heterotrimeric G Proteins Regulate Immunity by Directly Coupling to the FLS2 Receptor. eLife 2016, 5, e13568. [Google Scholar] [CrossRef]
- Kim, H.-S.; Desveaux, D.; Singer, A.U.; Patel, P.; Sondek, J.; Dangl, J.L. The Pseudomonas Syringae Effector AvrRpt2 Cleaves Its C-Terminally Acylated Target, RIN4, from Arabidopsis Membranes to Block RPM1 Activation. Proc. Natl. Acad. Sci. USA 2005, 102, 6496–6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, A.J.; da Cunha, L.; Mackey, D. Separable Fragments and Membrane Tethering of Arabidopsis RIN4 Regulate Its Suppression of PAMP-Triggered Immunity. Plant Cell 2011, 23, 3798–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Elmore, J.M.; Lin, Z.-J.D.; Coaker, G. A Receptor-like Cytoplasmic Kinase Phosphorylates the Host Target RIN4, Leading to the Activation of a Plant Innate Immune Receptor. Cell Host Microbe 2011, 9, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.-H.; El-Kasmi, F.; He, Y.; Loehr, A.; Dangl, J.L. A Plant Phosphoswitch Platform Repeatedly Targeted by Type III Effector Proteins Regulates the Output of Both Tiers of Plant Immune Receptors. Cell Host Microbe 2014, 16, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardar, A.; Nandi, A.K.; Chattopadhyay, D. CBL-Interacting Protein Kinase 6 Negatively Regulates Immune Response to Pseudomonas Syringae in Arabidopsis. J. Exp. Bot. 2017, 68, 3573–3584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Gilmour, S.J.; Chao, L.; Park, S.; Thomashow, M.F. Arabidopsis CAMTA Transcription Factors Regulate Pipecolic Acid Biosynthesis and Priming of Immunity Genes. Mol. Plant 2020, 13, 157–168. [Google Scholar] [CrossRef]
- Sun, T.; Huang, J.; Xu, Y.; Verma, V.; Jing, B.; Sun, Y.; Orduna, A.R.; Tian, H.; Huang, X.; Xia, S.; et al. Redundant CAMTA Transcription Factors Negatively Regulate the Biosynthesis of Salicylic Acid and N-Hydroxypipecolic Acid by Modulating the Expression of SARD1 and CBP60g. Mol. Plant 2020, 13, 144–156. [Google Scholar] [CrossRef]
- Jacob, F.; Kracher, B.; Mine, A.; Seyfferth, C.; Blanvillain-Baufumé, S.; Parker, J.E.; Tsuda, K.; Schulze-Lefert, P.; Maekawa, T. A Dominant-Interfering Camta3 Mutation Compromises Primary Transcriptional Outputs Mediated by Both Cell Surface and Intracellular Immune Receptors in Arabidopsis Thaliana. New Phytol. 2018, 217, 1667–1680. [Google Scholar] [CrossRef] [Green Version]
- Irieda, H.; Inoue, Y.; Mori, M.; Yamada, K.; Oshikawa, Y.; Saitoh, H.; Uemura, A.; Terauchi, R.; Kitakura, S.; Kosaka, A.; et al. Conserved Fungal Effector Suppresses PAMP-Triggered Immunity by Targeting Plant Immune Kinases. Proc. Natl. Acad. Sci. USA 2019, 116, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-H.; Abd-El-Haliem, A.; Bozkurt, T.O.; Belhaj, K.; Terauchi, R.; Vossen, J.H.; Kamoun, S. NLR Network Mediates Immunity to Diverse Plant Pathogens. Proc. Natl. Acad. Sci. USA 2017, 114, 8113–8118. [Google Scholar] [CrossRef]
- Baggs, E.; Dagdas, G.; Krasileva, K. NLR Diversity, Helpers and Integrated Domains: Making Sense of the NLR IDentity. Curr. Opin. Plant Biol. 2017, 38, 59–67. [Google Scholar] [CrossRef]
- Steuernagel, B.; Witek, K.; Krattinger, S.G.; Ramirez-Gonzalez, R.H.; Schoonbeek, H.; Yu, G.; Baggs, E.; Witek, A.I.; Yadav, I.; Krasileva, K.V.; et al. The NLR-Annotator Tool Enables Annotation of the Intracellular Immune Receptor Repertoire. Plant Physiol. 2020, 183, 468–482. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.-X.; Meyers, B.C.; Chen, J.-Q.; Tian, D.; Yang, S. Tracing the Origin and Evolutionary History of Plant Nucleotide-Binding Site–Leucine-Rich Repeat (NBS-LRR) Genes. New Phytol. 2012, 193, 1049–1063. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, W.; Zhang, T.; Gong, Z.; Zhao, H.; Han, G.-Z. Out of Water: The Origin and Early Diversification of Plant R-Genes. Plant Physiol. 2018, 177, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Van Ooijen, G.; Mayr, G.; Kasiem, M.M.A.; Albrecht, M.; Cornelissen, B.J.C.; Takken, F.L.W. Structure-Function Analysis of the NB-ARC Domain of Plant Disease Resistance Proteins. J. Exp. Bot. 2008, 59, 1383–1397. [Google Scholar] [CrossRef] [Green Version]
- Jubic, L.M.; Saile, S.; Furzer, O.J.; El Kasmi, F.; Dangl, J.L. Help Wanted: Helper NLRs and Plant Immune Responses. Curr. Opin. Plant Biol. 2019, 50, 82–94. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Dong, O.X.; Xia, S.; Liang, W.; Bao, Y.; Wasteneys, G.; Li, X. Differential Regulation of TNL-mediated Immune Signaling by Redundant Helper CNLs. New Phytol. 2019, 222, 938–953. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, Z.-M. A Unique RPW8-Encoding Class of Genes That Originated in Early Land Plants and Evolved through Domain Fission, Fusion, and Duplication. Sci. Rep. 2016, 6, 32923. [Google Scholar] [CrossRef] [Green Version]
- Cannon, S.B.; Zhu, H.; Baumgarten, A.M.; Spangler, R.; May, G.; Cook, D.R.; Young, N.D. Diversity, Distribution, and Ancient Taxonomic Relationships within the TIR and Non-TIR NBS-LRR Resistance Gene Subfamilies. J. Mol. Evol. 2002, 54, 548–562. [Google Scholar] [CrossRef]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR Proteins: Adaptable Guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The Plant Hypersensitive Response: Concepts, Control and Consequences. Mol. Plant Pathol. 2019, mpp.12821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourelis, J.; Adachi, H. Activation and Regulation of NLR Immune Receptor Networks. Plant Cell Physiol. 2022, pcac116. [Google Scholar] [CrossRef] [PubMed]
- Ao, K.; Li, X. Indirect Recognition of Pathogen Effectors by NLRs. Essays Biochem. 2022, 66, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Derevnina, L.; Kamoun, S. NLR Singletons, Pairs, and Networks: Evolution, Assembly, and Regulation of the Intracellular Immunoreceptor Circuitry of Plants. Curr. Opin. Plant Biol. 2019, 50, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongus, J.A.; Parker, J.E. EDS1 Signalling: At the Nexus of Intracellular and Surface Receptor Immunity. Curr. Opin. Plant Biol. 2021, 62, 102039. [Google Scholar] [CrossRef] [PubMed]
- Baudin, M.; Hassan, J.A.; Schreiber, K.J.; Lewis, J.D. Analysis of the ZAR1 Immune Complex Reveals Determinants for Immunity and Molecular Interactions. Plant Physiol. 2017, 174, 2038–2053. [Google Scholar] [CrossRef] [Green Version]
- Bi, G.; Su, M.; Li, N.; Liang, Y.; Dang, S.; Xu, J.; Hu, M.; Wang, J.; Zou, M.; Deng, Y.; et al. The ZAR1 Resistosome is a Calcium-Permeable Channel Triggering Plant Immune Signaling. Cell 2021, 184, 3528–3541.e12. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Hu, M.; Wu, S.; Qi, J.; Wang, G.; Han, Z.; Qi, Y.; Gao, N.; Wang, H.-W.; et al. Ligand-Triggered Allosteric ADP Release Primes a Plant NLR Complex. Science 2019, 364, eaav5868. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.-W.; Zhou, J.-M.; Chai, J. Reconstitution and Structure of a Plant NLR Resistosome Conferring Immunity. Science 2019, 364, eaav5870. [Google Scholar] [CrossRef]
- Reuber, T.L.; Ausubel, F.M. Isolation of Arabidopsis Genes That Differentiate between Resistance Responses Mediated by the RPS2 and RPM1 Disease Resistance Genes. Plant Cell 1996, 8, 241–249. [Google Scholar] [CrossRef]
- Axtell, M.J.; Staskawicz, B.J. Initiation of RPS2-Specified Disease Resistance in Arabidopsis Is Coupled to the AvrRpt2-Directed Elimination of RIN4. Cell 2003, 112, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Afzal, A.J.; Kim, J.H.; Mackey, D. The Role of NOI-Domain Containing Proteins in Plant Immune Signaling. BMC Genom. 2013, 14, 327. [Google Scholar] [CrossRef] [Green Version]
- Mackey, D.; Holt, B.F.; Wiig, A.; Dangl, J.L. RIN4 Interacts with Pseudomonas Syringae Type III Effector Molecules and is Required for RPM1-Mediated Resistance in Arabidopsis. Cell 2002, 108, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Redditt, T.J.; Chung, E.-H.; Karimi, H.Z.; Rodibaugh, N.; Zhang, Y.; Trinidad, J.C.; Kim, J.H.; Zhou, Q.; Shen, M.; Dangl, J.L.; et al. AvrRpm1 Functions as an ADP-Ribosyl Transferase to Modify NOI-Domain Containing Proteins, Including Arabidopsis and Soybean RPM1-Interacting Protein 4. Plant Cell 2019, 31, 2664–2681. [Google Scholar] [CrossRef] [Green Version]
- Coppinger, P.; Repetti, P.P.; Day, B.; Dahlbeck, D.; Mehlert, A.; Staskawicz, B.J. Overexpression of the Plasma Membrane-Localized NDR1 Protein Results in Enhanced Bacterial Disease Resistance in Arabidopsis Thaliana: Characterization of NDR1. Plant J. 2004, 40, 225–237. [Google Scholar] [CrossRef]
- Knepper, C.; Savory, E.A.; Day, B. The Role of NDR1 in Pathogen Perception and Plant Defense Signaling. Plant Signal. Behav. 2011, 6, 1114–1116. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Hirabuchi, A.; Sugihara, Y.; Abe, A.; Takeda, T.; Kobayashi, M.; Hiraka, Y.; Kanzaki, E.; Oikawa, K.; Saitoh, H.; et al. A Genetically Linked Pair of NLR Immune Receptors Shows Contrasting Patterns of Evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2116896119. [Google Scholar] [CrossRef]
- Castel, B.; Ngou, P.-M.; Cevik, V.; Redkar, A.; Kim, D.-S.; Yang, Y.; Ding, P.; Jones, J.D.G. Diverse NLR Immune Receptors Activate Defence via the RPW8-NLR NRG1. New Phytol. 2019, 222, 966–980. [Google Scholar] [CrossRef]
- Saile, S.C.; Jacob, P.; Castel, B.; Jubic, L.M.; Salas-Gonzáles, I.; Bäcker, M.; Jones, J.D.G.; Dangl, J.L.; Kasmi, F.E. Two Unequally Redundant “Helper” Immune Receptor Families Mediate Arabidopsis Thaliana Intracellular “Sensor” Immune Receptor Functions. PLoS Biol. 2020, 18, e3000783. [Google Scholar] [CrossRef]
- Bernoux, M.; Ellis, J.G.; Dodds, P.N. New Insights in Plant Immunity Signaling Activation. Curr. Opin. Plant Biol. 2011, 14, 512–518. [Google Scholar] [CrossRef]
- Williams, S.J.; Sohn, K.H.; Wan, L.; Bernoux, M.; Sarris, P.F.; Segonzac, C.; Ve, T.; Ma, Y.; Saucet, S.B.; Ericsson, D.J.; et al. Structural Basis for Assembly and Function of a Heterodimeric Plant Immune Receptor. Science 2014, 344, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jia, A.; Song, W.; Hessler, G.; Meng, Y.; Sun, Y.; Xu, L.; Laessle, H.; Jirschitzka, J.; Ma, S.; et al. Identification and Receptor Mechanism of TIR-Catalyzed Small Molecules in Plant Immunity. Science 2022, 377, eabq3297. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lapin, D.; Liu, L.; Sun, Y.; Song, W.; Zhang, X.; Logemann, E.; Yu, D.; Wang, J.; Jirschitzka, J.; et al. Direct Pathogen-Induced Assembly of an NLR Immune Receptor Complex to Form a Holoenzyme. Science 2020, 370, eabe3069. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Song, W.; Tan, E.Y.J.; Liu, L.; Cao, Y.; Jirschitzka, J.; Li, E.; Logemann, E.; Xu, C.; Huang, S.; et al. TIR Domains of Plant Immune Receptors Are 2′,3′-CAMP/CGMP Synthetases Mediating Cell Death. Cell 2022, 185, 2370–2386. [Google Scholar] [CrossRef] [PubMed]
- Lapin, D.; Bhandari, D.D.; Parker, J.E. Origins and Immunity Networking Functions of EDS1 Family Proteins. Annu. Rev. Phytopathol. 2020, 58, 253–276. [Google Scholar] [CrossRef]
- Dongus, J.A.; Bhandari, D.D.; Penner, E.; Lapin, D.; Stolze, S.C.; Harzen, A.; Patel, M.; Archer, L.; Dijkgraaf, L.; Shah, J.; et al. Cavity Surface Residues of PAD4 and SAG101 Contribute to EDS1 Dimer Signaling Specificity in Plant Immunity. Plant J. Cell Mol. Biol. 2022, 110, 1415–1432. [Google Scholar] [CrossRef]
- Voss, M.; Toelzer, C.; Bhandari, D.D.; Parker, J.E.; Niefind, K. Arabidopsis Immunity Regulator EDS1 in a PAD4/SAG101-Unbound Form is a Monomer with an Inherently Inactive Conformation. J. Struct. Biol. 2019, 208, 107390. [Google Scholar] [CrossRef]
- Wagner, S.; Stuttmann, J.; Rietz, S.; Guerois, R.; Brunstein, E.; Bautor, J.; Niefind, K.; Parker, J.E. Structural Basis for Signaling by Exclusive EDS1 Heteromeric Complexes with SAG101 or PAD4 in Plant Innate Immunity. Cell Host Microbe 2013, 14, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Gantner, J.; Ordon, J.; Kretschmer, C.; Guerois, R.; Stuttmann, J. An EDS1-SAG101 Complex Is Essential for TNL-Mediated Immunity in Nicotiana Benthamiana. Plant Cell 2019, 31, 2456–2474. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, M.; Serrano, I.; Rodibaugh, N.; Bhandari, D.D.; Bautor, J.; Parker, J.E.; Innes, R.W. Arabidopsis EDR1 Protein Kinase Regulates the Association of EDS1 and PAD4 to Inhibit Cell Death. Mol. Plant-Microbe Interact. 2020, 33, 693–703. [Google Scholar] [CrossRef]
- Jacob, P.; Kim, N.H.; Wu, F.; El-Kasmi, F.; Chi, Y.; Walton, W.G.; Furzer, O.J.; Lietzan, A.D.; Sunil, S.; Kempthorn, K.; et al. Plant “Helper” Immune Receptors Are Ca2+-Permeable Nonselective Cation Channels. Science 2021, 373, 420–425. [Google Scholar] [CrossRef]
- Jia, A.; Huang, S.; Song, W.; Wang, J.; Meng, Y.; Sun, Y.; Xu, L.; Laessle, H.; Jirschitzka, J.; Hou, J.; et al. TIR-Catalyzed ADP-Ribosylation Reactions Produce Signaling Molecules for Plant Immunity. Science 2022, 377, eabq8180. [Google Scholar] [CrossRef]
- Sun, X.; Lapin, D.; Feehan, J.M.; Stolze, S.C.; Kramer, K.; Dongus, J.A.; Rzemieniewski, J.; Blanvillain-Baufumé, S.; Harzen, A.; Bautor, J.; et al. Pathogen Effector Recognition-Dependent Association of NRG1 with EDS1 and SAG101 in TNL Receptor Immunity. Nat. Commun. 2021, 12, 3335. [Google Scholar] [CrossRef]
- Lapin, D.; Kovacova, V.; Sun, X.; Dongus, J.A.; Bhandari, D.; von Born, P.; Bautor, J.; Guarneri, N.; Rzemieniewski, J.; Stuttmann, J.; et al. A Coevolved EDS1-SAG101-NRG1 Module Mediates Cell Death Signaling by TIR-Domain Immune Receptors. Plant Cell 2019, 31, 2430–2455. [Google Scholar] [CrossRef] [Green Version]
- Lapin, D.; Johanndrees, O.; Wu, Z.; Li, X.; Parker, J.E. Molecular Innovations in Plant TIR-Based Immunity Signaling. Plant Cell 2022, 34, 1479–1496. [Google Scholar] [CrossRef]
- Maruta, N.; Burdett, H.; Lim, B.Y.J.; Hu, X.; Desa, S.; Manik, M.K.; Kobe, B. Structural Basis of NLR Activation and Innate Immune Signalling in Plants. Immunogenetics 2022, 74, 5–26. [Google Scholar] [CrossRef]
- Ma, W.; Smigel, A.; Tsai, Y.-C.; Braam, J.; Berkowitz, G.A. Innate Immunity Signaling: Cytosolic Ca2+ Elevation is Linked to Downstream Nitric Oxide Generation through the Action of Calmodulin or a Calmodulin-like Protein. Plant Physiol. 2008, 148, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Dalio, R.J.D.; Paschoal, D.; Arena, G.D.; Magalhães, D.M.; Oliveira, T.S.; Merfa, M.V.; Maximo, H.J.; Machado, M.A. Hypersensitive Response: From NLR Pathogen Recognition to Cell Death Response. Ann. Appl. Biol. 2021, 178, 268–280. [Google Scholar] [CrossRef]
- Domínguez-Ferreras, A.; Kiss-Papp, M.; Jehle, A.K.; Felix, G.; Chinchilla, D. An Overdose of the Arabidopsis Coreceptor BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 or Its Ectodomain Causes Autoimmunity in a SUPPRESSOR OF BIR1-1-Dependent Manner. Plant Physiol. 2015, 168, 1106–1121. [Google Scholar] [CrossRef] [Green Version]
- Lolle, S.; Greeff, C.; Petersen, K.; Roux, M.; Jensen, M.K.; Bressendorff, S.; Rodriguez, E.; Sømark, K.; Mundy, J.; Petersen, M. Matching NLR Immune Receptors to Autoimmunity in Camta3 Mutants Using Antimorphic NLR Alleles. Cell Host Microbe 2017, 21, 518–529. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Mu, Z.; Mawsheng, C.; Xuewei, C.; Jing, W. Deciphering Rice Lesion Mimic Mutants to Understand Molecular Network Governing Plant Immunity and Growth. Rice Sci. 2020, 27, 278–288. [Google Scholar] [CrossRef]
- Freh, M.; Gao, J.; Petersen, M.; Panstruga, R. Plant Autoimmunity—Fresh Insights into an Old Phenomenon. Plant Physiol. 2022, 188, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xiong, J.; Xu, L.; Chen, X.; Li, W. Recent advances in plant immunity with cell death: A review. J. Integr. Agric. 2021, 21, 610–620. [Google Scholar] [CrossRef]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A Core Function of EDS1 with PAD4 Is to Protect the Salicylic Acid Defense Sector in Arabidopsis Immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Meier, N.; Dinesh-Kumar, S.P. Parasite Effectors Target Helper NLRs in Plants to Suppress Immunity-Related Cell Death. PLoS Biol. 2021, 19, e3001395. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Du, J.; Zhan, Y.; Sun, D.; Zhao, J.; Zhang, S.; Li, J.; He, K. Both Light-Induced SA Accumulation and ETI Mediators Contribute to the Cell Death Regulated by BAK1 and BKK1. Front. Plant Sci. 2017, 8, 622. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Gao, Y.; Zhan, Y.; Kui, H.; Liu, H.; Yan, L.; Kemmerling, B.; Zhou, J.-M.; He, K.; Li, J. Loss of the Common Immune Coreceptor BAK1 Leads to NLR-Dependent Cell Death. Proc. Natl. Acad. Sci. USA 2020, 117, 27044–27053. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, L.; Liu, X.; Zhang, Y.; Li, X. TIR Signal Promotes Interactions between Lipase-like Proteins and ADR1-L1 Receptor and ADR1-L1 Oligomerization. Plant Physiol. 2021, 187, 681–686. [Google Scholar] [CrossRef]
- Roux, M.; Schwessinger, B.; Albrecht, C.; Chinchilla, D.; Jones, A.; Holton, N.; Malinovsky, F.G.; Tör, M.; de Vries, S.; Zipfel, C. The Arabidopsis Leucine-Rich Repeat Receptor-like Kinases BAK1/SERK3 and BKK1/SERK4 Are Required for Innate Immunity to Hemibiotrophic and Biotrophic Pathogens. Plant Cell 2011, 23, 2440–2455. [Google Scholar] [CrossRef] [Green Version]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual Potentiation of Plant Immunity by Cell-Surface and Intracellular Receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Kadota, Y.; Liebrand, T.W.H.; Goto, Y.; Sklenar, J.; Derbyshire, P.; Menke, F.L.H.; Torres, M.; Molina, A.; Zipfel, C.; Coaker, G.; et al. Quantitative Phosphoproteomic Analysis Reveals Common Regulatory Mechanisms between Effector- and PAMP-triggered Immunity in Plants. New Phytol. 2019, 221, 2160–2175. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI Crosstalk: An Integrative View of Plant Immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.-F. Pattern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Clough, S.J.; Fengler, K.A.; Yu, I.; Lippok, B.; Smith, R.K.; Bent, A.F. The Arabidopsis Dnd1 “Defense, No Death” Gene Encodes a Mutated Cyclic Nucleotide-Gated Ion Channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Tang, Y.; Wang, J.; Zeng, Y.; Sun, H.; Zheng, Z.; Su, R.; Schneeberger, K.; Parker, J.E.; Cui, H. A Mis-Regulated Cyclic Nucleotide-Gated Channel Mediates Cytosolic Calcium Elevation and Activates Immunity in Arabidopsis. New Phytol. 2021, 230, 1078–1094. [Google Scholar] [CrossRef]
- Dong, X. NPR1, All Things Considered. Curr. Opin. Plant Biol. 2004, 7, 547–552. [Google Scholar] [CrossRef]
- Nawrath, C.; Heck, S.; Parinthawong, N.; Métraux, J.-P. EDS5, an Essential Component of Salicylic Acid–Dependent Signaling for Disease Resistance in Arabidopsis, is a Member of the MATE Transporter Family. Plant Cell 2002, 14, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tsuda, K.; Sato, M.; Cohen, J.D.; Katagiri, F.; Glazebrook, J. Arabidopsis CaM Binding Protein CBP60g Contributes to MAMP-Induced SA Accumulation and is Involved in Disease Resistance against Pseudomonas Syringae. PLoS Pathog. 2009, 5, e1000301. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tsuda, K.; Truman, W.; Sato, M.; Nguyen, L.V.; Katagiri, F.; Glazebrook, J. CBP60g and SARD1 Play Partially Redundant Critical Roles in Salicylic Acid Signaling: Role of CBP60 Proteins in Salicylic Acid Signaling. Plant J. 2011, 67, 1029–1041. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of Salicylic Acid in Plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.; Li, Y.; Zhang, Q.; Ding, Y.; Zhang, Y. ChIP-Seq Reveals Broad Roles of SARD1 and CBP60g in Regulating Plant Immunity. Nat. Commun. 2015, 6, 10159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurkowski, G.I.; Smith, R.K.; Yu, I.; Ham, J.H.; Sharma, S.B.; Klessig, D.F.; Fengler, K.A.; Bent, A.F. Arabidopsis DND2, a Second Cyclic Nucleotide-Gated Ion Channel Gene for Which Mutation Causes the “Defense, No Death” Phenotype. Mol. Plant-Microbe Interact. 2004, 17, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, J.E.; Hessler, G.; Cui, H. A New Biochemistry Connecting Pathogen Detection to Induced Defense in Plants. New Phytol. 2021, 234, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Iakovidis, M.; Chung, E.-H.; Saile, S.C.; Sauberzweig, E.; El Kasmi, F. The Emerging Frontier of Plant Immunity’s Core Hubs. FEBS J. 2022, 289. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wu, Z.; Chen, S.; Ao, K.; Huang, W.; Yaghmaiean, H.; Sun, T.; Xu, F.; Zhang, Y.; Wang, S.; et al. Activation of TIR Signalling Boosts Pattern-Triggered Immunity. Nature 2021, 598, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Locci, F.; Wanke, F.; Zhang, L.; Saile, S.C.; Joe, A.; Karelina, D.; Hua, C.; Fröhlich, K.; Wan, W.-L.; et al. The EDS1–PAD4–ADR1 Node Mediates Arabidopsis Pattern-Triggered Immunity. Nature 2021, 598, 495–499. [Google Scholar] [CrossRef]
- Bjornson, M.; Pimprikar, P.; Nürnberger, T.; Zipfel, C. The Transcriptional Landscape of Arabidopsis Thaliana Pattern-Triggered Immunity. Nat. Plants 2021, 7, 579–586. [Google Scholar] [CrossRef]
- Nandety, R.S.; Caplan, J.L.; Cavanaugh, K.; Perroud, B.; Wroblewski, T.; Michelmore, R.W.; Meyers, B.C. The Role of TIR-NBS and TIR-X Proteins in Plant Basal Defense Responses. Plant Physiol. 2013, 162, 1459–1472. [Google Scholar] [CrossRef] [Green Version]
- López-Márquez, D.; Del-Espino, Á.; López-Pagán, N.; Rodríguez-Negrete, E.A.; Rubio-Somoza, I.; Ruiz-Albert, J.; Bejarano, E.R.; Beuzón, C.R. MiR825-5p Targets the TIR-NBS-LRR Gene MIST1 and down-Regulates Basal Immunity against Pseudomonas Syringae in Arabidopsis. J. Exp. Bot. 2021, 72, 7316–7334. [Google Scholar] [CrossRef]
- Wiermer, M.; Feys, B.J.; Parker, J.E. Plant Immunity: The EDS1 Regulatory Node. Curr. Opin. Plant Biol. 2005, 8, 383–389. [Google Scholar] [CrossRef]
- Bonardi, V.; Tang, S.; Stallmann, A.; Roberts, M.; Cherkis, K.; Dangl, J.L. Expanded Functions for a Family of Plant Intracellular Immune Receptors beyond Specific Recognition of Pathogen Effectors. Proc. Natl. Acad. Sci. USA 2011, 108, 16463–16468. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.-Q.; Xue, J.-Y.; Wu, P.; Zhang, Y.-M.; Wu, Y.; Hang, Y.-Y.; Wang, B.; Chen, J.-Q. Large-Scale Analyses of Angiosperm Nucleotide-Binding Site-Leucine-Rich Repeat Genes Reveal Three Anciently Diverged Classes with Distinct Evolutionary Patterns. Plant Physiol. 2016, 170, 2095–2109. [Google Scholar] [CrossRef] [Green Version]
- Andolfo, G.; Villano, C.; Errico, A.; Frusciante, L.; Carputo, D.; Aversano, R.; Ercolano, M.R. Inferring RPW8-NLRs’s Evolution Patterns in Seed Plants: Case Study in Vitis Vinifera. Planta 2019, 251, 32. [Google Scholar] [CrossRef]
- Baggs, E.L.; Monroe, J.G.; Thanki, A.S.; O’Grady, R.; Schudoma, C.; Haerty, W.; Krasileva, K.V. Convergent Loss of an EDS1/PAD4 Signaling Pathway in Several Plant Lineages Reveals Coevolved Components of Plant Immunity and Drought Response [OPEN]. Plant Cell 2020, 32, 2158–2177. [Google Scholar] [CrossRef]
- Collier, S.M.; Hamel, L.-P.; Moffett, P. Cell Death Mediated by the N-Terminal Domains of a Unique and Highly Conserved Class of NB-LRR Protein. Mol. Plant-Microbe Interact. MPMI 2011, 24, 918–931. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zeng, Z.; Zhang, Y.-M.; Li, Q.; Jiang, X.-M.; Jiang, Z.; Tang, J.-H.; Chen, D.; Wang, Q.; Chen, J.-Q.; et al. An Angiosperm NLR Atlas Reveals That NLR Gene Reduction is Associated with Ecological Specialization and Signal Transduction Component Deletion. Mol. Plant 2021, 14, 2015–2031. [Google Scholar] [CrossRef]
- Chini, A.; Grant, J.J.; Seki, M.; Shinozaki, K.; Loake, G.J. Drought Tolerance Established by Enhanced Expression of the CC-NBS-LRR Gene, ADR1, Requires Salicylic Acid, EDS1 and ABI1. Plant J. 2004, 38, 810–822. [Google Scholar] [CrossRef]
- Wituszyńska, W.; Ślesak, I.; Vanderauwera, S.; Szechyńska-Hebda, M.; Kornaś, A.; van der Kelen, K.; Mühlenbock, P.; Karpińska, B.; Maćkowski, S.; van Breusegem, F.; et al. Lesion Simulating Disease1, Enhanced Disease Susceptibility1, and Phytoalexin Deficient4 Conditionally Regulate Cellular Signaling Homeostasis, Photosynthesis, Water Use Efficiency, and Seed Yield in Arabidopsis. Plant Physiol. 2013, 161, 1795–1805. [Google Scholar] [CrossRef] [Green Version]
- Szechyńska-Hebda, M.; Czarnocka, W.; Hebda, M.; Karpiński, S. PAD4, LSD1 and EDS1 Regulate Drought Tolerance, Plant Biomass Production, and Cell Wall Properties. Plant Cell Rep. 2016, 35, 527–539. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Holmes, E.C.; Rajniak, J.; Kim, J.-G.; Tang, S.; Fischer, C.R.; Mudgett, M.B.; Sattely, E.S. N-Hydroxy-Pipecolic Acid is a Mobile Metabolite That Induces Systemic Disease Resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4920–E4929. [Google Scholar] [CrossRef]
- Johanndrees, O.; Baggs, E.L.; Uhlmann, C.; Locci, F.; Läßle, H.L.; Melkonian, K.; Käufer, K.; Dongus, J.A.; Nakagami, H.; Krasileva, K.V.; et al. Differential EDS1 Requirement for Cell Death Activities of Plant TIR-Domain Proteins. bioRxiv 2021. [Google Scholar] [CrossRef]
- Meyers, B.C.; Morgante, M.; Michelmore, R.W. TIR-X and TIR-NBS Proteins: Two New Families Related to Disease Resistance TIR-NBS-LRR Proteins Encoded in Arabidopsis and Other Plant Genomes: TX and TN Proteins. Plant J. 2002, 32, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.E.; Mesarich, C.H.; Thomma, B.P.H.J. Understanding Plant Immunity as a Surveillance System to Detect Invasion. Annu. Rev. Phytopathol. 2015, 53, 541–563. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Finch, P.; Butt, V.S.; Whitelegge, J.P.; Souda, P.; Ausubel, F.M.; Bolwell, G.P. A Peroxidase-Dependent Apoplastic Oxidative Burst in Cultured Arabidopsis Cells Functions in MAMP-Elicited Defense. Plant Physiol. 2012, 158, 2013–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammarella, N.D.; Cheng, Z.; Fu, Z.Q.; Daudi, A.; Bolwell, G.P.; Dong, X.; Ausubel, F.M. Apoplastic Peroxidases Are Required for Salicylic Acid-Mediated Defense against Pseudomonas Syringae. Phytochemistry 2015, 112, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, F.; Tsuda, K. Understanding the Plant Immune System. Mol. Plant-Microbe Interact. 2010, 23, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Chen, X.; Lin, W.; Chen, S.; Lu, D.; Niu, Y.; Li, L.; Cheng, C.; McCormack, M.; Sheen, J.; et al. Bifurcation of Arabidopsis NLR Immune Signaling via Ca2+-Dependent Protein Kinases. PLoS Pathog. 2013, 9, e1003127. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.A.J.; Laarhoven, L.J.J.; Harren, F.J.M.; Hall, M.A.; Smith, A.R. Nitric Oxide Interacts with Salicylate to Regulate Biphasic Ethylene Production during the Hypersensitive Response. Plant Physiol. 2008, 148, 1537–1546. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A. ROS in Biotic Interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.-M. Apoplastic ROS Signaling in Plant Immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose Deposition: A Multifaceted Plant Defense Response. Mol. Plant-Microbe Interact. 2011, 24, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Voigt, C.A. Callose-Mediated Resistance to Pathogenic Intruders in Plant Defense-Related Papillae. Front. Plant Sci. 2014, 5, 443–448. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the Generation of Reactive Oxygen Species in Plant Defence—A Broad Perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Vainonen, J.P.; Wrzaczek, M.; Kangasjärvi, J. ROS-Talk—How the Apoplast, the Chloroplast, and the Nucleus Get the Message Through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-Induced Reactive Oxygen Species Compartmentalization, Perception and Signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and Extracellular Journey of the Phytohormone Salicylic Acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network Properties of Robust Immunity in Plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X. Salicylic Acid: Biosynthesis, Perception, and Contributions to Plant Immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Li, X.; Zhang, Y. Biosynthesis and Regulation of Salicylic Acid and N-Hydroxypipecolic Acid in Plant Immunity. Mol. Plant 2020, 13, 31–41. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachroo, A.; Robin, G.P. Systemic Signaling during Plant Defense. Curr. Opin. Plant Biol. 2013, 16, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling Mechanisms Underlying Systemic Acquired Resistance to Microbial Pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-Derived Biosynthesis of the Plant Stress Hormone Salicylic Acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.-K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Vogelmann, K.; Drechsel, G.; Bergler, J.; Subert, C.; Philippar, K.; Soll, J.; Engelmann, J.C.; Engelsdorf, T.; Voll, L.M.; Hoth, S. Early Senescence and Cell Death in Arabidopsis Saul1 Mutants Involves the PAD4 -Dependent Salicylic Acid Pathway. Plant Physiol. 2012, 159, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Qiu, J.; Zhou, Y.; Bhandari, D.D.; Zhao, C.; Bautor, J.; Parker, J.E. Antagonism of Transcription Factor MYC2 by EDS1/PAD4 Complexes Bolsters Salicylic Acid Defense in Arabidopsis Effector-Triggered Immunity. Mol. Plant 2018, 11, 1053–1066. [Google Scholar] [CrossRef]
- Zhou, N.; Tootle, T.L.; Tsui, F.; Klessig, D.F.; Glazebrook, J. PAD4 Functions Upstream from Salicylic Acid to Control Defense Responses in Arabidopsis. Plant Cell 1998, 10, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Seyfferth, C.; Tsuda, K. Salicylic Acid Signal Transduction: The Initiation of Biosynthesis, Perception and Transcriptional Reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Borad, V.; Sriram, S. Pathogenesis-Related Proteins for the Plant Protection. Asian J. Exp. Sci. 2008, 22, 189–196. [Google Scholar]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 Transcription Factor: A Node of Convergence for Jasmonate-Mediated and Salicylate-Mediated Signals in Plant Defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; van Hulten, M.; Martin, J.; Pieterse, C.M.J.; van Wees, S.C.M.; Ton, J. Genetic Dissection of Basal Defence Responsiveness in Accessions of Arabidopsis Thaliana: Genetic Dissection of Defence Responsiveness. Plant Cell Environ. 2011, 34, 1191–1206. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.e15. [Google Scholar] [CrossRef]
- Jirage, D.; Tootle, T.L.; Reuber, T.L.; Frost, L.N.; Feys, B.J.; Parker, J.E.; Ausubel, F.M.; Glazebrook, J. Arabidopsis Thaliana PAD4 Encodes a Lipase-like Gene That Is Important for Salicylic Acid Signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 13583–13588. [Google Scholar] [CrossRef] [Green Version]
- Feys, B.J. Direct Interaction between the Arabidopsis Disease Resistance Signaling Proteins, EDS1 and PAD4. EMBO J. 2001, 20, 5400–5411. [Google Scholar] [CrossRef]
- Gimenez-Ibanez, S.; Zamarreño, A.M.; García-Mina, J.M.; Solano, R. An Evolutionarily Ancient Immune System Governs the Interactions between Pseudomonas Syringae and an Early-Diverging Land Plant Lineage. Curr. Biol. 2019, 29, 2270–2281.e4. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Tanaka, S.; Han, X.; Kahmann, R. Microbial Effectors Target Multiple Steps in the Salicylic Acid Production and Signaling Pathway. Front. Plant Sci. 2015, 6, 349. [Google Scholar] [CrossRef] [Green Version]
- Mishina, T.E.; Zeier, J. The Arabidopsis Flavin-Dependent Monooxygenase FMO1 Is an Essential Component of Biologically Induced Systemic Acquired Resistance. Plant Physiol. 2006, 141, 1666–1675. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Zeier, J. l-Lysine Metabolism to N -Hydroxypipecolic Acid: An Integral Immune-Activating Pathway in Plants. Plant J. 2018, 96, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.; Shivnauth, V.; Castroverde, C.D.M. Salicylic Acid and N-Hydroxypipecolic Acid at the Fulcrum of the Plant Immunity-Growth Equilibrium. Front. Plant Sci. 2022, 13, 841688. [Google Scholar] [CrossRef] [PubMed]
- Zeier, J. Metabolic Regulation of Systemic Acquired Resistance. Curr. Opin. Plant Biol. 2021, 62, 102050. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Zeier, J. N-Hydroxypipecolic Acid and Salicylic Acid: A Metabolic Duo for Systemic Acquired Resistance. Curr. Opin. Plant Biol. 2019, 50, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, N.M.; Jung, H.W.; Engle, N.L.; Tschaplinski, T.J.; Greenberg, J.T. ALD1 Regulates Basal Immune Components and Early Inducible Defense Responses in Arabidopsis. Mol. Plant-Microbe Interact. 2015, 28, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, R.; Lim, G.-H.; de Lorenzo, L.; Yu, K.; Zhang, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Pipecolic Acid Confers Systemic Immunity by Regulating Free Radicals. Sci. Adv. 2018, 4, eaar4509. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, I.; Mantz, M.; Hartmann, M.; Zeier, T.; Kessel, J.; Thurow, C.; Gatz, C.; Petzsch, P.; Köhrer, K.; Zeier, J. The Mobile SAR Signal N-Hydroxypipecolic Acid Induces NPR1-Dependent Transcriptional Reprogramming and Immune Priming. Plant Physiol. 2021, 186, 1679–1705. [Google Scholar] [CrossRef]
- Nair, A.; Goyal, I.; Voß, E.; Mrozek, P.; Prajapati, S.; Thurow, C.; Tietze, L.; Tittmann, K.; Gatz, C. N-Hydroxypipecolic Acid-Induced Transcription Requires the Salicylic Acid Signaling Pathway at Basal SA Levels. Plant Physiol. 2021, 187, 2803–2819. [Google Scholar] [CrossRef]
- Tsuda, K.; Mine, A.; Bethke, G.; Igarashi, D.; Botanga, C.J.; Tsuda, Y.; Glazebrook, J.; Sato, M.; Katagiri, F. Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in Arabidopsis Thaliana. PLoS Genet. 2013, 9, e1004015. [Google Scholar] [CrossRef]
- Gloggnitzer, J.; Akimcheva, S.; Srinivasan, A.; Kusenda, B.; Riehs, N.; Stampfl, H.; Bautor, J.; Dekrout, B.; Jonak, C.; Jiménez-Gómez, J.M.; et al. Nonsense-Mediated MRNA Decay Modulates Immune Receptor Levels to Regulate Plant Antibacterial Defense. Cell Host Microbe 2014, 16, 376–390. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential Transphosphorylation of the BRI1/BAK1 Receptor Kinase Complex Impacts Early Events in Brassinosteroid Signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef]
- De León, I.P.; Montesano, M. Adaptation Mechanisms in the Evolution of Moss Defenses to Microbes. Front. Plant Sci. 2017, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Carella, P.; Schornack, S. Manipulation of Bryophyte Hosts by Pathogenic and Symbiotic Microbes. Plant Cell Physiol. 2018, 59, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Mackey, A.J.; Vermunt, J.K.; Roos, D.S. Assessing Performance of Orthology Detection Strategies Applied to Eukaryotic Genomes. PLoS ONE 2007, 2, e383. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Hagelsieb, G.; Latimer, K. Choosing BLAST Options for Better Detection of Orthologs as Reciprocal Best Hits. Bioinformatics 2008, 24, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Kuzniar, A.; van Ham, R.C.H.J.; Pongor, S.; Leunissen, J.A.M. The Quest for Orthologs: Finding the Corresponding Gene across Genomes. Trends Genet. 2008, 24, 539–551. [Google Scholar] [CrossRef]
- Tamborski, J.; Krasileva, K.V. Evolution of Plant NLRs: From Natural History to Precise Modifications. Annu. Rev. Plant Biol. 2020, 71, 355–378. [Google Scholar] [CrossRef] [Green Version]
- Rieseberg, T.P.; Dadras, A.; Fürst-Jansen, J.M.R.; Ashok, A.D.; Darienko, T.; de Vries, S.; Irisarri, I.; de Vries, J. Crossroads in the Evolution of Plant Specialized Metabolism. Semin. Cell Dev. Biol. 2022, in press. [Google Scholar] [CrossRef]
- Forslund, K.; Pekkari, I.; Sonnhammer, E.L. Domain Architecture Conservation in Orthologs. BMC Bioinform. 2011, 12, 326. [Google Scholar] [CrossRef] [Green Version]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s Conserved Domain Database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler-Bauer, A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinforma. 2020, 69, e90. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into Land Plant Evolution Garnered from the Marchantia Polymorpha Genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Betts, H.C.; Puttick, M.N.; Clark, J.W.; Williams, T.A.; Donoghue, P.C.J.; Pisani, D. Integrated Genomic and Fossil Evidence Illuminates Life’s Early Evolution and Eukaryote Origin. Nat. Ecol. Evol. 2018, 2, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Guarino, F.; Marchi, L.; Muto, A.; Piro, A.; Degola, F. Bryo-Activities: A Review on How Bryophytes Are Contributing to the Arsenal of Natural Bioactive Compounds against Fungi. Plants 2021, 10, 203. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Xue, J.-Y.; Wang, Y.; Wu, P.; Wang, Q.; Yang, L.-T.; Pan, X.-H.; Wang, B.; Chen, J.-Q. A Primary Survey on Bryophyte Species Reveals Two Novel Classes of Nucleotide-Binding Site (NBS) Genes. PLoS ONE 2012, 7, e36700. [Google Scholar] [CrossRef] [Green Version]
- Carella, P.; Gogleva, A.; Hoey, D.J.; Bridgen, A.J.; Stolze, S.C.; Nakagami, H.; Schornack, S. Conserved Biochemical Defenses Underpin Host Responses to Oomycete Infection in an Early-Divergent Land Plant Lineage. Curr. Biol. 2019, 29, 2282–2294. [Google Scholar] [CrossRef]
- Otero-Blanca, A.; Pérez-Llano, Y.; Reboledo-Blanco, G.; Lira-Ruan, V.; Padilla-Chacon, D.; Folch-Mallol, J.L.; Sánchez-Carbente, M.D.; de León, I.P.; Batista-García, R.A. Physcomitrium Patens Infection by Colletotrichum Gloeosporioides: Understanding the Fungal–Bryophyte Interaction by Microscopy, Phenomics and RNA Sequencing. J. Fungi 2021, 7, 677. [Google Scholar] [CrossRef]
- Reboledo, G.; Agorio, A.D.; Vignale, L.; Batista-García, R.A.; de León, I.P. Transcriptional Profiling Reveals Conserved and Species-Specific Plant Defense Responses during the Interaction of Physcomitrium Patens with Botrytis Cinerea. Plant Mol. Biol. 2021, 107, 365–385. [Google Scholar] [CrossRef]
- de León, I.P.; Schmelz, E.A.; Gaggero, C.; Castro, A.; Álvarez, A.; Montesano, M. Physcomitrella Patens Activates Reinforcement of the Cell Wall, Programmed Cell Death and Accumulation of Evolutionary Conserved Defence Signals, Such as Salicylic Acid and 12-Oxo-Phytodienoic Acid, but Not Jasmonic Acid, upon Botrytis Cinerea Infection: P. Patens Defence Responses against B. Cinerea. Mol. Plant Pathol. 2012, 13, 960–974. [Google Scholar] [CrossRef]
- Bressendorff, S.; Azevedo, R.; Kenchappa, C.S.; de León, I.P.; Olsen, J.V.; Rasmussen, M.W.; Erbs, G.; Newman, M.-A.; Petersen, M.; Mundy, J. An Innate Immunity Pathway in the Moss Physcomitrella Patens. Plant Cell 2016, 28, 1328–1342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-C.; Cannon, S.B.; Stacey, G. Evolutionary Genomics of LysM Genes in Land Plants. BMC Evol. Biol. 2009, 9, 183. [Google Scholar] [CrossRef] [Green Version]
- De Vries, J.; Curtis, B.A.; Gould, S.B.; Archibald, J.M. Embryophyte Stress Signaling Evolved in the Algal Progenitors of Land Plants. Proc. Natl. Acad. Sci. USA 2018, 115, E3471–E3480. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.S.; Bowman, C.E.; Villani, P.J.; Dolan, T.E.; Hauck, N.R. Systemic Acquired Resistance in Moss: Further Evidence for Conserved Defense Mechanisms in Plants. PLoS ONE 2014, 9, e101880. [Google Scholar] [CrossRef]
- Upson, J.L.; Zess, E.K.; Białas, A.; Wu, C.; Kamoun, S. The Coming of Age of EvoMPMI: Evolutionary Molecular Plant–Microbe Interactions across Multiple Timescales. Curr. Opin. Plant Biol. 2018, 44, 108–116. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Zavaleta, C.Y.; García-Barrera, L.J.; Rodríguez-Verástegui, L.L.; Arrieta-Flores, D.; Gregorio-Jorge, J. An Overview of PRR- and NLR-Mediated Immunities: Conserved Signaling Components across the Plant Kingdom That Communicate Both Pathways. Int. J. Mol. Sci. 2022, 23, 12974. https://doi.org/10.3390/ijms232112974
Ramírez-Zavaleta CY, García-Barrera LJ, Rodríguez-Verástegui LL, Arrieta-Flores D, Gregorio-Jorge J. An Overview of PRR- and NLR-Mediated Immunities: Conserved Signaling Components across the Plant Kingdom That Communicate Both Pathways. International Journal of Molecular Sciences. 2022; 23(21):12974. https://doi.org/10.3390/ijms232112974
Chicago/Turabian StyleRamírez-Zavaleta, Candy Yuriria, Laura Jeannette García-Barrera, Lizette Liliana Rodríguez-Verástegui, Daniela Arrieta-Flores, and Josefat Gregorio-Jorge. 2022. "An Overview of PRR- and NLR-Mediated Immunities: Conserved Signaling Components across the Plant Kingdom That Communicate Both Pathways" International Journal of Molecular Sciences 23, no. 21: 12974. https://doi.org/10.3390/ijms232112974
APA StyleRamírez-Zavaleta, C. Y., García-Barrera, L. J., Rodríguez-Verástegui, L. L., Arrieta-Flores, D., & Gregorio-Jorge, J. (2022). An Overview of PRR- and NLR-Mediated Immunities: Conserved Signaling Components across the Plant Kingdom That Communicate Both Pathways. International Journal of Molecular Sciences, 23(21), 12974. https://doi.org/10.3390/ijms232112974





