Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CARD Aqueous Extract
2.2. Liquid Chromatography Time-of-Flight Mass Spectrometry (LC-TOF-MS) Analysis
2.3. Radical Scavenging Properties of Cardamom
2.4. Animal’s Acquisition and Ethical Statement
2.5. Experimental Design
2.6. Histopathological Investigation
2.7. Hepatic Function Tests Determination
2.8. Hepatic Oxidative Stress Status Determination
2.9. Determination of Inflammation and Apoptotic Signaling Markers
2.10. Gene Expression Experiments (Real-Time PCR)
2.11. Statistical Analysis
3. Results
3.1. LC-TOF-MS Analysis of CARD Extract
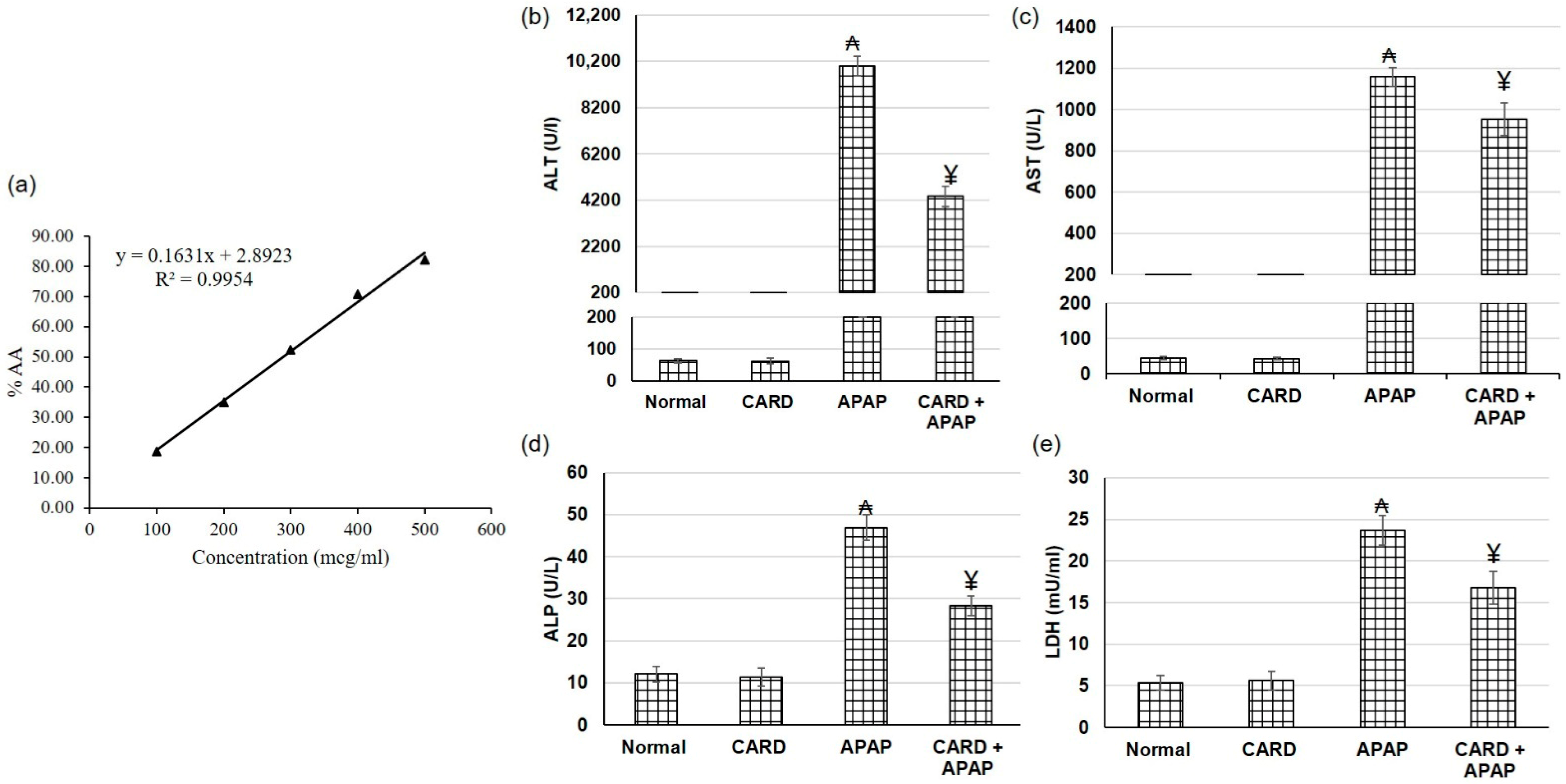
3.2. DPPH Assay of CARD Extract
3.3. CARD Extract Alleviated APAP-Induced Acute Hepatic Failure
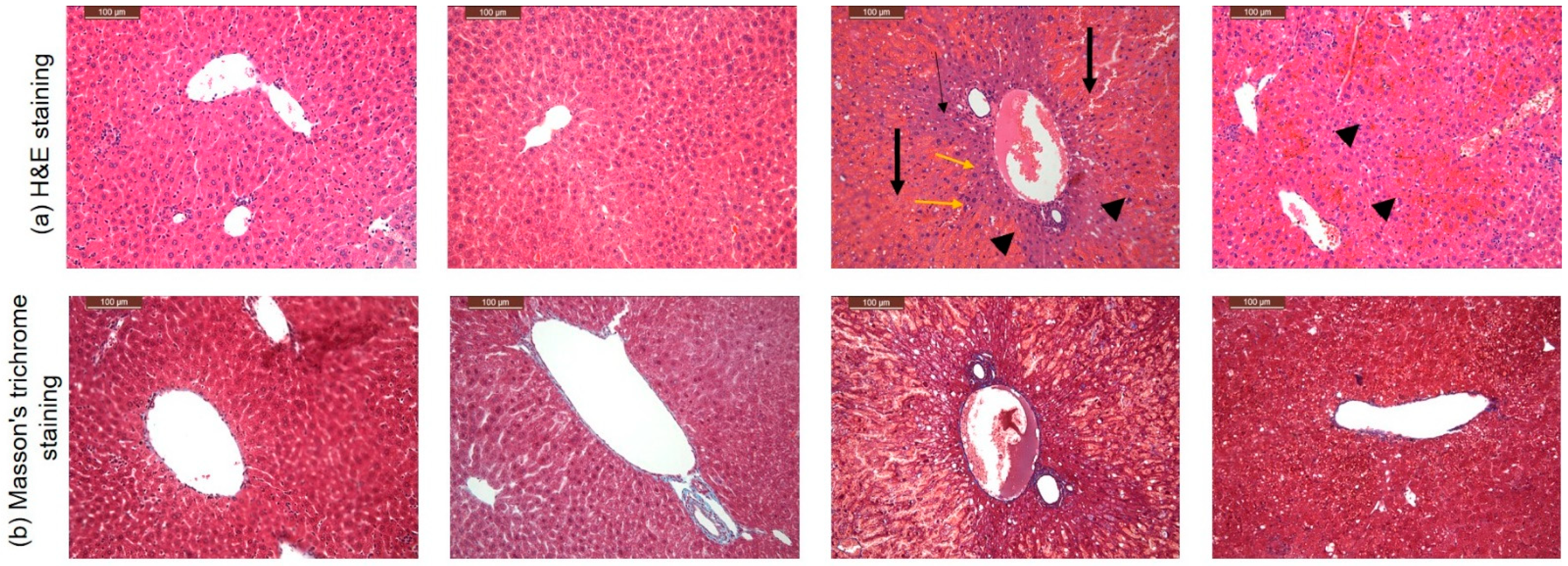
3.4. CARD Mitigated APAP-Induced Acute Hepatic Failure Alterations in Histopathological Examination
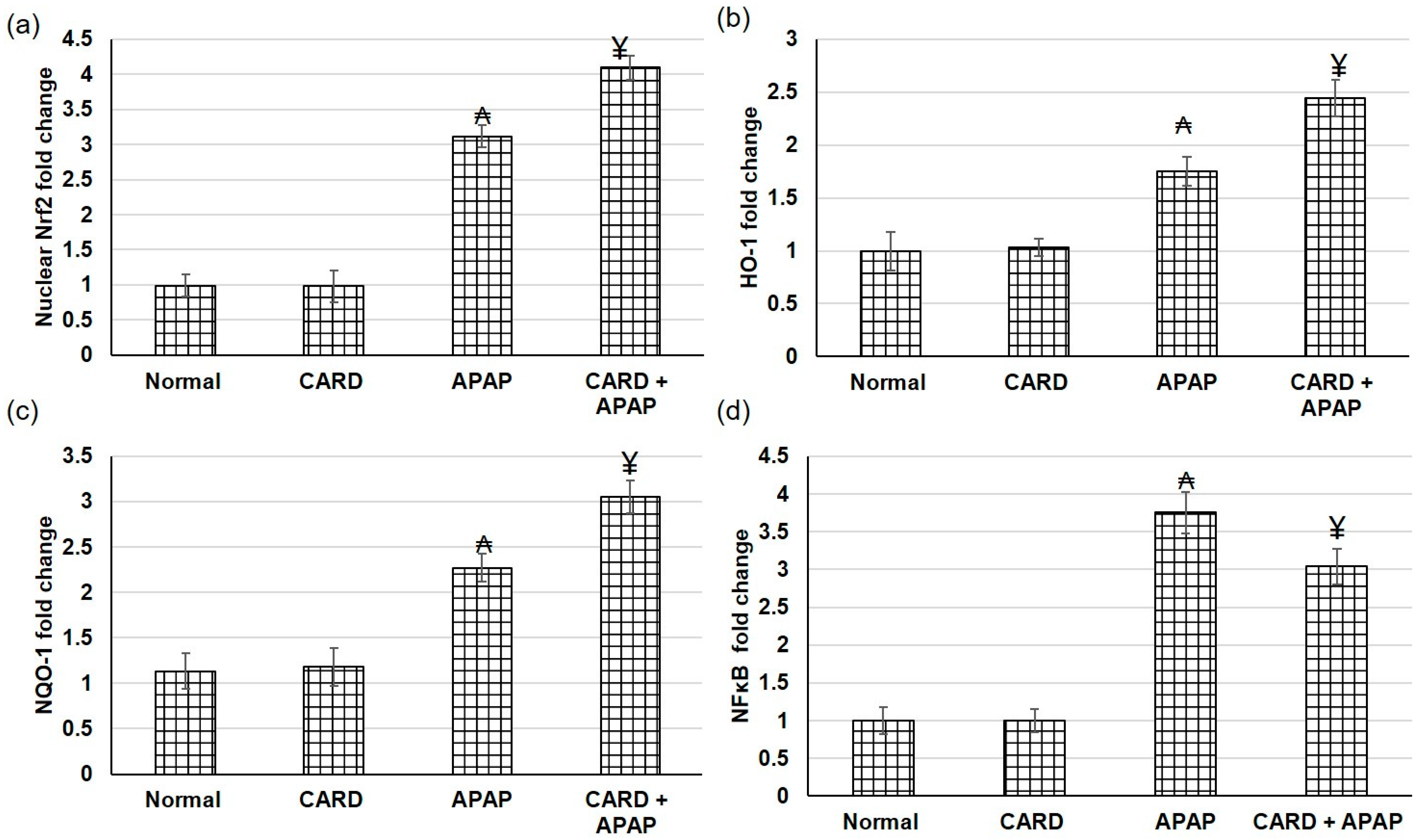
3.5. CARD Amplified Nrf2/HO-1/NQO-1/NF-κB Pathway Gene Expression in APAP-Induced Acute Hepatic Failure
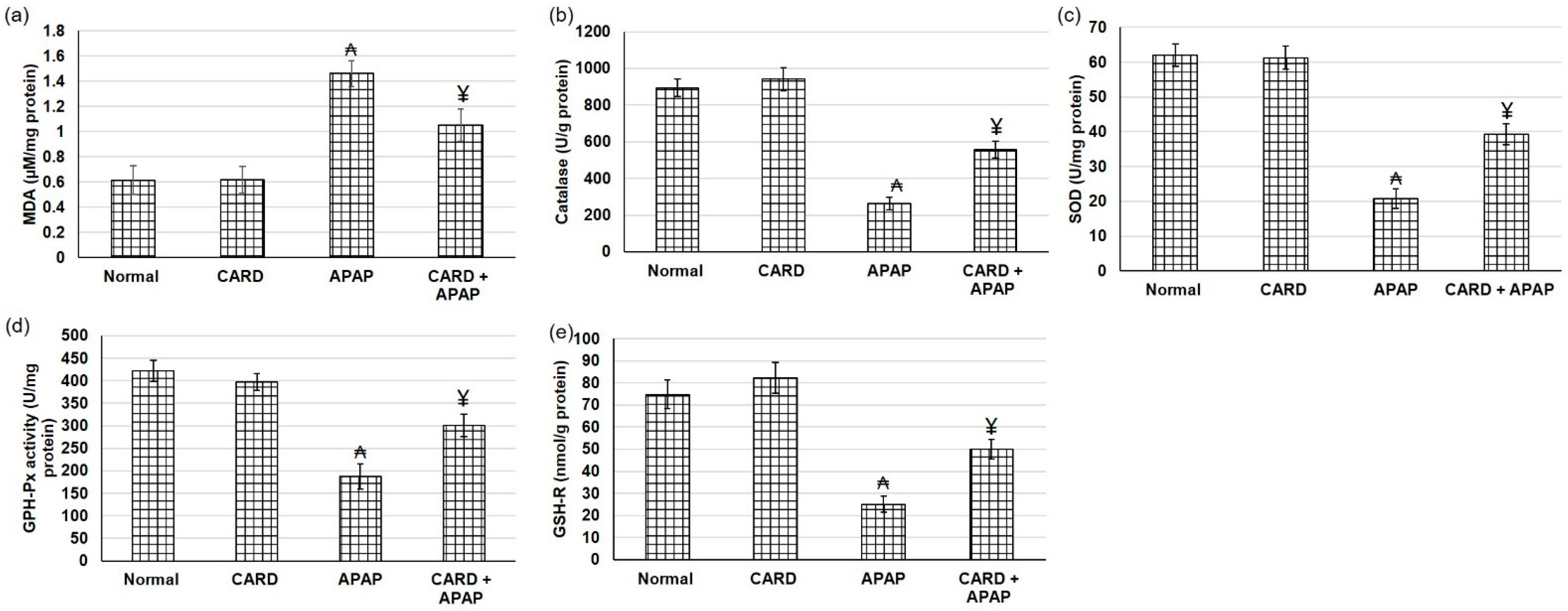
3.6. CARD Alleviated Oxidative Stress in APAP-Induced Acute Hepatic Failure
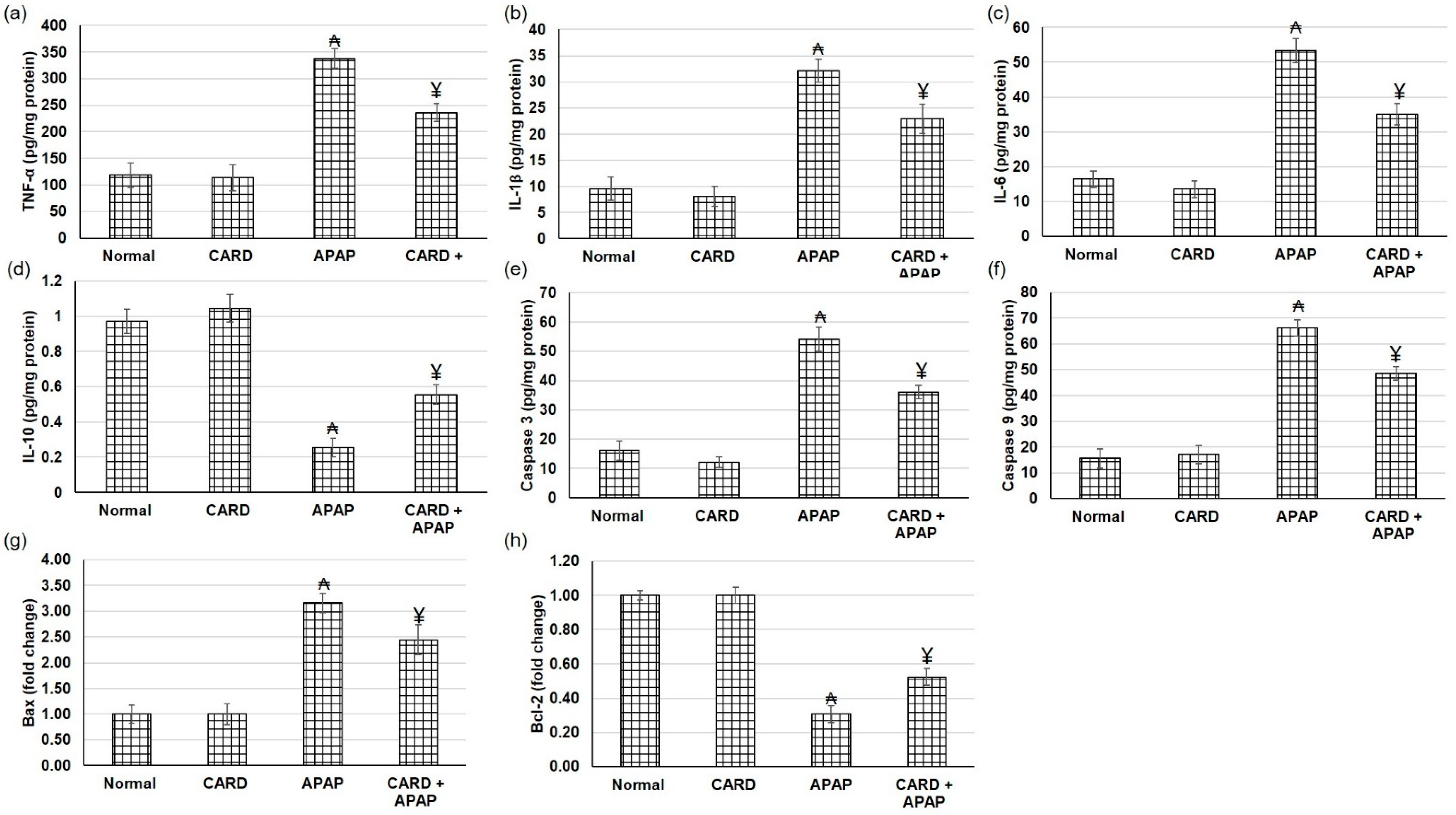
3.7. CARD Alleviated Hepatic Inflammation and Apoptosis Responses in APAP-Induced Acute Hepatic Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramachandran, A.; Jaeschke, H. Acetaminophen Hepatotoxicity. Semin. Liver. Dis. 2019, 39, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar] [PubMed]
- Fisher, E.S.; Curry, S.C. Evaluation and treatment of acetaminophen toxicity. Adv. Pharmacol. 2019, 85, 263–272. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, J.; Choo, B.K.; Kim, I.S.; Ahn, D.; Tae, H.J.; Islam, M.S.; Park, B.Y. Camellia japonica diminishes acetaminophen-induced acute liver failure by attenuating oxidative stress in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 57192–57206. [Google Scholar] [CrossRef]
- McGill, M.R.; Yan, H.M.; Ramachandran, A.; Murray, G.J.; Rollins, D.E.; Jaeschke, H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 2011, 53, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.A.; Eldeeb, H.M.; Mohammed, H.A.; Al-Omar, M.S.; Almahmoud, S.A.; El-Readi, M.Z.; Ragab, E.A.; Sulaiman, G.M.; Aly, M.S.A.; Khan, R.A. Roles of Suaeda vermiculata Aqueous-Ethanolic Extract, Its Subsequent Fractions, and the Isolated Compounds in Hepatoprotection against Paracetamol-Induced Toxicity as Compared to Silymarin. Oxid. Med. Cell. Longev. 2021, 2021, 6174897. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2017, 66, 836–848. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Abdel Moneim, A.E.; Hafez, T.A.; Mubaraki, M.A.; Mohamed, W.F.; Thagfan, F.A.; Al-Quraishy, S. Myristica fragrans Kernels Prevent Paracetamol-Induced Hepatotoxicity by Inducing Anti-Apoptotic Genes and Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2019, 20, 993. [Google Scholar] [CrossRef]
- Eugenio-Pérez, D.; Montes de Oca-Solano, H.A.; Pedraza-Chaverri, J. Role of food-derived antioxidant agents against acetaminophen-induced hepatotoxicity. Pharm. Biol. 2016, 54, 2340–2352. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The keap1-nrf2 cellular defense pathway: Mechanisms of regulation and role in protection against drug-induced toxicity. Advers. Drug React. 2010, 196, 233–266. [Google Scholar]
- Sengupta, A.; Ghosh, S.; Bhattacharjee, S. Dietary cardamom inhibits the formation of azoxymethane-induced aberrant crypt foci in mice and reduces COX-2 and iNOS expression in the colon. Asian Pac. J. Cancer Prev. 2005, 6, 118–122. [Google Scholar] [PubMed]
- Das, I.; Acharya, A.; Berry, D.L.; Sen, S.; Williams, E.; Permaul, E.; Sengupta, A.; Bhattacharya, S.; Saha, T. Antioxidative effects of the spice cardamom against non-melanoma skin cancer by modulating nuclear factor erythroid-2-related factor 2 and NF-κB signalling pathways. Br. J. Nutr. 2012, 108, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Zahedi, S.; Koohdani, F.; Qorbani, M.; Siassi, F.; Keshavarz, A.; Nasli-Esfahani, E.; Aghasi, M.; Khoshamal, H.; Sotoudeh, G. The effects of green cardamom supplementation on blood pressure and endothelium function in type 2 diabetic patients: A study protocol for a randomized controlled clinical trial. Medicine 2020, 99, e11005. [Google Scholar] [CrossRef] [PubMed]
- Aghasi, M.; Koohdani, F.; Qorbani, M.; Nasli-Esfahani, E.; Ghazi-Zahedi, S.; Khoshamal, H.; Keshavarz, A.; Sotoudeh, G. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: A randomized double-blind placebo controlled clinical trial. J. Sci. Food Agric. 2019, 99, 3933–3940. [Google Scholar] [CrossRef]
- Darwish, M.M.; Abd El Azime, A.S. Role of Cardamom (Elettaria cardamomum) in Ameliorating Radiation Induced Oxidative Stress In Rats. Arab. J. Nucl. Sci. Appl. 2013, 46, 232–239. [Google Scholar]
- Rahman, M.M.; Alam, M.N.; Ulla, A.; Sumi, F.A.; Subhan, N.; Khan, T.; Sikder, B.; Hossain, H.; Reza, H.M.; Alam, M.A. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 2017, 16, 151. [Google Scholar] [CrossRef]
- Elguindy, N.M.; Yacout, G.A.; El Azab, E.F.; Maghraby, H.K. Chemoprotective Effect of Elettaria Cardamomum against Chemically induced Hepatocellular Carcinoma in Rats by Inhibiting NF-κB, Oxidative Stress, and Activity of Ornithine Decarboxylase. S. Afr. J. Bot. 2016, 105, 251–258. [Google Scholar] [CrossRef]
- Kader, S.M.A.; Bauomi, A.A.; Abdel-Rahman, M.; Mohammaden, T.F.; Rezk, M.M. Antioxidant potentials of (Elletaria cardamomum) cardamom against uranium hazards. Int. J. Basic Life Sci. 2015, 3, 164–181. [Google Scholar]
- Kanthlal, S.K.; Joseph, J.; Paul, B. Antioxidant and vasorelaxant effects of aqueous extract of large cardamom in L-NAME induced hypertensive rats. Clin. Exp. Hypertens 2020, 42, 581–589. [Google Scholar] [CrossRef]
- Kaushik, P.; Goyal, P.; Chauhan, A.; Chauhan, G. In Vitro Evaluation of Antibacterial Potential of Dry FruitExtracts of Elettaria cardamomum Maton (Chhoti Elaichi). Iran J. Pharm. Res. 2010, 9, 287–292. [Google Scholar] [PubMed]
- El-Segaey, O.; Abdallah, A.; Al-Nooman, S. Experimental study of antioxidant and Hepatoprotective effects of clove and cardamom in ethanol induced hepatotoxicity, Tanta Med. Sci. J. 2007, 2, 27–36. [Google Scholar]
- Cárdenas Garza, G.R.; Elizondo Luévano, J.H.; Bazaldúa Rodríguez, A.F.; Chávez Montes, A.; Pérez Hernández, R.A.; Martínez Delgado, A.J.; López Villarreal, S.M.; Rodríguez Rodríguez, J.; Sánchez Casas, R.M.; Castillo Velázquez, U.; et al. Benefits of Cardamom (Elettaria cardamomum (L.) Maton) and Turmeric (Curcuma longa L.) Extracts for Their Applications as Natural Anti-Inflammatory Adjuvants. Plants 2021, 10, 1908. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Carr, R.I. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). J. Med. Food 2010, 13, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Rathod, M.; Director, D. An in vitro study of the immunomodulatory effects of Piper nigrum (black pepper) and Elettaria cardamomum (cardamom) extracts using a murine macrophage cell line. AIJRFANS 2014, 8, 18–27. [Google Scholar]
- Abu Gazia, M.; El-Magd, M.A. Ameliorative Effect of Cardamom Aqueous Extract on Doxorubicin-Induced Cardiotoxicity in Rats. Cells Tissues Organs 2018, 206, 62–72. [Google Scholar] [CrossRef]
- Goyal, S.N.; Sharma, C.; Mahajan, U.B.; Patil, C.R.; Agrawal, Y.O.; Kumari, S.; Arya, D.S.; Ojha, S. Protective Effects of Cardamom in Isoproterenol-Induced Myocardial Infarction in Rats. Int. J. Mol. Sci. 2015, 16, 27457–27469. [Google Scholar] [CrossRef]
- Faiza, M.-H.; Mokhtaria Yasmina, B.; Soumia, K.; Abdelkader, H. Chemical Composition and Antimicrobial Properties of Elettaria cardamomum Extract. Pharmacogn. J. 2020, 12, 1058–1063. [Google Scholar]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Papackova, Z.; Heczkova, M.; Dankova, H.; Sticova, E.; Lodererova, A.; Bartonova, L.; Poruba, M.; Cahova, M. Silymarin prevents acetaminophen-induced hepatotoxicity in mice. PLoS ONE 2018, 13, e0191353. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, A.; Bibi, T.; Ahmad, S.; Shehzad, O.; Ali, H.; Seo, E.K.; Khan, S. Comprehensive in vivo and in silico approaches to explore the hepatoprotective activity of poncirin against paracetamol toxicity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 195–215. [Google Scholar] [CrossRef]
- Abo El-Magd, N.F.; Eraky, S.M. The molecular mechanism underlining the preventive effect of vitamin D against hepatic and renal acute toxicity through the NrF2/ BACH1/ HO-1 pathway. Life Sci. 2020, 244, 117331. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, R.S.; Abdel-Rahman, N. Dimethyl fumarate ameliorates acetaminophen-induced hepatic injury in mice dependent of Nrf-2/HO-1 pathway. Life Sci. 2019, 217, 251–260. [Google Scholar] [CrossRef]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef]
- Chan, K.; Han, X.-D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616. [Google Scholar] [CrossRef]
- Abdelmageed, N.; Twafik, W.A.; Seddek, A.L.; Morad, S.A.F. Vinpocetine-based therapy is an attractive strategy against oxidative stress-induced hepatotoxicity in vitro by targeting Nrf2/HO-1 pathway. Excli. J. 2021, 20, 550–561. [Google Scholar] [CrossRef]
- Eraky, S.M.; Abo El-Magd, N.F. Omega-3 fatty acids protect against acetaminophen-induced hepatic and renal toxicity in rats through HO-1-Nrf2-BACH1 pathway. Arch. Biochem. Biophys. 2020, 687, 108387. [Google Scholar] [CrossRef]
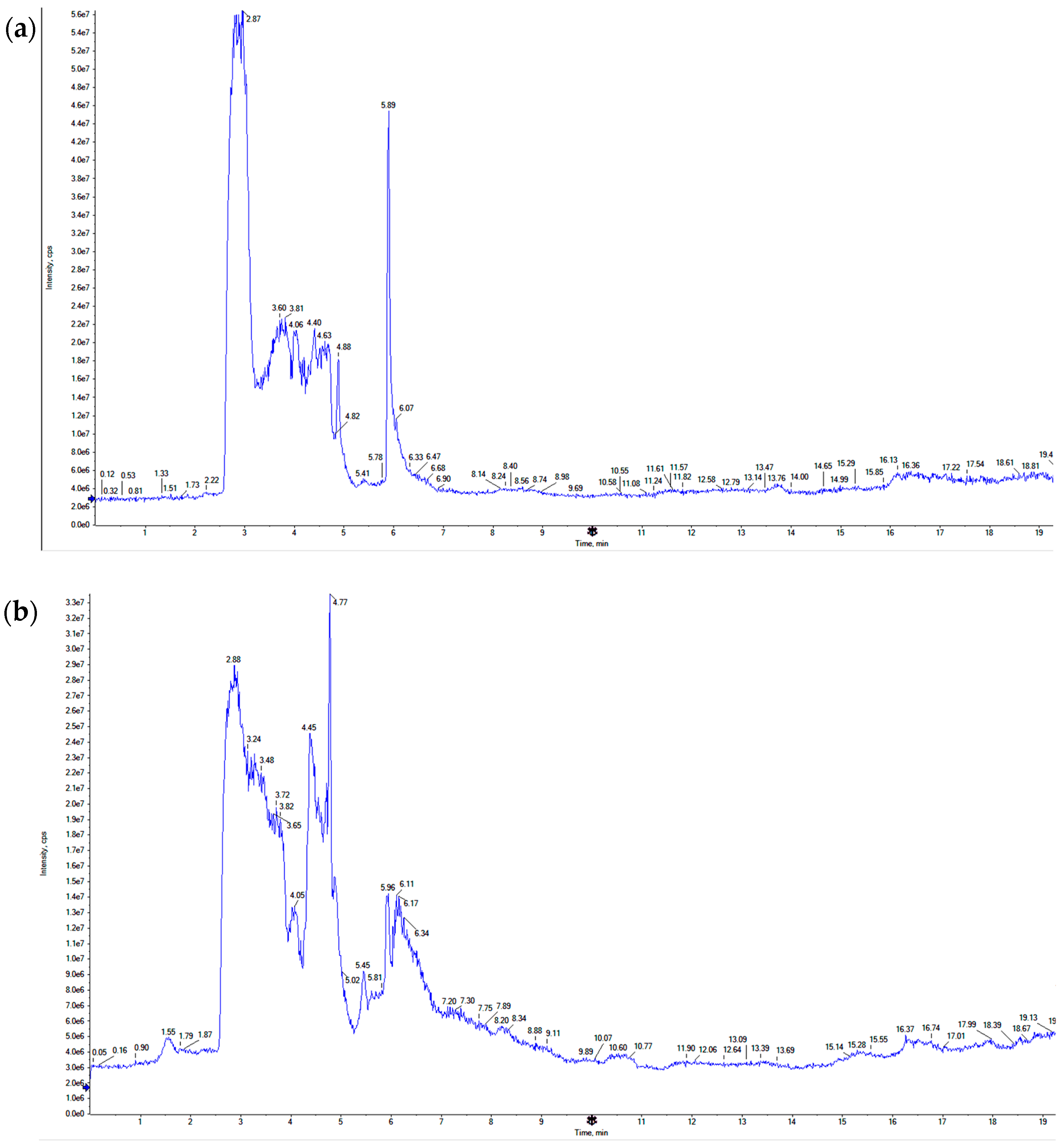
| # | Retention Time (Minutes) | Compound Name | Peak Area Percentage |
|---|---|---|---|
| 1 | 2.888 | Gallic acid | 8.18 |
| 2 | 2.904 | Catechin | 4.295 |
| 4 | 3.014 | Ferulic acid | 3.972 |
| 5 | 3.024 | Quercetin | 5.372 |
| 6 | 3.037 | Luteolin | 9.275 |
| 7 | 3.09 | Cafeic acid | 1.236 |
| 8 | 3.166 | Abscisic alcohol | 1.834 |
| 9 | 3.63 | Trans-cinnamic acid | 4.188 |
| 10 | 3.688 | Davidigenin | 1.124 |
| 11 | 3.762 | Lunularic acid | 2.706 |
| 12 | 3.949 | Patuletin | 1.547 |
| 13 | 3.984 | Ellagic acid | 1.765 |
| 14 | 4.091 | 8-methoxyquercetin | 4.654 |
| 15 | 4.144 | Apigeninidin | 3.701 |
| 16 | 4.309 | trans-Cinnamoyl beta-D-glucoside | 1.094 |
| 17 | 4.532 | Glutamyl-glutamic acid | 3.583 |
| 18 | 5.388 | Traumatic acid | 0.493 |
| 20 | 5.895 | Hydrocinnamamide | 1.128 |
| 21 | 5.914 | Myricetin | 3.243 |
| 22 | 6.108 | cis-Citral | 1.007 |
| 23 | 16.539 | (+)-Camphor | 1.616 |
| 24 | 16.667 | trans-Carveol | 1.04 |
| 25 | 19.148 | Limonene-1,2-epoxide | 1.176 |
| Total | 68.229 | ||
| Compound group name | Area percentage | ||
| Flavonoids | 32.087 | ||
| Polyphenols and phenolic acids | 45.862 | ||
| Cinnamic acid derivatives | 15.448 | ||
| Essential oil components | 4.839 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhalifah, E.A.R.; Alobaid, A.A.; Almajed, M.A.; Alomair, M.K.; Alabduladheem, L.S.; Al-Subaie, S.F.; Akbar, A.; Attimarad, M.V.; Younis, N.S.; Mohamed, M.E. Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway. Curr. Issues Mol. Biol. 2022, 44, 5390-5404. https://doi.org/10.3390/cimb44110365
Alkhalifah EAR, Alobaid AA, Almajed MA, Alomair MK, Alabduladheem LS, Al-Subaie SF, Akbar A, Attimarad MV, Younis NS, Mohamed ME. Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway. Current Issues in Molecular Biology. 2022; 44(11):5390-5404. https://doi.org/10.3390/cimb44110365
Chicago/Turabian StyleAlkhalifah, Essraa A. R., Amjad A. Alobaid, Marwah A. Almajed, Manar K. Alomair, Lama S. Alabduladheem, Sarah F. Al-Subaie, Abdullah Akbar, Mahesh V. Attimarad, Nancy S. Younis, and Maged E. Mohamed. 2022. "Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway" Current Issues in Molecular Biology 44, no. 11: 5390-5404. https://doi.org/10.3390/cimb44110365
APA StyleAlkhalifah, E. A. R., Alobaid, A. A., Almajed, M. A., Alomair, M. K., Alabduladheem, L. S., Al-Subaie, S. F., Akbar, A., Attimarad, M. V., Younis, N. S., & Mohamed, M. E. (2022). Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway. Current Issues in Molecular Biology, 44(11), 5390-5404. https://doi.org/10.3390/cimb44110365







