Is Pathologic Axillary Staging Valid If Lymph Nodes Are Less than 10 with Axillary Lymph Node Dissection after Neoadjuvant Chemotherapy?
Abstract
:1. Introduction
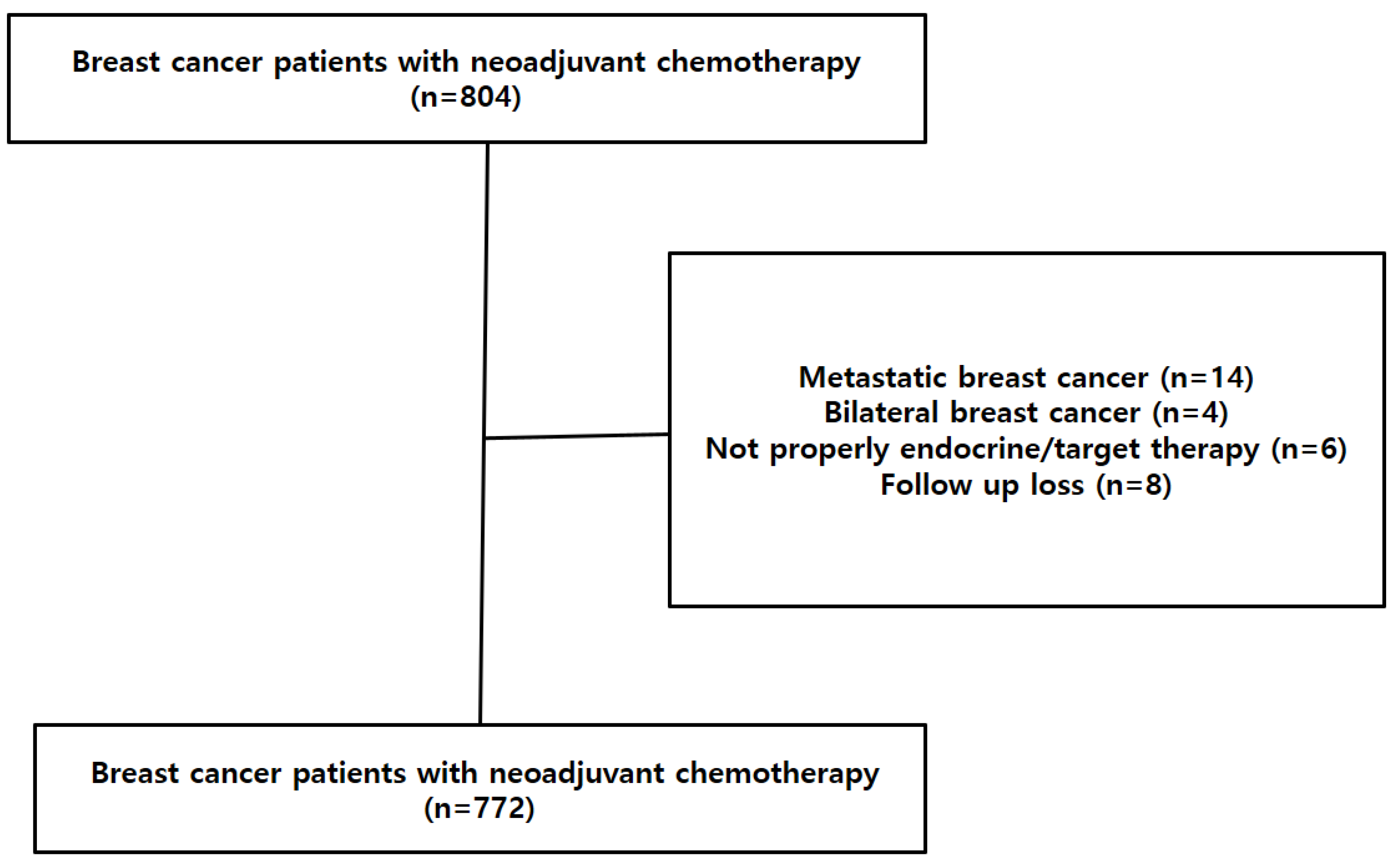
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shimizu, C.; Ando, M.; Kouno, T.; Katsumata, N.; Fujiwara, Y. Current trends and controversies over pre-operative chemotherapy for women with operable breast cancer. Jpn. J. Clin. Oncol. 2007, 37, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuschek, C.; Jazmati, D.; Bölke, E.; Tamaskovics, B.; Corradini, S.; Budach, W.; Krug, D.; Mohrmann, S.; Ruckhäberle, E.; Fehm, T.; et al. Post-Neoadjuvant Treatment Strategies in Breast Cancer. Cancers 2022, 14, 1246. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, L.H.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Fayanju, O.M.; Hwang, E.S.; Plichta, J.K. Axillary lymph node dissection in node-positive breast cancer: Are ten nodes adequate and when is enough, enough? Breast Cancer Res. Treat. 2020, 179, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.E.; Paik, H.J.; Ryu, J.M.; Bae, S.Y.; Lee, S.K.; Kim, S.W.; Nam, S.J. Feasibility and Prognostic Effect of Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy in Cytology-Proven, Node-Positive Breast Cancer. Clin. Breast Cancer 2017, 17, e19–e29. [Google Scholar] [CrossRef] [PubMed]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ruano Perez, R.; Rebollo Aguirre, A.C.; Garcia-Talavera San Miguel, P.; Diaz Exposito, R.; Vidal-Sicart, S.; Cordero Garcia, J.M.; Carrera Salazar, D.; Rioja Martin, M.E. Review of the role of the sentinel node biopsy in neoadjuvant chemotherapy in women with breast cancer and negative or positive axillary node at diagnosis. Rev. Esp. De Med. Nucl. E Imagen Mol. 2018, 37, 63–70. [Google Scholar] [CrossRef]
- Uyan, M.; Koca, B.; Yuruker, S.; Ozen, N. Effect of Neoadjuvant Chemotherapy on Axillary Lymph Node Positivity and Numbers in Breast Cancer Cases. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 1181–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. (2018) NCCN, Invasive Cancer, Surgical Axillary Staging. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 4 January 2019).
- Brackstone, M.; Baldassarre, F.G.; Perera, F.E.; Cil, T.; Gregor, M.C.M.; Dayes, I.S.; Engel, J.; Horton, J.K.; King, T.A.; Kornecki, A.; et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J. Clin. Oncol. 2021, 39, 3056–3082. [Google Scholar] [CrossRef] [PubMed]
- Swisher, S.K.; Vila, J.; Tucker, S.L.; Bedrosian, I.; Shaitelman, S.F.; Litton, J.K.; Smith, B.D.; Caudle, A.S.; Kuerer, H.M.; Mittendorf, E.A. Locoregional Control According to Breast Cancer Subtype and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients Undergoing Breast-conserving Therapy. Ann. Surg. Oncol. 2016, 23, 749–756. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Moliterni, A.; Vazquez, F.; Byakhov, M.J.; Lichinitser, M.; et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014, 15, 640–647. [Google Scholar] [CrossRef]
- De Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Van Nijnatten, T.J.; Simons, J.M.; Moossdorff, M.; de Munck, L.; Lobbes, M.B.; van der Pol, C.C.; Koppert, L.B.; Luiten, E.J.; Smidt, M.L. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: Isolated tumor cells and micrometastases carry a better prognosis than macrometastases. Breast Cancer Res. Treat. 2017, 163, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuschek, C.; Bolke, E.; Roth, S.L.; Orth, K.; Lang, I.; Bojar, H.; Janni, J.W.; Audretsch, W.; Nestle-Kraemling, C.; Lammering, G.; et al. Long-term outcome after neoadjuvant radiochemotherapy in locally advanced noninflammatory breast cancer and predictive factors for a pathologic complete remission: Results of a multivariate analysis. Strahlenther. Und Onkol. 2012, 188, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Kiricuta, C.I.; Tausch, J. A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer 1992, 69, 2496–2501. [Google Scholar] [CrossRef]
- Vinh-Hung, V.; Cserni, G.; Burzykowski, T.; van de Steene, J.; Voordeckers, M.; Storme, G. Effect of the number of uninvolved nodes on survival in early breast cancer. Oncol. Rep. 2003, 10, 363–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.G.; Sun, J.Y.; Zhou, J.; Li, F.Y.; Lin, Q.; Lin, H.X.; Guan, X.X.; He, Z.Y. Number of negative lymph nodes is associated with disease-free survival in patients with breast cancer. BMC Cancer 2015, 15, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, P.; Cole, B.F.; Price, K.N.; Coates, A.S.; Castiglione-Gertsch, M.; Gusterson, B.A.; Murray, E.; Lindtner, J.; Collins, J.P.; Holmberg, S.B.; et al. The role of the number of uninvolved lymph nodes in predicting locoregional recurrence in breast cancer. J. Clin. Oncol. 2007, 25, 2019–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.; Bertoni, D.; Hernandez-Boussard, T.; Telli, M.L.; Wapnir, I.L. Lymph Node Ratio Analysis After Neoadjuvant Chemotherapy is Prognostic in Hormone Receptor-Positive and Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2016, 23, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Li, Q.; Zhou, J.; Sun, J.Y.; Li, F.Y.; Lin, Q.; Lin, H.X.; Gaun, X.X.; He, Z.Y. Using the Lymph Node Ratio to Evaluate the Prognosis of Stage II/III Breast Cancer Patients Who Received Neoadjuvant Chemotherapy and Mastectomy. Cancer Res. Treat. 2015, 47, 757–764. [Google Scholar] [CrossRef] [PubMed]



| Variable | <10 Nodes (n = 123) No. (%) | ≥10 Nodes (n = 649) No. (%) | Total (n = 772) No. (%) | p-Value |
|---|---|---|---|---|
| Age (years) | 45.8 ± 10.1 | 45.1 ± 9.4 | 45.2 ± 9.5 | 0.419 |
| BMI | 23.2 ± 3.1 | 24.1 ± 3.4 | 23.9 ± 3.4 | 0.011 |
| Type of surgery | 0.272 | |||
| Conserving surgery | 69 (56.1) | 329 (50.7) | 398 (51.6) | |
| Mastectomy | 54 (43.9) | 320 (43.9) | 374 (48.4) | |
| ER status | 0.117 | |||
| Negative | 69 (56.1) | 314 (48.4) | 383 (49.6) | |
| Positive | 54 (43.9) | 335 (51.6) | 389 (50.4) | |
| PR status | 0.143 | |||
| Negative | 82 (66.7) | 387 (59.6) | 469 (60.8) | |
| Positive | 41 (33.3) | 262 (40.4) | 303 (39.2) | |
| HER2 status | 0.219 | |||
| Negative | 76 (61.8) | 438 (67.5) | 514 (66.6) | |
| Positive | 47 (38.2) | 211 (32.5) | 140 (33.5) | |
| Pathologic tumor stage | 0.028 | |||
| ypT0-is | 36 (29.2) | 124 (19.1) | 160 (20.7) | |
| ypT1 | 43 (35.0) | 205 (31.6) | 248 (32.1) | |
| ypT2 | 29 (23.6) | 178 (27.4) | 207 (26.8) | |
| ypT3 ypT4 Pathologic node stage ypN0 ypN1 ypN2 ypN3 | 14 (11.4) 1 (0.8) 69 (56.1) 39 (31.7) 15 (12.2) | 131 (20.2) 11 (1.7) 216 (33.3) 219 (33.7) 120 (18.5) 94 (14.5) | 145 (18.8) 12 (1.6) 285 (39.9) 258 (33.4) 165 (17.5) 94 (12.2) | <0.001 |
| Adjuvant Radiotherapy | 0.016 | |||
| Absent | 14 (11.4) | 36 (5.5) | 50 (6.5) | |
| Present | 109 (88.6) | 613 (94.5) | 722 93.5 |
| Variable | <10 Nodes (n = 69) No. (%) | ≥10 Nodes (n = 216) No. (%) | Total (n = 285) No. (%) | p-Value |
|---|---|---|---|---|
| Age (years) | 45.6 ± 10.1 | 45.4 ± 9.7 | 45.5 ± 9.7 | 0.867 |
| BMI | 23.5 ± 2.9 | 24.2 ± 3.4 | 24.0 ± 3.3 | 0.123 |
| Type of surgery | 0.697 | |||
| Conserving surgery | 41 (59.4) | 134 (62.0) | 175 (61.4) | |
| Mastectomy | 28 (40.6) | 82 (37.9) | 110 (38.6) | |
| ER status | 0.684 | |||
| Negative | 45 (65.2) | 135 (62.5) | 180 (63.2) | |
| Positive | 24 (34.8) | 81 (37.5) | 105 (36.8) | |
| PR status | 0.997 | |||
| Negative | 49 (71.0) | 153 (70.8) | 202 (70.1) | |
| Positive | 20 (29.0) | 63 (29.2) | 83 (29.1) | |
| HER2 status | 0.997 | |||
| Negative | 40 (58.0) | 125 (57.9) | 165 (57.9) | |
| Positive | 29 (42.0) | 91 (42.1) | 120 (42.1) | |
| Pathologic tumor stage | 0.854 | |||
| ypT0-is | 30 (43.5) | 93 (43.0) | 123 (43.2) | |
| ypT1 | 25 (36.2) | 68 (31.5) | 93 (32.6) | |
| ypT2 | 11 (15.9) | 38 (17.6) | 49 (17.2) | |
| ypT3 | 3 (4.3) | 16 (7.4) | 19 (6.7) | |
| ypT4 | 0 (0.0) | 1 (0.5) | 1 (0.3) | |
| Radiotherapy | 0.324 | |||
| Absent | 10 (14.5) | 22 (10.2) | 32 (11.2) | |
| Present | 59 (85.5) | 194 (89.8) | 253 (88.8) |
| Variable | <10 Nodes (n = 54) No. (%) | ≥10 Nodes (n = 433) No. (%) | Total (n = 487) No. (%) | p-Value |
|---|---|---|---|---|
| Age (years) | 46.15 ± 10.14 | 44.96 ± 9.26 | 45.09 ± 9.36 | 0.379 |
| BMI | 23.83 ± 3.38 | 23.98 ± 3.45 | 23.85 ± 3.46 | 0.202 |
| Type of surgery | 0.343 | |||
| Conserving surgery | 28 (51.85) | 195 (45.03) | 223 (45.79) | |
| Mastectomy | 26 (48.15) | 238 (54.97) | 264 (54.21) | |
| ER status | 0.663 | |||
| Negative | 24 (44.44) | 179 (41.34) | 203 (41.68) | |
| Positive | 30 (55.56) | 254 (58.66) | 284 (58.32) | |
| PR status | 0.325 | |||
| Negative | 33 (61.11) | 234 (54.04) | 267 (54.83) | |
| Positive | 21 (38.89) | 199 (45.96) | 220 (45.17) | |
| HER2 status | 0.388 | |||
| Negative | 36 (66.67) | 313 (72.29) | 349 (71.66) | |
| Positive | 18 (33.33) | 120 (27.71) | 138 (28.34) | |
| Pathologic tumor stage | 0.737 | |||
| ypT0-is | 6 (11.11) | 31 (7.16) | 37 (7.60) | |
| ypT1 | 18 (33.33) | 137 (31.64) | 155 (31.83) | |
| ypT2 | 18 (33.33) | 140 (32.33) | 158 (32.44) | |
| ypT3 | 11 (20.37) | 115 (2.56) | 126 (25.87) | |
| ypT4 | 1 (1.85) | 10 (2.31) | 11 (2.26) | |
| Radiotherapy | 0.125 | |||
| Absent | 4 (7.41) | 14 (3.23) | 18 (3.70) | |
| Present | 50 (92.59) | 419 (96.77) | 469 (96.30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.J.; Ryu, J.M.; Lee, J.H.; Bang, Y.; Oh, J.; Chae, B.-J.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Lee, S.K.; et al. Is Pathologic Axillary Staging Valid If Lymph Nodes Are Less than 10 with Axillary Lymph Node Dissection after Neoadjuvant Chemotherapy? J. Clin. Med. 2022, 11, 6564. https://doi.org/10.3390/jcm11216564
Choi HJ, Ryu JM, Lee JH, Bang Y, Oh J, Chae B-J, Nam SJ, Kim SW, Lee JE, Lee SK, et al. Is Pathologic Axillary Staging Valid If Lymph Nodes Are Less than 10 with Axillary Lymph Node Dissection after Neoadjuvant Chemotherapy? Journal of Clinical Medicine. 2022; 11(21):6564. https://doi.org/10.3390/jcm11216564
Chicago/Turabian StyleChoi, Hee Jun, Jai Min Ryu, Jun Ho Lee, Yoonju Bang, Jongwook Oh, Byung-Joo Chae, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Se Kyung Lee, and et al. 2022. "Is Pathologic Axillary Staging Valid If Lymph Nodes Are Less than 10 with Axillary Lymph Node Dissection after Neoadjuvant Chemotherapy?" Journal of Clinical Medicine 11, no. 21: 6564. https://doi.org/10.3390/jcm11216564
APA StyleChoi, H. J., Ryu, J. M., Lee, J. H., Bang, Y., Oh, J., Chae, B.-J., Nam, S. J., Kim, S. W., Lee, J. E., Lee, S. K., & Yu, J. (2022). Is Pathologic Axillary Staging Valid If Lymph Nodes Are Less than 10 with Axillary Lymph Node Dissection after Neoadjuvant Chemotherapy? Journal of Clinical Medicine, 11(21), 6564. https://doi.org/10.3390/jcm11216564





