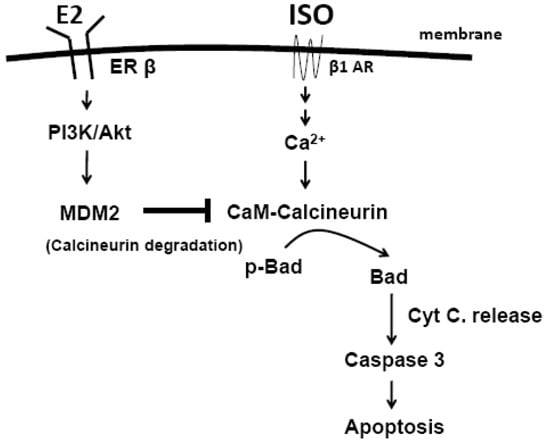
E2/ER β Enhances Calcineurin Protein Degradation and PI3K/Akt/MDM2 Signal Transduction to Inhibit ISO-Induced Myocardial Cell Apoptosis
Abstract
:1. Introduction
2. Results
2.1. 17β-Estradiol (E2)/Estrogen Receptor Beta (ERβ) Inhibits Isoproterenol (ISO)-Induced Cellular Apoptosis in Tet-On ERβ H9c2 Myocardial Cells
2.2. E2/ERβ Inhibits ISO-Induced Apoptosis Associated Caspase Activation and Cytochrome c Release in Tet-On ERβ H9c2 Myocardial Cells
2.3. E2/ERβ Attenuates ISO Induced Calcium Accumulation in H9c2 Cells
2.4. Calcineurin Plays an Important Role in ISO-Induced Cellular Apoptosis Signaling
2.5. E2 Enhances Calcineurin Protein Degradation via Estrogen Receptor β
2.6. E2/ERβ Enhances Calcineurin Protein Degradation via PI3K/Akt/MDM2 Signaling
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Cardiomyocyte Culture
4.3. Construct Tet-On Gene Expression System
4.4. Western-Blot Analysis
4.5. 4',6-Diamidino-2-phenylindole DAPI Staining and In Situ Terminal Deoxynucleotide Transferase-Mediated dUTP Nick End-Labeling TUNEL Assay
4.6. Intercellular Calcium Staining
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, age, cardiovascular risk factors, and coronary heart disease: A prospective follow-up study of 14786 middle-aged men and women in Finland. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Levin, E.R. Estrogen signaling in the cardiovascular system. Nucl. Recept. Signal. 2006, 4, e013. [Google Scholar] [CrossRef] [PubMed]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor β: An overview and update. Nucl. Recept. Signal. 2008, 6, e003. [Google Scholar] [CrossRef] [PubMed]
- Korte, T.; Fuchs, M.; Arkudas, A.; Geertz, S.; Meyer, R.; Gardiwal, A.; Klein, G.; Niehaus, M.; Krust, A.; Chambon, P.; et al. Female mice lacking estrogen receptor β display prolonged ventricular repolarization and reduced ventricular automaticity after myocardial infarction. Circulation 2005, 111, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, T.; Loza, P.A.; Hu, K.; Bayer, B.; Dienesch, C.; Calvillo, L.; Couse, J.F.; Korach, K.S.; Neyses, L.; Ertl, G. Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction. Circulation 2005, 111, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.R.; Cheng, W.C.; Su, Y.M.; Chiu, C.H.; Liou, Y.M. Molecular targets for anti-oxidative protection of green tea polyphenols against myocardial ischemic injury. BioMedicine 2014, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Itoh, G.; Tamura, J.; Suzuki, M.; Suzuki, Y.; Ikeda, H.; Koike, M.; Nomura, M.; Jie, T.; Ito, K. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am. J. Pathol. 1995, 146, 1325–1331. [Google Scholar] [PubMed]
- Bialik, S.; Geenen, D.L.; Sasson, I.E.; Cheng, R.; Horner, J.W.; Evans, S.M.; Lord, E.M.; Koch, C.J.; Kitsis, R.N. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J. Clin. Investig. 1997, 100, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Kajstura, J.; Nitahara, J.A.; Li, B.; Reiss, K.; Liu, Y.; Clark, W.A.; Krajewski, S.; Reed, J.C.; Olivetti, G.; et al. Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp. Cell Res. 1996, 226, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Palojoki, E.; Saraste, A.; Eriksson, A.; Pulkki, K.; Kallajoki, M.; Voipio-Pulkki, L.M.; Tikkanen, I. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2726–H2731. [Google Scholar] [PubMed]
- Chu, S.H.; Goldspink, P.; Kowalski, J.; Beck, J.; Schwertz, D.W. Effect of estrogen on calcium-handling proteins, β-adrenergic receptors, and function in rat heart. Life Sci. 2006, 79, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Levin, E.R. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J. Biol. Chem. 2005, 280, 26339–26348. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lo, J.F.; Kuo, C.H.; Chu, C.H.; Chen, L.M.; Tsai, F.J.; Tsai, C.H.; Tzang, B.S.; Kuo, W.W.; Huang, C.Y. Akt mediates 17β-estradiol and/or estrogen receptor-α inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFκB pathway. J. Cell. Mol. Med. 2009, 13, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Eschenhagen, T.; Jones, L.R.; Linck, B.; Schmitz, W.; Scholz, H.; Zimmermann, N. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell. Cardiol. 1997, 29, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.C.; Kass, D.A. Calcium handler mishandles heart. Nat. Med. 2004, 10, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Luo, C.; Hogan, P.G. Transcription factors of the NFAT family: Regulation and function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, F.; Kondo, E.; Akagi, T.; McKeon, F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature 1997, 386, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Zha, J.; Jockel, J.; Boise, L.H.; Thompson, C.B.; Korsmeyer, S.J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995, 80, 285–291. [Google Scholar] [CrossRef]
- Del Peso, L.; GonzalezGarcia, M.; Page, C.; Herrera, R.; Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278, 687–689. [Google Scholar]
- Saito, S.; Hiroi, Y.; Zou, Y.Z.; Aikawa, R.; Toko, H.; Shibasaki, F.; Yazaki, Y.; Nagai, R.; Komuro, I. β-adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J. Biol. Chem. 2000, 275, 34528–34533. [Google Scholar] [CrossRef] [PubMed]
- Jahns, R.; Boivin, V.; Siegmund, C.; Inselmann, G.; Lohse, M.J.; Boege, F. Autoantibodies activating human β1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation 1999, 99, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Jahns, R.; Boivin, V.; Krapf, T.; Wallukat, G.; Boege, F.; Lohse, M.J. Modulation of β1-adrenoceptor activity by domain-specific antibodies and heart failure-associated autoantibodies. J. Am. Coll. Cardiol. 2000, 36, 1280–1287. [Google Scholar] [CrossRef]
- Hjalmarson, A.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; El Allaf, D.; Vitovec, J.; Aldershvile, J.; et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000, 283, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Fagerberg, B.; Hjalmarson, A.; Kjekshus, J.; Waagstein, F.; Wedel, H.; Wikstrand, J.; Group, M.H.S. Metoprolol controlled release/extended release in patients with severe heart failure: Analysis of the experience in the MERIT-HF study. J. Am. Coll. Cardiol. 2001, 38, 932–938. [Google Scholar] [CrossRef]
- Zhang, G.X.; Ohmori, K.; Nagai, Y.; Fujisawa, Y.; Nishiyama, A.; Abe, Y.; Kimura, S. Role of AT1 receptor in isoproterenol-induced cardiac hypertrophy and oxidative stress in mice. J. Mol. Cell. Cardiol. 2007, 42, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Long, Z.; Wang, C.; Wang, L.; Sun, P.; Li, P.; Wang, T. Hydrogen (H2) Inhibits Isoproterenol-Induced Cardiac Hypertrophy via Antioxidative Pathways. Front. Pharmacol. 2016, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Vakili, B.A.; Okin, P.M.; Devereux, R.B. Prognostic implications of left ventricular hypertrophy. Am. Heart J. 2001, 141, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Communal, C.; Singh, K.; Pimentel, D.R.; Colucci, W.S. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation 1998, 98, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Shizukuda, Y.; Buttrick, P.M.; Geenen, D.L.; Borczuk, A.C.; Kitsis, R.N.; Sonnenblick, E.H. β-adrenergic stimulation causes cardiocyte apoptosis: Influence of tachycardia and hypertrophy. Am. J. Physiol. 1998, 275, H961–H968. [Google Scholar] [PubMed]
- Singh, K.; Xiao, L.; Remondino, A.; Sawyer, D.B.; Colucci, W.S. Adrenergic regulation of cardiac myocyte apoptosis. J. Cell. Phys. 2001, 189, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, C.; Eder, S.; Baker, C.; Aronovitz, M.J.; Weiss, A.D.; Hall-Porter, M.; Wang, F.; Ackerman, A.; Karas, R.H.; Molkentin, J.D.; et al. Estrogen Attenuates Left Ventricular and Cardiomyocyte Hypertrophy by an Estrogen Receptor-Dependent Pathway That Increases Calcineurin Degradation. Circ. Res. 2009, 104, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Kedar, V.; Zhang, C.; McDonough, H.; Arya, R.; Wang, D.Z.; Patterson, C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J. Clin. Investig. 2004, 114, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.S.; Patterson, C. Into the heart: The emerging role of the ubiquitin-proteasome system. J. Mol. Cell. Cardiol. 2006, 41, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Zolk, O.; Schenke, C.; Sarikas, A. The ubiquitin-proteasome system: Focus on the heart. Cardiovasc. Res. 2006, 70, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Lokkegaard, E.; Jovanovic, Z.; Heitmann, B.L.; Keiding, N.; Ottesen, B.; Pedersen, A.T. The association between early menopause and risk of ischaemic heart disease: Influence of Hormone Therapy. Maturitas 2006, 53, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Skegg, D.C.G. Hormone therapy and heart disease after the menopause. Lancet 2001, 358, 1196–1197. [Google Scholar] [CrossRef]
- Narula, J.; Pandey, P.; Arbustini, E.; Haider, N.; Narula, N.; Kolodgie, F.D.; Dal Bello, B.; Semigran, M.J.; Bielsa-Masdeu, A.; Dec, G.W.; et al. Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl. Acad. Sci. USA 1999, 96, 8144–8149. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Kuo, W.W.; Ho, Y.J.; Lin, A.C.; Tsai, C.H.; Wang, H.F.; Kuo, C.H.; Yang, A.L.; Huang, C.Y.; Hwang, J.M. Cardiac Fas-dependent and mitochondria-dependent apoptosis in ovariectomized rats. Maturitas 2008, 61, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Arenas, I.A.; Armstrong, S.J.; Plahta, W.C.; Xu, H.; Davidge, S.T. Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-α. Cardiovasc. Res. 2006, 69, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Smith, J.A.; Gibson, C.; Varma, A.K.; Ray, S.K.; Banik, N.L. Estrogen receptor agonists and estrogen attenuate TNF-α-induced apoptosis in VSC4.1 motoneurons. J. Endocrinol. 2011, 208, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Morkuniene, R.; Arandarcikaite, O.; Ivanoviene, L.; Borutaite, V. Estradiol-induced protection against ischemia-induced heart mitochondrial damage and caspase activation is mediated by protein kinase G. BBA Bioenerg. 2010, 1797, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.M.; Yang, A.L.; Kuo, C.H.; Tin, H.; Huang, C.Y.; Lee, S.D. Effects of 17β-estradiol on cardiac apoptosis in ovariectomized rats. Cell Biochem. Funct. 2010, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Komuro, I.; Yamazaki, T.; Kudoh, S.; Uozumi, H.; Kadowaki, T.; Yazaki, Y. Both Gs and Gi Proteins Are Critically Involved in Isoproterenol-induced Cardiomyocyte Hypertrophy. J. Biol. Chem. 1999, 274, 9760–9770. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.Z.; Wu, Y.; Ni, Y.J.; Liu, J.H.; Gong, M.; Wang, X.H.; Wei, F.; Wang, T.Z.; Yuan, Z.; Ma, A.Q.; et al. Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis 2013, 18, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Freund, C.; Schmidt-Ullrich, R.; Baurand, A.; Dunger, S.; Schneider, W.; Loser, P.; El-Jamali, A.; Dietz, R.; Scheidereit, C.; Bergmann, M.W. Requirement of nuclear factor-κB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 2005, 111, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yao, A.; Zhu, W.; Kudoh, S.; Hiroi, Y.; Shimoyama, M.; Uozumi, H.; Kohmoto, O.; Takahashi, T.; Shibasaki, F.; et al. Isoproterenol Activates Extracellular Signal–Regulated Protein Kinases in Cardiomyocytes Through Calcineurin. Circulation 2001, 104, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, X.; Xiao, W.; Li, B.; Wang, J.; Jin, L.; Lian, J.; Zhou, L.; Liu, J. Endoplasmic Reticulum Stress is Involved in DFMO Attenuating Isoproterenol-Induced Cardiac Hypertrophy in Rats. Cell. Physiol. Biochem. 2016, 38, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Weil, B.; Abarbanell, A.; Herrmann, J.; Tan, J.; Kelly, M.; Meldrum, D.R. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R972–R978. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Tamaskovic, R.; Yang, Z.Z.; Brazil, D.P.; Merlo, A.; Hess, D.; Hemmings, B.A. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 2004, 279, 35510–35517. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.L.; Yang, Y.S.; Wang, C.M.; Hui, N.; Gu, L.H.; Zhong, H.J.; Cai, Z.J.; Wang, Q.Q.; Zhang, Q.H.; Li, N.; et al. Endogenous Human CaMKII Inhibitory Protein Suppresses Tumor Growth by Inducing Cell Cycle Arrest and Apoptosis through Downregulation of the Phosphatidylinositide 3-Kinase/Akt/HDM2 Pathway. J. Biol. Chem. 2009, 284, 24773–24782. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, T.; Du, Q.Y.; Jovanovic, S.; Neemo, A.; Holmes, R.; Sinha, S.; Jovanovic, A. Testosterone protects female embryonic heart H9c2 cells against severe metabolic stress by activating estrogen receptors and upregulating IES SUR2B. Int. J. Biochem. Cell Biol. 2013, 45, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sukhodub, A.; Sudhir, R.; Du, Q.Y.; Jovanovic, S.; Reyes, S.; Jovanovic, A. Nicotinamide-rich diet improves physical endurance by upregulating SUR2A in the heart. J. Cell. Mol. Med. 2011, 15, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Shameem, K.; Abdul, M.; Jovanovic, S.; Du, Q.Y.; Sukhodub, A.; Jovanovic, A. A link between ATP and SUR2A: A novel mechanism explaining cardioprotection at high altitude. Int. J. Cardiol. 2015, 189, 73–76. [Google Scholar]
- Smith, K. Heart Disease in Women. Circulation 2004, 109, e207. [Google Scholar] [CrossRef]
- Huxley, V.H. Sex and the cardiovascular system: The intriguing tale of how women and men regulate cardiovascular function differently. Adv. Physiol. Educ. 2007, 31, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Michos, E.D.; Karas, R.H. Hormone replacement therapy and the cardiovascular system—Lessons learned and unanswered questions. J. Am. Coll. Cardiol. 2006, 47, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Ares-Carrasco, S.; Picatoste, B.; Camafeita, E.; Carrasco-Navarro, S.; Zubiri, I.; Ortiz, A.; Egido, J.; Lopez, J.A.; Tunon, J.; Lorenzo, O. Proteome changes in the myocardium of experimental chronic diabetes and hypertension: Role of PPARalpha in the associated hypertrophy. J. Proteom. 2012, 75, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.J.; Kuo, W.W.; Lai, Y.P.; Shibu, M.A.; Shen, C.Y.; Pai, P.; Yeh, Y.L.; Lin, J.Y.; Viswanadha, V.P.; Huang, C.Y. 17β-Estradiol and/or Estrogen Receptor β Attenuate the Autophagic and Apoptotic Effects Induced by Prolonged Hypoxia Through HIF-1α-Mediated BNIP3 and IGFBP-3 Signaling Blockage. Cell. Physiol. Biochem. 2015, 36, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H. Anti-glycative effects of asiatic acid in human keratinocyte cells. BioMedicine 2014, 4, 19. [Google Scholar] [CrossRef] [PubMed]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-H.; Kuo, W.-W.; Shibu, M.A.; Day, C.-H.; Hsieh, Y.-L.; Chung, L.-C.; Chen, R.-J.; Wen, S.-Y.; Viswanadha, V.P.; Huang, C.-Y. E2/ER β Enhances Calcineurin Protein Degradation and PI3K/Akt/MDM2 Signal Transduction to Inhibit ISO-Induced Myocardial Cell Apoptosis. Int. J. Mol. Sci. 2017, 18, 892. https://doi.org/10.3390/ijms18040892
Lin K-H, Kuo W-W, Shibu MA, Day C-H, Hsieh Y-L, Chung L-C, Chen R-J, Wen S-Y, Viswanadha VP, Huang C-Y. E2/ER β Enhances Calcineurin Protein Degradation and PI3K/Akt/MDM2 Signal Transduction to Inhibit ISO-Induced Myocardial Cell Apoptosis. International Journal of Molecular Sciences. 2017; 18(4):892. https://doi.org/10.3390/ijms18040892
Chicago/Turabian StyleLin, Kuan-Ho, Wei-Wen Kuo, Marthandam Asokan Shibu, Cecilia-Hsuan Day, You-Liang Hsieh, Li-Chin Chung, Ray-Jade Chen, Su-Ying Wen, Vijaya Padma Viswanadha, and Chih-Yang Huang. 2017. "E2/ER β Enhances Calcineurin Protein Degradation and PI3K/Akt/MDM2 Signal Transduction to Inhibit ISO-Induced Myocardial Cell Apoptosis" International Journal of Molecular Sciences 18, no. 4: 892. https://doi.org/10.3390/ijms18040892
APA StyleLin, K.-H., Kuo, W.-W., Shibu, M. A., Day, C.-H., Hsieh, Y.-L., Chung, L.-C., Chen, R.-J., Wen, S.-Y., Viswanadha, V. P., & Huang, C.-Y. (2017). E2/ER β Enhances Calcineurin Protein Degradation and PI3K/Akt/MDM2 Signal Transduction to Inhibit ISO-Induced Myocardial Cell Apoptosis. International Journal of Molecular Sciences, 18(4), 892. https://doi.org/10.3390/ijms18040892