Abstract
Study Design: Descriptive cross-sectional. Objective: The origin of the lingual artery (LA) has been well studied due to its implication in neck dissection, but the course thereafter to the oral cavity is less described. This cadaveric study traced the journey of the LA from the external carotid artery to its terminal branches in the tongue. Methods: Following bilateral neck dissections in 35 black Kenyan cadavers, the incidence of Beclard’s, Lesser’s and Pirogoff’s triangles, the types of LA origin with its length, relationship to the hyoglossus muscle and anastomosis with other vessels were documented. Results: Beclard’s triangle was found in 64 dissections (91.42%), Lesser’s in 46 dissections (65.71%) and Pirogoff’s in 39 dissections (55.71%). The LA presented either as a solitary branch (67.15%) or as a branch of either the linguofacial (LFT–24.29%), thyrolingual (TLT–2.72%) or thyrolinguofacial (TLFT–2.86%) trunk. The solitary LA was the longest at 6.93 mm, followed by the TLT branch (6.58 mm), LFT branch (6.12 mm) and TLFT branch (5.65 mm). The majority of solitary LA and LA branches of LFT and TLFT passed through the hyoglossus, while all LA branches of the TLT coursed medial to the muscle. All variants of LA have been found to anastomose with the submental artery (SMA) at frequencies that ranged from 11.10% to 100%. Conclusions: The LA was found in all cadavers and all Beclards’ triangles. There is a significant incidence of LFT and TLFT variants in the Kenyan population. The LA passed either through or medial to the hyoglossus with no lateral relationship being observed.
Introduction
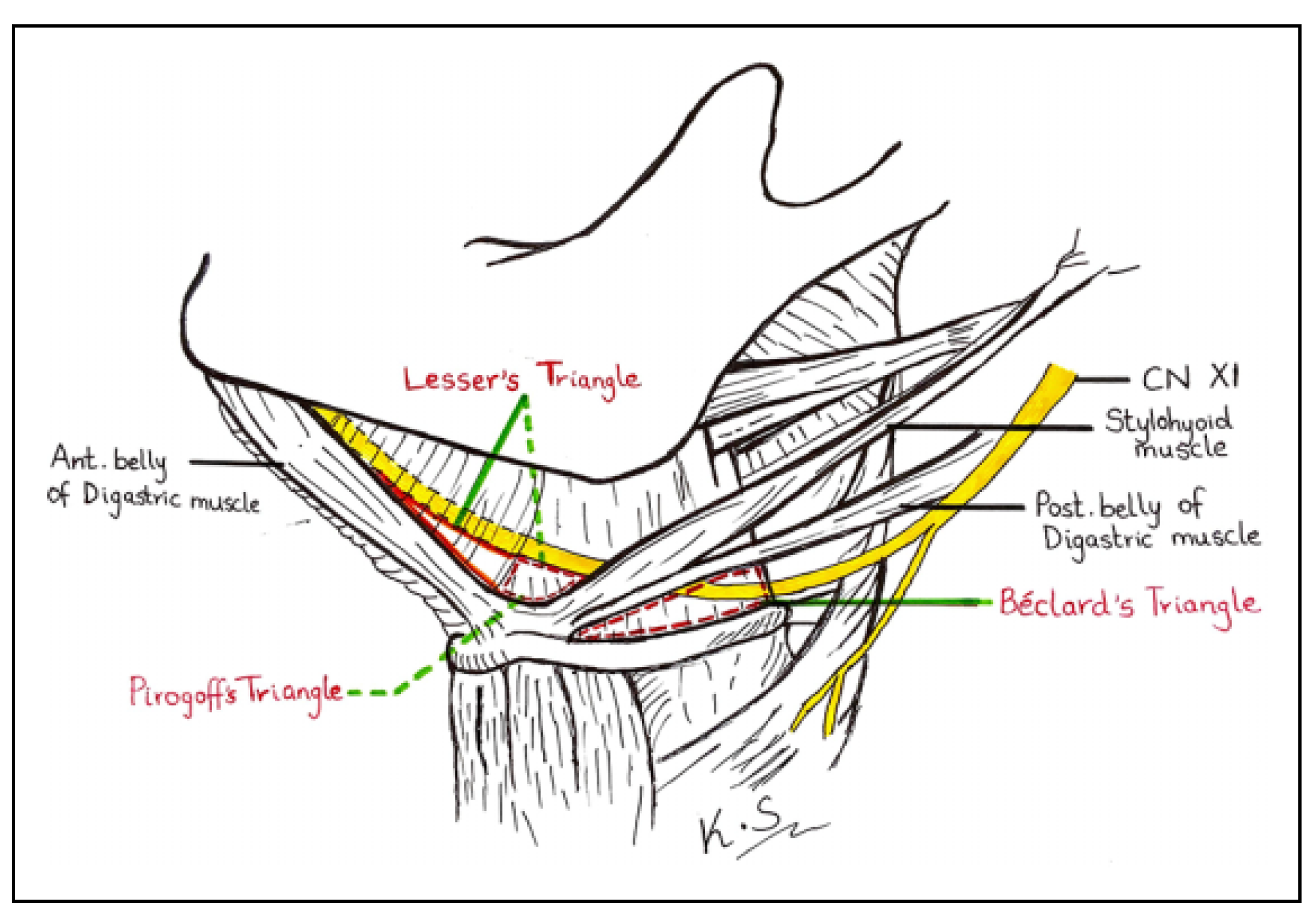
The lingual artery (LA) is an anteromedial branch of the external carotid artery (ECA) that provides perfusion to the oral cavity, especially the tongue and floor of the mouth. It arises adjacent to the greater cornu of the hyoid bone (GCHB) between the superior thyroid artery (STA) and facial artery (FA), either alone (solitary) or from a common trunk with either one or both of these vessels.[1] It runs parallel and immediately deep to the hypoglossal nerve.[2] The hyo- glossus muscle divides the LA into descriptive thirds.[1] In its first part, the artery courses within the Beclard’s triangle, a subdivision of the much larger carotid triangle. In its second part, the artery lies deep to the hyoglossus muscle, tendons of digastric and the stylohyoid muscle. At this point, the vessel is described to lie within the confines of both Lesser’s and Pirogoff’s triangles (the latter forms the posterior portion of the former bigger triangle) (Figure 1). In its third part, the vessel turns sharply upward at the anterior border of the hyoglossus and divides into the sublingual and deep lingual arteries which may form an anastomosis with other vessels beneath the frenulum of the tongue. However, this course is subjected to considerable variation and is far from the so- called norm described in many texbooks.[3]
Figure 1.
Figure showing the borders of Beclard’s, Pirogoff’s and Lesser’s triangles.
Beclard’s, Lesser’s and Pirogoff’s triangles are 3 of the numerous anatomical triangles of the neck that have been described just after the turn of the last century, but have gone out of acceptance due to the general adoption of the nomenclature subscribed by modern head and neck sur- geons.[2,4] For example, the digastric triangle is the term used to describe the subdivision of the submandibular tri- angle, instead of Pirogoff’s triangle. Figure 1 provides a visual summary of the anatomy of these 3 triangles, which have been described in detail by Tubbs et al one decade ago.[4] The importance of these triangles cannot be overem- phasized due to the fact that they act as vital surgical land- marks of the LA that may be useful to locate the vessel during emergency LA ligation. Traditionally, Pirogoff’s triangle was deemed the ideal site of exposure for the liga- tion of LA in cases of severe tongue hemorrhage.[5] Clini- cians can easily identify these triangles with palpation by using the hyoid bone as the anchor.[6] There is, however, a wide range of variations in both the presence of these triangles and the presence of the LA within them.
Population specific variations in the length of both the LA and its variant trunks have been described.[7,8,9,10,11] This knowl- edge is crucial in order to prevent severe iatrogenic injury when performing the procedures described above. Such knowledge is also important when interpreting angiograms and of course, to avoid inflicting vessel perforation or rupture during angiography.[7,12] There is, however, scarcity in regional data on the variations of the origin, length and course of the LA, with only 2 studies found describing the origin (but not the course) of the LA in African populations.[11,13] There- fore, this study aims to determine the type of origin of the LA, its length, incidence of Beclard’s, Lesser’s and Pirogoff’s triangles, course from the neck to the oral cavity and the presence of anastomosis with other vessels.
Materials and Methods
This is a cadaveric study that received ethical approval from the Department of Human Anatomy, University of Nairobi as per the Kenyan constitution. A total of 70 bilat- eral neck dissections were performed on 35 donated human cadavers, whose age of death range from 20 to 35 years. Cadavers with evidence of having undergone surgical pro- cedures in the neck or suffered from traumatic injuries to the neck were excluded from the study. For the dissection, the modified Y incision made during autopsy was extended superiorly to the mastoid process. The resulting skin flap was reflected along the inferior border of the mandible thus exposing the structures beneath, bilaterally. The carotid triangle was outlined, and the origin of the lingual artery was gently exposed within this triangle. The presence or absence of Beclard’s, Pirogoff’s and Lesser’s triangles (Figure 1) were recorded and the observation of the LA within each triangle was noted. Dissection thereafter was continued along its course to the point of termination where it divided into the sublingual and the deep lingual arteries. The length of the artery was measured based on its pattern of origin (solitary or from a trunk). In cases where the artery originated from a common trunk, the length of both the trunk and the LA branch were recorded. The relation- ship of the LA to the hyoglossus muscle throughout its course was determined as being either lateral, medial or coursing through the muscle. Finally, any anastomosis with other vessels e.g. the submental artery (SMA), was recorded. Representative photos of the vessel and its varia- tions were taken for documentation purposes.
All measurements were made bilaterally using a Vernier callipers (Mitutoyo 500-196-30 600 AOS Digital Calliper). The average of each measurement was calculated and ana- lyzed using SPSS (IBM version 27). A paired t-test was used to compare the right and left sides for any statistically significant difference between the results. A P-value of 0.05 was considered as significant.
Results
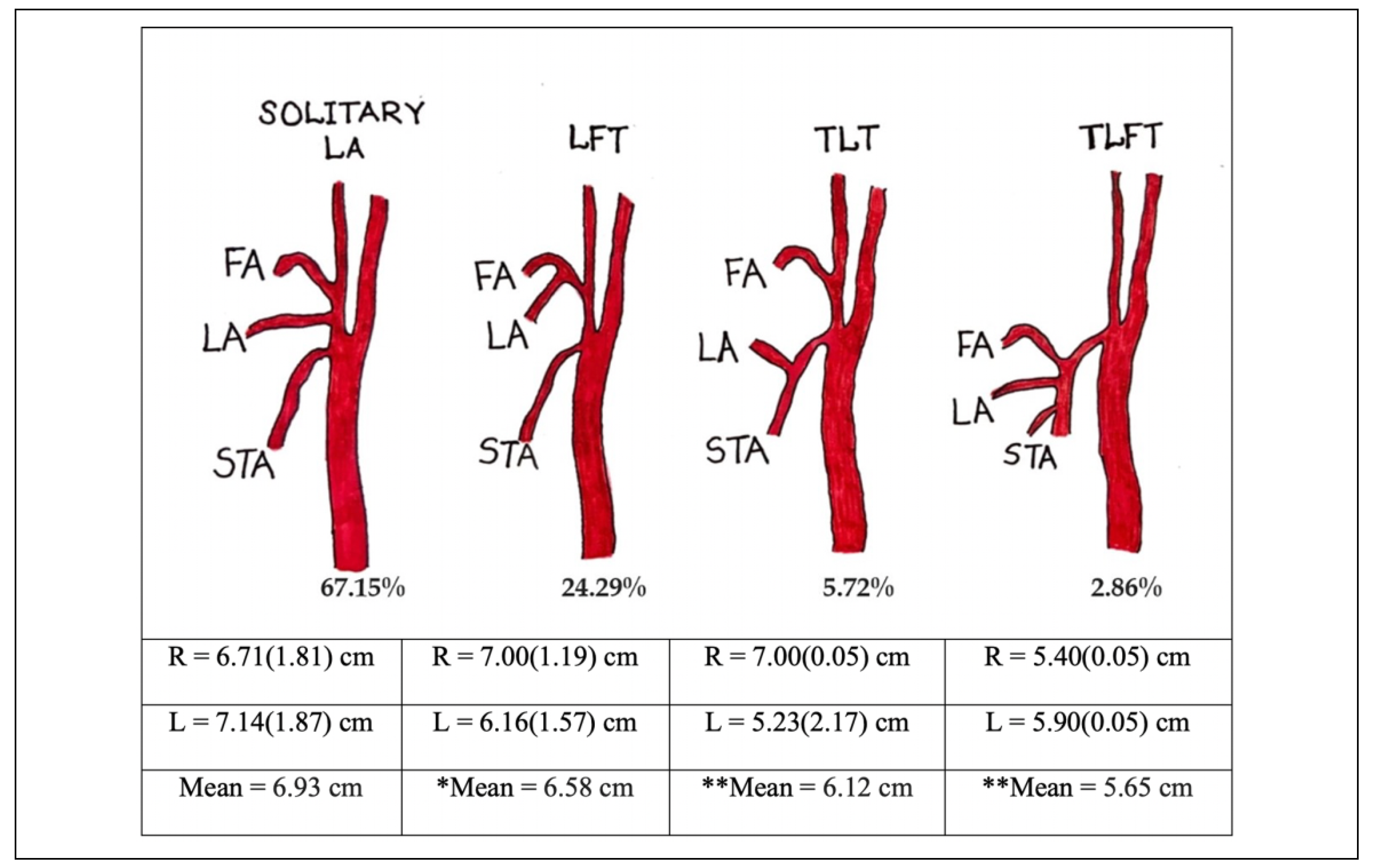
The LA was found to be present in all cadavers dissected, with more than half of them (57%) not being symmetrical in their pattern of origin. The LA was found to be present either as a solitary branch from the ECA or as a branch of either the linguofacial (LFT), thyrolingual (TLT) or thyr- olinguofacial (TLFT) trunk. The occurrence of each of these variants has been described in detail in the previous paper by Sarna et al,[14] so only pertinent information will be mentioned in the current report. The length of the LA and the incidence of variant trunks is summarized in Figure 2. The results reveal that solitary LA was slightly longer on the left than the right and of the 3 variant trunks, the TLT was found to be the longest followed by the LFT and TLFT respectively. The trunk length for the LF variant was 1.14 + 0.52 mm on the right and 1.14 + 0.51 mm on the left side. Corresponding trunk length for the TL variant was 1.50 + 0.05 mm and 1.43 + 1.12 mm while that of the TLF variant were 0.30 + 0.05 mm and 0.20 + 0.05 mm on the right and left sides respectively. All differences related to the sides were not statistically significant (P > 0.05)
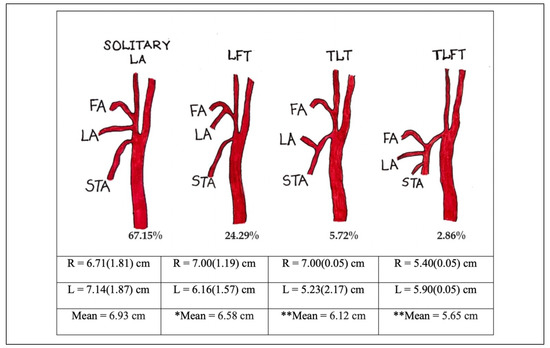
Figure 2.
The incidence and lengths of Solitary LA and LA branches from various trunks. There was significant difference between the 2 sides for *LFT (P ¼ 0.013), **TLT and TLFT (P < 0.01). LA—Lingual artery, LFT—Linguofacial trunk, TLT—Thyrolingual trunk, TLFT— Thyrolinguofacial trunk, FA—Facial artery, STA—Superior thyroid artery.
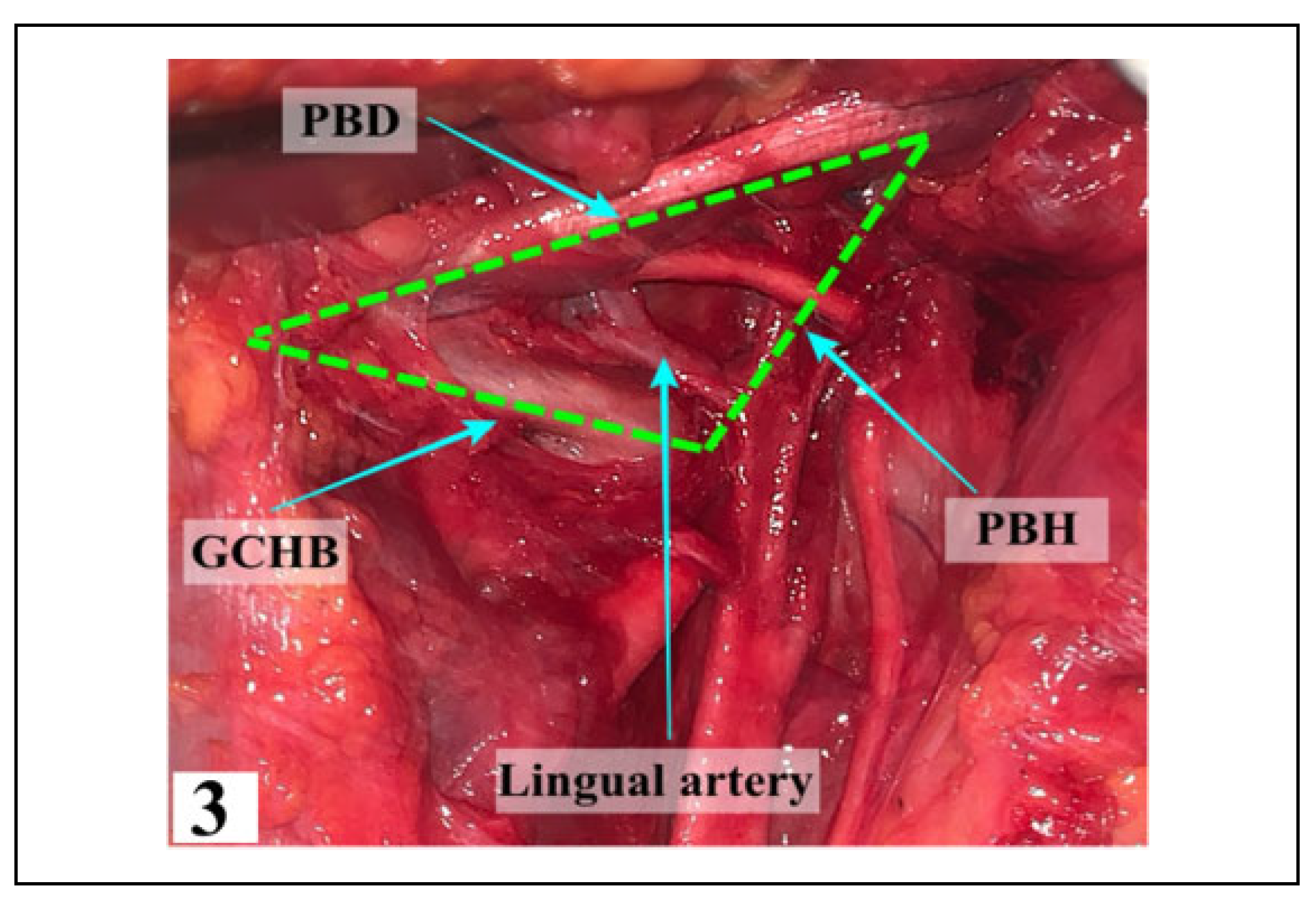
All 3 triangles (Beclard’s, Pirogoff’s and Lesser’s trian- gles) were found variably. Beclard’s triangle (Figure 3) was found in 64 dissections (91.42%) with the remainder (8.58%) being absent because the posterior belly of the digastric muscle did not touch the hyoid bone or because the posterior belly of the digastric muscle was directly over the greater cornu of the hyoid bone (Table 1). The LA was always found to be present in the triangle seen, located 13.20 + 2.75 mm above the greater cornu of the hyoid bone.
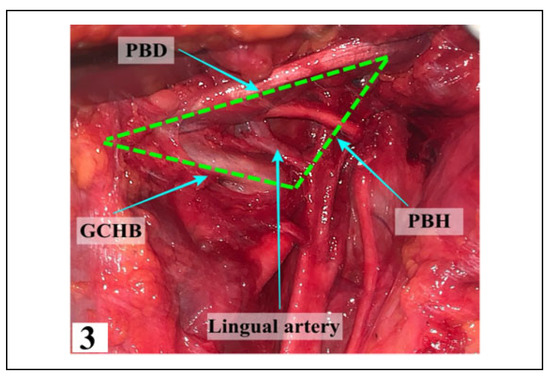
Figure 3.
The boundaries of Beclard’s triangle with the LA coursing through. PBD—Posterior Belly of Digastric, GCHB— Greater Cornu of Hyoid Bone, PBH—Posterior Border of Hyoglossus.

Table 1.
Distribution of the Lingual Artery Within the Triangles.
In comparison, Pirogoff’s triangle was identified in 46 dissections (65.71%) while in the remainder of dissections it was absent when the hypoglossal nerve was located infer- ior to superior border of the digastric tendon. The LA was not always present in Pirogoff’s triangle (Table 1). The average distance of the LA to hyoid bone was 8.57+ 2.70 mm. The LA has variable relationship with the hypo- glossal nerve within this triangle. In the 65.71% of cases, the LA was found at a distance of 4.50 + 1.45 mm below the hypoglossal nerve, however in the 34.29% of cases, the LA was at an average distance of 2.50 + 1.20 mm above the nerve.
Lesser’s triangle was the least encountered triangle. It was found in 39 dissections (55.71%) but was absent when the hypoglossal nerve coursed below the belly of the ante- rior digastric muscle. The results show that the LA was absent from the triangle if the triangle itself was not found and not because of a variation in vessel course.
The solitary LA and the LA branch of the variant trunks were noted to pass either through or medial to the hyoglos- sus with, no lateral relationship being observed. As pertain- ing to the solitary LA, LA branch of LFT and TLFT, the majority passed through the muscle, while in all cases the LA branch of the TLT coursed medial to the muscle (Table 2).

Table 2.
Relationship of the LA to the Hyoglossus Muscle. .
The results reveal that 16.00% and 13.60% of the soli- tary LA on the right and left sides respectively anastomosed with the submental artery (SMA). As pertains the variant trunks, the LA branch of the LFT and TLT did not form an anastomosis on the right side in contrast to the results found on the left. The TLFT anastomosed with the SMA in all cases on both sides of the neck (Table 3)

Table 3.
Percentage of Anastomosis of the LA with the SMA.
Discussion
The LA, as the second branch of the ECA often arises as a solitary artery opposite the tip of GCHB. Sometimes it arises from a common trunk with the FA, STA or both. It usually lies inferior to the hypoglossal nerve although inci- dence of superiorly located LA has been reported.[15] The LA may get lacerated during intraoral procedures, resulting in profuse hemorrhage. Intraoral ligature of the LA may be necessary, but it is not an easy procedure due to the confine of the tight access in the oral cavity.[6] Alternatively, extra- oral ligation may be needed to stop the uncontrollable hemorrhage.[15] However, one needs to be aware of the pos- sible presence of any of these variants in order to not cause untoward events. This is more so, when current surgical procedures become more minimally invasive, with smaller incisions being made. In recent years, transoral robotic surgery (TORS) for example, has been used for the removal of base of tongue cancer.[16] TORS allows a clearer view of the surgical field and better visualization of structures through minimal approach. However, one complication reported to be associated with this surgical technique is hemorrhage, with the lingual artery being affected in the resection of tongue base cancer.[17] This is the situation where prophylactic ligation of the ECA or its branches has been proposed.[18] In this scenario, the application of knowl- edge derived from the current or similar study will be of great assistance to identify the appropriate landmark with- out the need to make a large neck incision.
Zu¨mre et al, in their study on human fetuses found a LFT in 20%, a TLT in 2.5% and a TLFT in 2.5% of the human fetuses studied.[19] Our current study follows this distribu- tion, albeit a higher percentage being observed for the LFT and TLFT. Because of this, the incidence of solitary LA is lower, in contrast to the majority of other studies which reported incidence between 77.1% and 90.0%.[20,21,22,23,24,25,26,27,28,29] The current finding is closer to the incidence of solitary LA observed in the Japanese.[30]
The LFT is the most commonly observed LA variant, followed by the TLT variant. TLFT, on the other hand is uncommon. [7,13,23,31,32] Several authors reported observing between 17.5% -19.9% of LFT and between 1%-10.5% of TLT. [7,21,23,31,33] The finding of the current study is not in agreement with most studies due to the higher prevalence of LFT and presence of TLFT in the current population. TLFT is rare, with most studies reporting below 1%.[7] The current study found similar incidence with that reported by Zumre et al [19] (2.5%), but is lower than that reported for the Kenyan population, which was 6.5%.[34]
More specifically, the incidence of 2.5% to 31.0% has been reported for the LFT by various authors, with most studies reporting it as the most common variant found. [13,21,23,28,32,35] Similar to TLFT, its incidence has been reported to be several times higher in Africans.[11,30] The incidence of LFT found in this study is slightly more than half of the incidence reported for the previous black Kenyan samples. Our finding of higher prevalence of LFT however, falls within the high incidence range observed for the Latin American and Japanese.[6,28,33] Lastly, the LA has been reported to also arise from the bifurcation level,[7,35] or even the common carotid artery [36] but no such occasions has been observed in the current study.
A recent publication from a Korean center reported that the LA was the least used recipient artery for free tissue transfer in head and neck reconstruction but no reason was provided.[37] This may be related to the fact that this artery is ligated or sacrificed during tumor removal or neck dissec- tion, a fact highlighted by Chia et al (2011).[38] Findings of the current study showed that the mean length of the LA (regardless of the type of trunk) and TLFT was lower than that reported in the Chinese [8,9] and Indian populations,10 which can be a hindering factor for anastomosis. However, the lengths of the LFT and TLT were higher than several other populations.[7,34,39,40]
Knowledge of the length of the LA as well as its trunks should also be kept in mind during procedures involving hemiglossectomy or total glossectomy. [7,41,42] For instance, knowing the length of the expected trunks once visualized, might help in locating the expected branching position of the LA.[30] The authors believe the current findings will be useful when performing these procedures in patients of Kenyan descent. The differences observed in the Kenyans could be due to population specific embryological factors governing the development of the vessel.
Beclard’s triangle has been found to be present in major- ity of cases with a study reporting an incidence of 82.5%.[4] This is in contrast to the present study which reported an even higher incidence of 91%. Studies have shown that the inferior portion of the hypoglossal nerve often approaches the bifurcation of the common carotid artery, and is crossed by the LA.[43] The results of this study showed that the LA was consistently present within this triangle as long as the triangle itself was present. Based on our results, the triangle could be a convenient and consistent landmark for the iden- tification of the LA and also the hypoglossal nerve during neck dissection.[44]
According to literature, Pirogoff’s triangle was found in 82.4% and 58.2% in the British and North American popu- lations respectively.[4,15] In the current setting it was encountered in 65.71% of cases, which falls between the ranges reported. In comparison to a study by Homze et al, the LA was present in the triangle in 84.6% of cases which lies in contrast to the present study.[15] Ligation of the LA within Pirogoff’s triangle has been used to control severe intraoral hemorrhage that would otherwise cause swelling in the floor of the mouth resulting in airway embarrass- ment.[45,46] Therefore, in consideration of the high reliability of the presence of the LA within the triangle and ease of access, it may be a suitable location to gain access to the artery during surgical procedures involving the head and neck region.[47]
Lastly, a study by Tubbs et al revealed that Lesser’s triangle was present in 88.23% of cases which stands in stark contrast to the present study in which it was found in only 55.71%.[4] The findings of that study revealed that the Lesser’s triangle did not contain any major structures as the lingual artery coursed deep to its floor, thus excluding it from the triangle. However, in this study, the LA was found in 57.14% on the left and 54.29% on the right side of the neck in cases where Lesser’s triangle was present.
The LA has been reported to travel lateral to the hyo- glossus muscle in a number of cases,[15] but none was observed in the current setting. The current findings were different from standard literature which dictates that the LA passes medial to the hyoglossus muscle.[47] While it was shown in a study done among the Indian population that the LA passed through the hyoglossus, it is crucial to note that this was a case report. In other studies done among the Indian and Japanese populations, the LA was mostly med- ial to the hyoglossus muscle.
The basis for the different possible relations of the LA to the hyoglossus might stem from anomaly in the embry- ological origin. It has been postulated that where the arteries coursing either medial, lateral, or penetrating the hyoglossus (once formed entirely), it is likely that, through some selection during development, the medial course, having the highest frequency of occurrence, remained preferentially as the route of LA.[3,48] The differ- ence between our results and those observed from other populations might be explained by specific molecular fac- tors whose presence is dependent upon either race or genetic make-up of individuals which might then affect development of the vessel.
Concerning its anastomosis with the SMA, the vessel was observed not to anastomose in a majority of cases when it originated as a single trunk, LFT or TLT bilaterally. However it was noted to anastomose in cases with a TLFT. There is paucity of data to the best of our knowledge, with only few studies assessing the anastomosis of the LA to the SMA. This anatomical information is crucial since the high rates of bleeding at the floor of the mouth may be attribu- table to this anastomosis and therefore the SMA has to be considered as the second artery to clamp when ligation of the seemingly normal lingual artery is not effective.[3,49]
Conclusion
The cross-sectional samples in this study showed a higher incidence of LFT and TLFT, with their trunk lengths (including that of the TLT) being longer than reported in other ethnicities. The mean length of solitary LA was 6.93 cm which was lower in comparison to other population groups. Beclard’s, Pirogoff’s and Lesser’s triangles were found in 91.42%, 65.71% and 55.71% of samples. The LA was found in all cadavers and all Beclards’ triangles. The LA passed either through or medial to the hyoglossus with no lateral relationship being observed. It may anastomose with the SMA.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Shangkuan H, Xinghai W, Zengxing W, Shizhen Z, Shiying J, Yishi C. Anatomic bases of tongue flaps. Surg Radiol Anat.
- Xu H, Jazayeri L, Matros E, Henderson PW. Anatomy, expo- sure, and preparation of recipient vessels in microsurgical head and neck reconstruction. J Reconstr Microsurg, 9: 37(2).
- Seki S, Sumida K, Yamashita K, Baba O, Kitamura S. Gross anatomical classification of the courses of the human lingual artery. Surg Radiol Anat.
- Tubbs RS, Rasmussen M, Loukas M, Shoja MM, Cohen- Gadol AA. Three nearly forgotten anatomical triangles of the neck: triangles of Beclard, Lesser and Pirogoff and their potential applications in surgical dissection of the neck. Surg Radiol Anat.
- Van Es RJJ, Thuau H. Pirogoff’s triangle revisited: an alter- native site for microvascular anastomosis to the lingual artery. A technical note. Int J Oral Maxillofac Surg, 2: 29(3).
- Lins ACCS, Cavalcanti SJ, do Nascimento LD. Extraoral ligature of lingual artery: anatomic and topographic study. Int J Morphol.
- Herrera-Nu’n˜ez M, Menchaca-Gutie’rrez JL, Pinales-Razo R, et al. Origin variations of the superior thyroid, lingual, and facial arteries: a computed tomography angiography study. Surg Radiol Anat, 1085.
- Guan J, Yin S, Yi H, et al. Anatomic characteristics and relationship of lingual artery and hypoglossal nerve with ton- gue base. Lin Chuang Er Bi Yan Hou Ke Za Zhi.
- Ye J, Wang J, He L, Han D. Anatomic characteristics of lingual artery and midline glossectomy. Zhonghua er bi yan hou ke za zhi.
- Baxla M, Kumari C, Kaler S. Bilateral thyrolinguofacial trunk: unusual and rare branching pattern of external carotid artery. Anat Cell Biol.
- Ogeng’o J, Misiani MK, Loyal P, et al. Variations in branch- ing pattern of external carotid artery in a black Kenyan pop- ulation. Anat J Africa.
- Wolf J, Mattila K, Hietanen J, Kozeltsev AL. A stereoangio- graphic study of the arterial variations in the external carotid system. Dentomaxillofac Radiol.
- Zunon-Kipre’ Y, Ouattara D, Broalet E, et al. Investigation of the collateral branches of the external carotid artery in pop- ulation of West Africa. About 60 cadavers dissections. Mor- phologie.
- Sarna K, Kamau M, Sonigra KJ, Amuti T. Anatomical variations in the origin of the lingual artery in the Kenyan population. Craniomaxillofac Trauma Reconstr, 6 January 2021. [CrossRef]
- Homze EJ, Harn SD, Bavitz BJ. Extraoral ligation of the lingual artery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
- Chung TK, Rosenthal EL, Magnuson JS, Carroll WR. Trans- oral robotic surgery for oropharyngeal and tongue cancer in the United States. Laryngoscope.
- Hay A, Migliacci J, Karassawa Zanoni D, et al. Haemorrhage following transoral robotic surgery. Clin Otolaryngol, 6: 43(2).
- Gleysteen J, Troob S, Light T, et al. The impact of prophy- lactic external carotid artery ligation on postoperative bleed- ing after transoral robotic surgery (TORS) for oropharyngeal squamous cell carcinoma. Oral Oncol.
- Zu¨mre O, Salbacak A, Cic¸ekcibas¸i AE, Tuncer I, Seker M. Investigation of the bifurcation level of the common carotid artery and variations of the branches of the external carotid artery in human fetuses. Ann Anat.
- Sanjeev IK, Anita H, Ashwini M, Mahesh U, Rairam GB. Branching pattern of external carotid artery in human cada- vers. J Cin Diagno Res, 3128.
- Mata JR, Mata FR, Souza MCR, Nishijo H, Ferreira TAA. Arrangement and prevalence of branches in the external car- otid artery in humans. Ital J Anat Embryol.
- Lohan Dg, Barkhordarain F, Saleh R, et al. MR Angiography at 3T for Assessment of the External carotid Artery System. Am J Roentegenol, 1088.
- Hayashi N, Hori E, Othani Y, Kuwayama N, Eudo S. Surgical Anatomy of the cervical Artery for carotid endarterectomy. Neurol Med Chir (Tokyo).
- Esakkiammal N, Renu C, Rakhee S. Implications of variable origin of external carotid artery branches and high level bifurcation of common carotid artery. Int J Anat Res, 3: 5(2.3), 3958.
- Heltzel S, Jelinek L, Jaynes D.Variation in the caudal branches of the external carotid artery: Comparison of sex and side. Med Res Arch.
- Anuradha M, Chitra S. A study of the common origin of lingual and facial artery from the external carotid artery. Int J Anat Res, 3656.
- Delic J, Savkovic A, Bajtarevic A, Isakovic E. Variations of ramification of external carotid artery—common trunks of collateral branches. Period Biol.
- Yonenaga K, Tohnai I, Mitsudo K, et al. Anatomical study of the external carotid artery and its branches for administration of superselective intra-arterial chemotherapy via the super- ficial temporal artery. Int J Clin Oncol.
- Ozgur Z, Govsa F, Ozgur T. Anatomic evaluation of the car- otid artery bifurcation in cadavers: Implications for open and endovascular therapy. Surg Rad Anat.
- Shintani S, Terakado N, Alcalde RE, et al. An anatomical study of the arteries for intraarterial chemotherapy of head and neck cancer. Int J Clin Oncol.
- Czerwinski, F. Variability of the course of external carotid artery and its rami in man in the light of anatomical and radiological studies. Folia Morphol (Warsz).
- Shima H, von Luedinghausen M, Ohno K, Michi K. Anatomy of microvascular anastomosis in the neck. Plast Reconstr Surg.
- Fazan VP, da Silva JH, Borges CT, Ribeiro RA, Caetano AG, Filho OA. An anatomical study on the lingual-facial trunk. Surg Radiol Anat.
- Ongeti KW, Ogeng’o JA. Variant origin of the superior thyroid artery in a Kenyan population. Clin Anat.
- Lucev N, Bobinac D, Maric I, Drescik I. Variations of the great arteries in carotid triangle. Otolaryngol. Head Neck Surg.
- Koneko K, Akita M, Murato E, Imai M, Sowa K.Unilateral anomalous left common carotid artery: a case report. Ann Anat.
- Chung JH, Kim KJ, Jung KY, Baek SK, Park SH, Yoon ES. Recipient vessel selection for head and neck reconstruction: a 30-year experience in a single institution. Arch Craniofac Surg.
- Chia HL, Wong CH, Tan BK, Tan KC, Ong YS. An algorithm for recipient vessel selection in microsurgical head and neck reconstruction. J Reconstr Microsurg.
- Lemaire V, Jacquemin G, Nelissen X, Heymans O. Tip of the greater horn of the hyoid bone: a landmark for cervical sur- gery. Surg Radiol Anat.
- Jadhav SD, Ambali MP, Patil RJ, Roy P. Thyrolingual trunk arising from the common carotid bifurcation. Singapore Med J.
- Mun MJ, Lee C-H, Lee B-J, et al. Histopathologic evaluations of the lingual artery in healthy tongue of adult cadaver. Clin Exp Otorhinolaryngol.
- Pons Y, Gauthier J, Cle’ment P, Conessa C. Ultrasonic partial glossectomy. Head Neck Oncol.
- Tubbs RS, Salter EG, Oakes WJ. Anatomic landmarks for nerves of the neck: a vade mecum for neurosurgeons. Neuro- surgery, 2: Suppl).
- Kikuta S, Iwanaga J, Kusukawa J, Tubbs RS. Triangles of the neck: a review with clinical/surgical applications. Anat Cell Biol.
- Saino M, Akasaka M, Nakajima M, et al. A case of a ruptured lingual artery aneurysm treated with endovascular surgery. No Shinkei Geka.
- Kaftan B, Snyder HS. Lingual artery hematoma resulting in upper airway obstruction. J Emerg Med.
- Lettau J, Bordoni B. Anatomy, head and neck, lingual artery. In: StatPearls [Internet]. StatPearls Publishing; 20. Updated July 27, 2020. Accessed January 14, 2021. https://www.ncbi.nlm.nih. 20 January 5545.
- Masui T, Seki S, Sumida K, Yamashita K, Kitamura S. Gross anatomical classification of the courses of the human sublin- gual artery. Anat Sci Int.
- Bavitz JB, Harn SD, Homze EJ. Arterial supply to the floor of the mouth and lingual gingiva. Oral Surg Oral Med Oral Pathol.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).