Patient-Specific Solutions for Cranial, Midface, and Mandible Reconstruction Following Ablative Surgery: Expert Opinion and a Consensus on the Guidelines and Workflow
Abstract
:1. Introduction
2. Workflow
2.1. Clinical Examination
2.2. Preoperative Imaging
2.2.1. Optimizing Data for Surgical Planning
2.2.2. Technical Considerations
2.3. 3D Modeling
2.3.1. Requirements for Planning Software
2.3.2. Modeling and Segmentation
2.4. Virtual Surgery Planning
2.4.1. Planning Mode
In-House Planning by the Surgeon
Service Model
Backward Planning
2.4.2. Virtual Planning for Resection
2.4.3. Planning the Reconstruction
- Determining whether opening or wedge osteotomies are feasible based on vascular supply and transplant type.
- Calculating precise bone segment sizes and positions to avoid nonunion and ensure adequate vascularization.
- Minimizing donor site morbidity by predefining bone length and shape, especially in the scapula and iliac crest.a
- Protecting perforators in combined soft tissue and bone transplants.
- Ensuring proper orientation of the vascular pedicle for easy connection to local vessels.
2.4.4. Dental Rehabilitation
Final Review and Documentation
2.5. Guides and Implants Design
2.5.1. Patient-Specific Resection and Drilling Guides
- Tissue compatibility and sterilizability: Guides are commonly made from materials like titanium, polyamide, or their combinations.
- Identification: Guides should be marked with a registration number, usage side, and, if applicable, color-coded for easy identification.
- Contamination prevention: During procedures involving saws, chisels, or piezoelectric devices, guides must not shed material chips into the wound.
- Cooling channels: Resection or drilling guides should include irrigation channels or holes to facilitate cooling during surgery.
- Perfect fit and precise positioning: Accurate placement depends on artifact-free imaging and anatomically distinct structures as reference points.
- Secure fixation: Guides must remain fixed in position, typically achieved through preplanned screw placements.
- Delicate yet stable design: Guides must balance fragility and robustness to ensure safe operation, even in restricted surgical areas.
- Angle considerations: Working angles dictated by soft tissue access must be factored into the guide design, particularly for sawing, drilling, or piezoelectric procedures.
- Drilling templates: These must include correctly sized drill channels and angles, with optional sleeves for dental implant placement.
- Technical support features: Resection templates should be equipped with flanges or slots to ensure precise cuts.
- Protection of vascularized transplants: Guides should safeguard the vascular pedicle and perforators in transplant procedures.
- Easy removal: Guides must be simple to remove after use.
- Cost efficiency: Manufacturing costs should remain low to make these guides widely accessible.
Technical Aspects of Resection and Drilling Guides
- Flange Guidance: A flange on the template defines the planned resection line by serving as a physical boundary.
- Slot Guidance: The resection is performed within a pre-designed slot in the template.
- Exact plate positioning via predefined screw holes.
- Maintenance of correct angulation to the bone surface.
- Controlled depth for bicortical fixation, ensuring structural stability.
- Avoidance of damage to critical structures like the bulbus oculi, tooth roots, or nerves during drilling.
Applications in Donor Sites
Fibula
- Angio-CT as a foundation: The data provide detailed information on blood circulation in the legs, enabling the selection of the optimal donor side and planning alternatives in case of vascular anomalies.
- Precise measurements and vascular assessment: Virtual planning allows exact measurement of the fibula’s dimensions, visualization of its vascular supply, and identification of necessary perforators for fasciocutaneous soft tissue portions. It also ensures preservation of critical structures, such as the caudal (6–8 cm) and proximal (8 cm) fibular stumps, to maintain ankle joint stability and protect the proximal fibula-tibial joint and the lateral collateral ligament of the knee.
- Contour optimization through osteotomies: Wedge osteotomy can be planned with precision, ensuring that all bone segments remain in the desired spatial plane under full contact. Flanges or guide slots in the sawing templates facilitate true-to-angle osteotomies.
- Drill guides for segment alignment: Integration of drill guides into cutting templates ensures the precise alignment of fibula segments to the desired shape, pre-drilled for attachment to patient-specific osteosynthesis plates. Drilling is usually monocortical to preserve vascular integrity.
- Time efficiency and standardization: These guides save time, standardize the procedure, reduce complications, and enable selection of the optimal bone segment for reconstruction.
Iliac Crest
- Angio-CT of the pelvis as a basis: The imaging provides detailed information about the vascular supply and conditions of the iliac and femoral arteries, helping to identify stenoses caused by arteriosclerosis. It also aids in selecting the optimal donor side, shape, and size of the graft.
- Template fixation and complex shapes: Templates are fixed with screws and simplify osteotomy for complex structures, such as the mandibular angle and ascending mandibular branch with the articular process. Templates also help determine whether the anterior iliac spine can be preserved.
- Angio-CT of the shoulder region as a basis: This imaging allows visualization of the scapula’s vascular supply, aiding in the selection of the donor site, such as the lateral scapular edge (a. circumflexa scapulae) or scapular tip (a. angularis scapulae). It also highlights the residual bone configuration, helping assess the risk of secondary fractures in narrow bone bridges.
- Virtual transplant fitting: The scapula is virtually modeled, transferred to the defect site, and adjusted to match the recipient area’s requirements. This ensures an optimal fit for complex reconstructions, such as basal maxillary defects requiring a horseshoe-shaped graft.
- Template supports and vascular preservation: Virtual planning determines the stilt-like supports for sawing and drilling templates, placed outside the transplant boundaries. These supports are screwed to the underlying bone after muscle repositioning, with careful attention to preserving the vascular supply along the lateral scapular edge.
- Guided osteotomies: Osteotomies are performed after muscle separation, guided by the template frame. For added flexibility, split stencils can be used when needed (Figure 2).
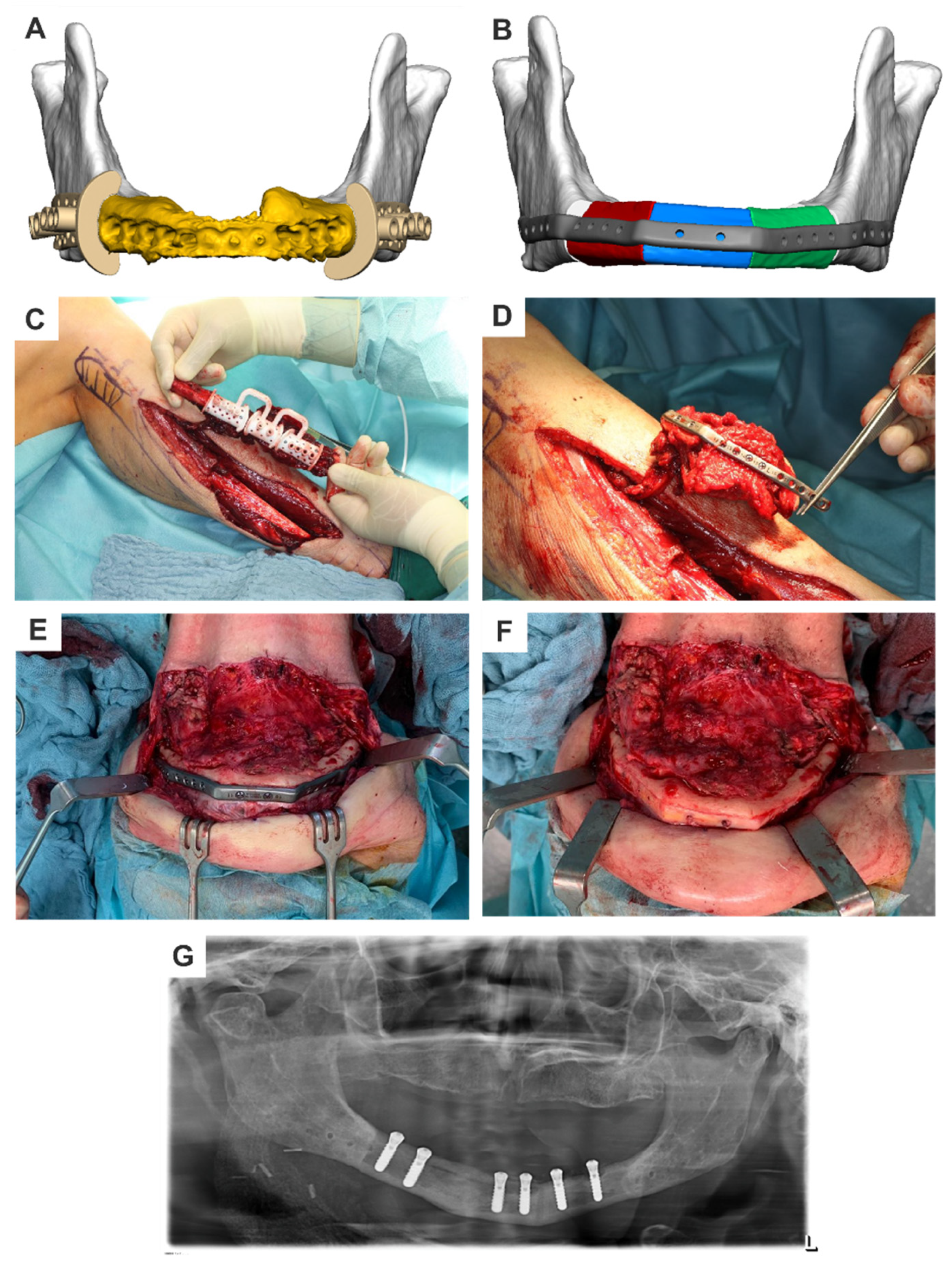
2.5.2. Patient-Specific Implants—Mandible
- a.
- Patient-specific reconstruction (PSR) plates
- Precise planning and visualization: Virtual planning ensures accurate positioning of the plate and screws, reducing the risk of damaging critical structures such as the inferior alveolar nerve, teeth, or dental implants.
- Optimal positioning: Factors like local bone availability, soft tissue conditions, additional interventions, and aesthetic considerations (e.g., avoiding chin over-projection) are integrated into the design.
- Time efficiency during surgery: Preoperative planning and the use of drilling templates eliminate the need for intraoperative plate bending.
- Material integrity: Plates are manufactured with a perfect fit, eliminating mechanical stress from overbending and reducing the risk of material breakage.
- Soft tissue compatibility: Patient-specific plates avoid unnecessary edges and corners, minimizing tissue irritation compared to prefabricated options.
- Improved force distribution: Plates allow free screw positioning, enhancing stability in cases of reduced bone density.
- Increased plate stability: Sections without required screw fixation are manufactured without screw holes, maintaining plate strength while optimizing thickness.
- Adaptability: PSR plates are particularly beneficial in cases with complex anatomical shapes or restricted adaptability of prefabricated plates, including controlled transoral or lingual applications.
- Time-intensive production: The design and manufacturing process requires significant time, making PSR plates unsuitable for emergencies.
- Irreversible errors: Planning mistakes, such as incorrect condyle positioning, cannot be corrected after the plate’s production.
- Limited flexibility: Plates configured for specific bone transplants may not accommodate alternative grafts if the initial transplant fails.
- Persistent complications: Issues such as plate breakage, screw loosening, and fractures near screws occur with PSR plates, though comparative data with prefabricated plates remain scarce.
- Cost: PSR plates are more expensive than prefabricated alternatives.
- Complex designs: Virtual planning can lead to overly complex solutions, particularly when used by inexperienced individuals. These designs may not align with soft tissue conditions, increasing the risk of complications.
- Lack of evidence-based data: Despite decades of use, there is limited data on optimal plate size, shape, or load conditions in varying scenarios. Conventional plates are often overengineered for maximum load, while CAD/CAM PSR plates demand tailored designs that remain underregulated for technical and legal reasons.
- PositionThe plate usually runs along the lower edge of the lower jaw body and along the posterior edge of the ascending lower jaw branch. To optimize force distribution, the plate can be doubled in a fork shape in the ascending ramus of the lower jaw and in the chin region, according to the bone trajectories. In the defect area, the plate’s course depends on whether a bone graft should be fixed or if the defect should only be closed with soft tissue.When using bone grafts, it is advantageous to slightly move the plate cranially. For fibula transplants, the bone should be relocated more into the alveolar process area, unless doubling (overbarrel and underbarrel) is planned. This facilitates later dental implant rehabilitation. In the case of iliac crest and scapula transplants, the vascular pedicle is spared.If the defect area is only closed with soft tissue, moving the part of the plate bridging the defect lingually can reduce tissue tension over the plate and lower the risk of material exposure [9].
- LengthPlate length is determined by the size of the defect. When using locking screws for plate fixation, there should be space for at least three screws. For non-locking screws, at least four screws are recommended if there is sufficient residual bone volume, with a minimum distance of five millimeters from the resection edge to the first screw. If conditions permit, increasing the number of screws will not do any harm. It will create a reserve in case bony resection becomes necessary.Regardless of the defect length, in case of edentulous, atrophic lower jaws, an attempt should be made to fix the screws in the better-supplied, muscle-covered areas of the chin and jaw angles/ascending lower jaw regions.
- ThicknessDifferent profile heights can be used depending on the procedure. Low profiles (2 mm) can be used for bony reconstructions, while thicker plates with higher profiles (2.5 mm) are recommended for continuity reconstructions without bone grafts.
- Screw holes/screwsAs previously described in connection with the type of screw used, the number of screw holes in the local bone varies. It is advisable to use at least three locking screws per side and at least four non-locking screws per side. These screws are typically fixed bicortically.
- Angulation and drilling vectorsTo protect functionally important structures such as nerves or teeth, drill holes can be angled up to a maximum of 15 degrees. The angulation is predetermined with the drilling templates, so only in cases of very loose bone structure it is necessary to ensure the desired angle is maintained during screwing.
- b.
- Condylar replacement
- Osteochondral rib grafts (costochondral transplants):Osteochondral rib grafts are indicated for short-length defects involving loss of the condyle and condylar process. Virtual resection planning enables precise design and implementation of these grafts:
- 3D Planning and Size Determination: The size and shape of the graft are defined using CT data and translated into sawing and drilling templates for either primary or secondary reconstruction.
- Patient-Specific Plates: These plates stabilize the relatively soft rib bones and support graft positioning, ensuring secure fixation and functional restoration.
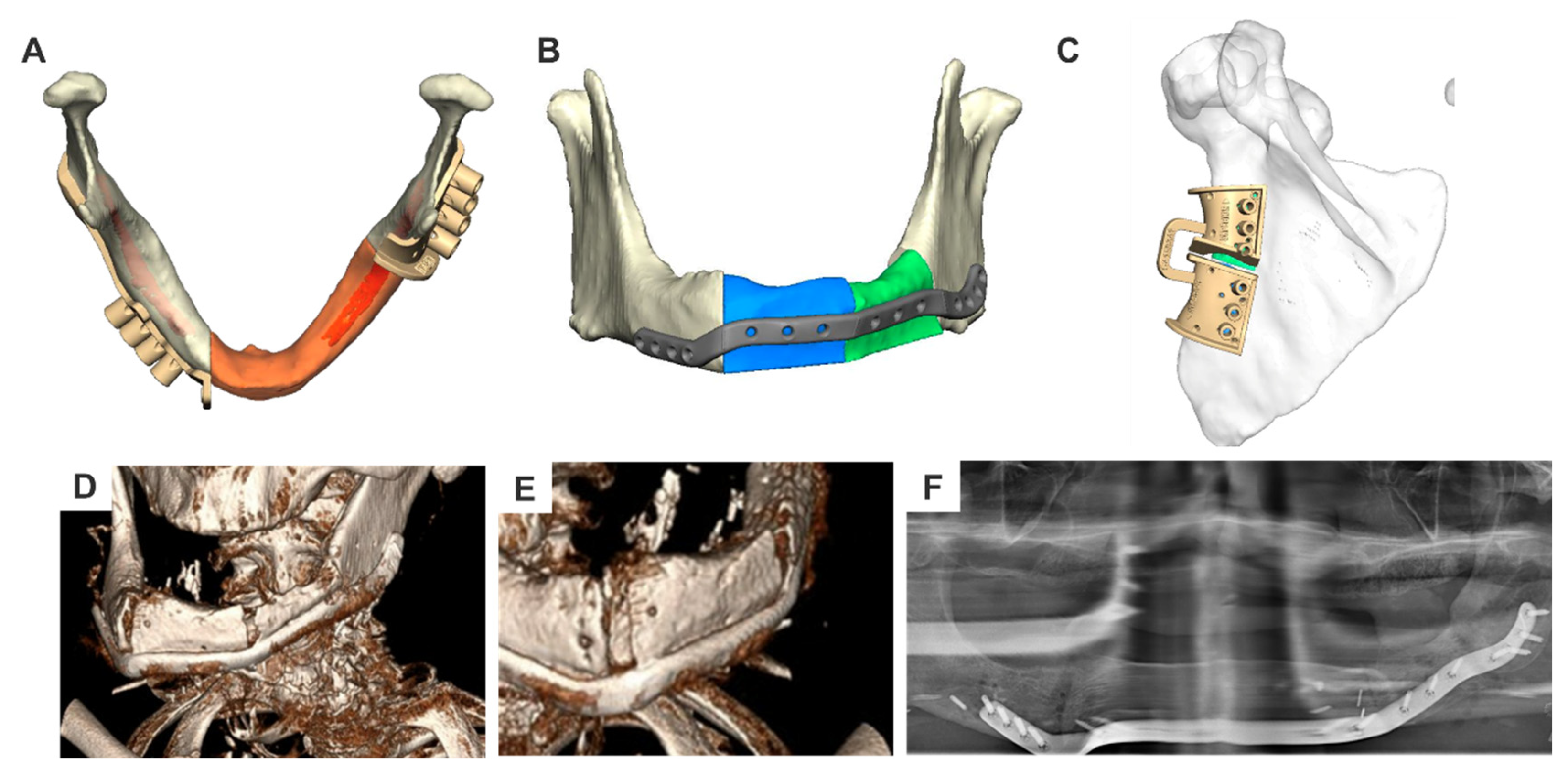
- Condylar plates or plate extensions with a condylar attachment:Condylar plates are useful when the pathological resection status is unclear or the disease prognosis is uncertain.
- Indications: Primarily considered as a temporary solution, condylar plates may be used intraoperatively if tumor infiltration requires resection of the condyle. In such cases, patient-specific solutions are often not feasible.
- Preoperative Planning: If identified preoperatively, condylar plates can be designed using 3D planning and CAD/CAM production. The condyle shape may be derived from the following:
- o
- The patient’s resected condyle (if unaffected by pathology).
- o
- A mirrored condyle from the opposite side.
- o
- A standardized condyle model.
- Production: The manufacturing process mirrors that of reconstruction plates. Ready-made condylar add-ons can also be used with patient-specific plates for added versatility.
- 3.
- Total temporomandibular joint replacement:If temporomandibular joint replacement is planned as a permanent solution, the use of a total joint implant is recommended. The components of the condylar fossa, the condylar head, and support plates for fixation are custom-made and manufactured with different materials adapted to the necessary requirements, such as titanium, cobalt-chromium-molybdenum, and ultra-high molecular weight polyethylene. The use of “metal-on-metal temporomandibular joint prostheses” should be avoided due to evidence indicating increased wear rates. Currently, manufacturing is time-consuming and expensive, and only a few recognized manufacturers offer a fully digital solution. The surgical procedure is complex and is typically reserved for experienced surgeons to minimize the risk of serious complications. Intraoperative navigation can be employed to support position control during implantation [11].
- 4.
- Free-ending revascularized bone grafts:Free-ending revascularized bone grafts are indicated for long-distance defects with loss of the condyle, condylar process, ascending ramus of the mandible, mandibular angle region, and the corpus. They are particularly suitable for patients both before and after radiation, as well as after the loss of alloplastic endoprostheses. The grafts must be designed to ensure that a free end of the graft replaces the condylar portion and adequately supports the posterior height of the lower jaw. Virtual 3D planning, followed by production of cutting and drilling guides for resection and flap rising, as well as reconstruction plates for fixation, greatly simplifies the approach. In addition to selecting the most suitable bone donor site (fibula, iliac crest, scapula), precise planning of the shape, size, the position of the vascular pedicle, and the production of patient-specific reconstruction plates are important to ensure optimal functional and aesthetic outcomes for the patient.
- c.
- Crib/Tray
2.5.3. Patient-Specific Implants—Cranium and Midface
| Implant Type | Use | Material | Advantages and Key Features | Risks and Considerations |
|---|---|---|---|---|
| Osteosynthesis Plates | Fixation of grafts/flaps in cranial and midfacial regions | Titanium, Mini-/Microplates |
|
|
| Orbital Plate | Reconstruction of orbital walls | (See AO Foundation White Paper) |
|
|
| Cranioplasty Implants/Meshes | Closing cranial bone defects | Titanium, PEEK, Polyethylene |
|
|
| Fixation Aids | Supporting epitheses, obturators, or dental prosthetics | Patient-specific Substructures |
|
|
- a.
- Osteosynthesis Plates for Graft/Flap FixationNumerous conventional plating systems are available for bony fixation in the cranial and midfacial areas. These plates are typically shaped, cut, and bent intraoperatively, which presents no technical challenges when using mini- or microplates. Screws of 4–6 mm are sufficient for most fixations.The advantage of patient-specific plates lies in their pre-planned design, which incorporates critical factors:
- Plate shape and position are optimized for bone contact.
- Vascular pedicles and supplying vessels are accounted for, especially in midface defect closures.
- Future dental implant positions can be anticipated and interference avoided.
- Pre-drilled holes in patient-specific plates allow simultaneous fixation and positioning of the graft, simplifying the procedure and reducing complications.
- b.
- Orbital PlateThe restoration of orbital wall structures is a well-established application of patient-specific 3D planning and implant manufacturing. Detailed information on orbital plates is available in a separate AO Foundation white paper [13].
- c.
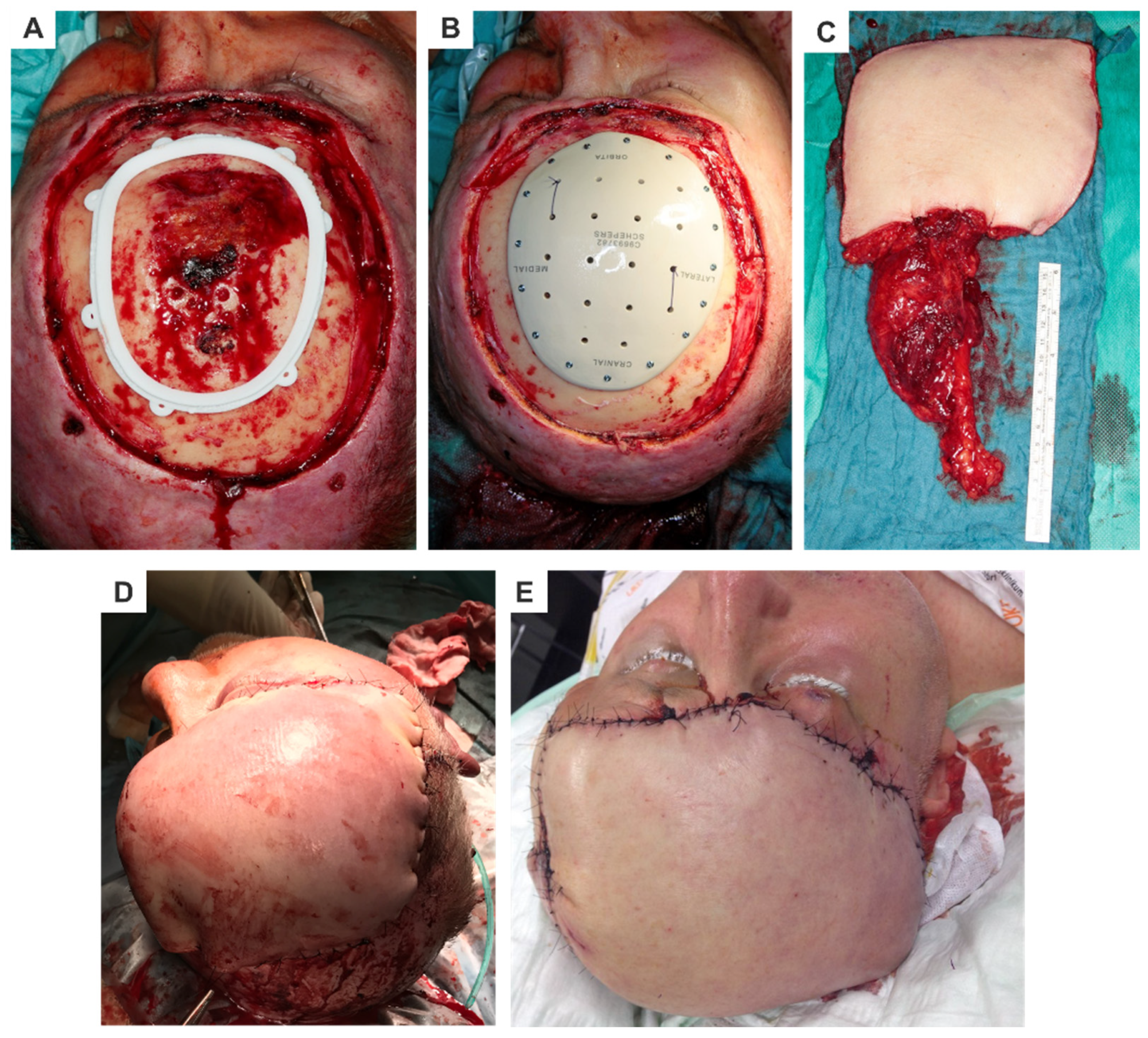
- Cranioplasty Implants and MeshesMaterials like titanium, polyethylene, and PEEK are commonly used for cranial reconstructions. These materials are sterilizable and can be CAD/CAM-processed or 3D-printed, enabling virtual planning and precise production. Cranioplasty implants are characterized by the following (Figure 3):
- Exceptional dimensional stability and accuracy.
- Pre-planned holes with optimized screw lengths and angles for fixation.
- d.
- Fixation Aids for Epitheses, Obturators, and Dental Prosthetics
2.5.4. Prototyping and Manufacturing
Subtractive Manufacturing (SM) Processes
- Applications: SM processes are primarily used in metal processing for producing titanium osteosynthesis plates and frameworks.
- Advantages: High precision and compatibility with standard industrial production.
- Challenges: High material consumption, as removed material cannot be reused directly. Prefabricated implants benefit from material-saving SM optimization, but similar efficiencies are not yet fully realized for patient-specific implants.
Additive Manufacturing (AM) Processes
- Polymerization (Stereolithography—SLA):Liquid photopolymers are hardened layer by layer using a laser beam. Common methods include the following:
- o
- Two-Photon Polymerization (2PP);
- o
- Polymer Jetting (Thermal Jet Printing);
- o
- Digital Light Processing (DLP).Applications: SLA techniques are primarily used for STL planning models and templates in reconstructive surgery.
- 2.
- Selective Laser Sintering (SLS)/Selective Laser Melting (SLM):These powder bed processes use a laser to melt metal or plastic powders selectively, based on a CAD model. Once solidified, the object is built layer by layer. SLM is particularly versatile and can process high-strength materials like titanium alloys (e.g., Ti6Al4V). Post-processing, such as heating, further optimizes the workpiece.Applications: Production of complex, patient-specific implants from metals or plastics.
- 3.
- Fused Layer Modeling (FLM):FLM involves extrusion of thermoplastic filaments through a heated nozzle, which deposits the material in dots or layers to form the structure. Applications: Rapid prototyping for models or stencils.
- 4.
- 3D Printing and Binder Jetting:A 3D printer uses a liquid binder to fuse powder layers, which are applied incrementally to form the workpiece. This method supports multi-color printing for enhanced visualization of anatomical structures like nerve canals or implants.
| Method | Process | Applications | Advantages | Challenges |
|---|---|---|---|---|
| Subtractive (SM) | Removal of material using CNC machines | Metal processing for plates and frameworks |
|
|
| Additive (AM) | Layering material (e.g., SLS, SLM, SLA) | Complex geometries and patient-specific implants |
|
|
| Binder Jetting (3D) | Binding powder layers with liquid adhesives | Detailed anatomical models |
|
|
Summary and Selection Criteria
- Material Requirements: SLS/SLM are preferred for high-strength metals, while SLA suits polymers.
- Workpiece Complexity: Additive methods handle intricate designs, while SM is ideal for simpler geometries.
- Desired Properties: Strength, biocompatibility, and aesthetic considerations influence the process selection.
2.6. Surgery
- Planning Documents: Ensure accessibility to planning documents via monitors or printouts in the operating room.
- Verification of Materials: Confirm that all implants, templates, and 3D models are complete, properly assigned to the patient, and sterilized preoperatively.
2.6.1. Approach
2.6.2. Recipient Vessels
2.6.3. Resection
2.6.4. Reconstruction
2.7. Accuracy Assessment
3. Evaluation
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagner, M.; Gander, T.; Blumer, M.; Valdec, S.; Schumann, P.; Essig, H.; Rücker, M. Die CAD/CAM-Revolution in der kraniofazialen Rekonstruktion. Praxis 2019, 108, 321–328. [Google Scholar] [CrossRef]
- Eibling, D.; Fried, M.; Blitzer, A.; Postma, G. Commentary on the role of expert opinion in developing evidence-based guidelines. Laryngoscope 2014, 124, 355–357. [Google Scholar] [CrossRef]
- Wenger, D.R. Limitations of evidence-based medicine: The role of experience and expert opinion. J. Pediatr. Orthop. 2012, 32 (Suppl. 2), S187–S192. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Zagalo, C.M.; Oliveira, M.L.; Correia, A.M.; Reis, A.R. Mandible reconstruction: History, state of the art and persistent problems. Prosthet. Orthot. Int. 2015, 39, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, Y.-C.; Kim, S.-G.; Garagiola, U. Advancements in Oral Maxillofacial Surgery: A Comprehensive Review on 3D Printing and Virtual Surgical Planning. Appl. Sci. 2023, 13, 9907. [Google Scholar] [CrossRef]
- Zoabi, A.; Redenski, I.; Oren, D.; Kasem, A.; Zigron, A.; Daoud, S.; Moskovich, L.; Kablan, F.; Srouji, S. 3D Printing and Virtual Surgical Planning in Oral and Maxillofacial Surgery. J. Clin. Med. 2022, 11, 2385. [Google Scholar] [CrossRef] [PubMed]
- Maglitto, F.; Dell’Aversana Orabona, G.; Committeri, U.; Salzano, G.; De Fazio, G.R.; Vaira, L.A.; Abbate, V.; Bonavolontà, P.; Piombino, P.; Califano, L. Virtual Surgical Planning and the “In-House” Rapid Prototyping Technique in Maxillofacial Surgery: The Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 1009. [Google Scholar] [CrossRef]
- Xi, T.; Baan, F.; Verhulst, A.C.; Liebregts, J.H. FInnovative methods and developments in oral care. 3D planning in oral and maxillofacial surgery. Ned. Tijdschr. Tandheelkd. 2023, 130, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.A.; Metzger, M.; Ehrenfeld, M.; Cornelius, C.P. Computer-Assisted Designed and Manufactured Procedures Facilitate the Lingual Application of Mandible Reconstruction Plates. J. Oral Maxillofac. Surg. 2016, 74, 1879–1895. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.P.; Giessler, G.A.; Wilde, F.; Metzger, M.C.; Mast, G.; Probst, F.A. Iterations of computer- and template assisted mandibular or maxillary reconstruction with free flaps containing the lateral scapular border—Evolution of a biplanar plug-on cutting guide. J. Craniomaxillofac. Surg. 2016, 44, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.P.; Ehrenfeld, M.; Mast, G.; Driemel, O.; Westermark, A.C.L. Combination of Temporo Mandibular Joint Replacement and Reconstructions in Mandibular Body or Zygoma Region with Extended, Custom-Made Endoprostheses. OP-Journal 2013, 29, 184–199. [Google Scholar]
- Marx, R.E. Mandibular reconstruction. J. Oral Maxillofac. Surg. 1993, 51, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.-C.; Gellrich, N.-C.; Grant, M.; Grant, M.; Matic, D.; Matic, D.; Korn, P.; Korn, P. Guidelines for Orbital Defect Assessment and Patient-Specific Implant Design: Introducing OA2 (Orbital Assessment Algorithm). Craniomaxillofacial Trauma Reconstr. 2024, 17, 298–318. [Google Scholar] [CrossRef]
- Müller, A.; Krishnan, K.G.; Uhl, E.; Mast, G. The application of rapid prototyping techniques in cranial reconstruction and preoperative planning in neurosurgery. J. Craniofac. Surg. 2003, 14, 899–914. [Google Scholar] [CrossRef]
- Ziegener, F. Stereolithografie—Lasersintern—Laserschmelzen—FLM. In Was Kann Additive Fertigung?—Alle Verfahren im Überblick; Konradin-Verlag Robert Kohlhammer: Leinfelden-Echterdingen, Germany, 2020. [Google Scholar]
- Rajasekhar, G.; Vura, N.G.; Sudhir, R.; Dhanala, S.; Alwala, A.M. Versatility of Dieffenbach’s Modification of Weber Fergusson’s Approach for Treatment of Maxillary Pathologies. J. Maxillofac. Oral Surg. 2012, 11, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Jütte, H.; Tannapfel, A. Intraoperativer Schnellschnitt—Wann sinnvoll, wann notwendig? Der. Chirurg. 2020, 91, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Seruya, M.; Fisher, M.; Rodriguez, E.D. Computer-assisted versus conventional free fibula flap technique for craniofacial reconstruction: An outcomes comparison. Plast. Reconstr. Surg. 2013, 132, 1219–1228. [Google Scholar] [CrossRef]
- Wilde, F.; Hanken, H.; Probst, F.; Schramm, A.; Heiland, M.; Cornelius, C.P. Multicenter study on the use of patient-specific CAD/CAM reconstruction plates for mandibular reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 2035–2051. [Google Scholar] [CrossRef] [PubMed]
- Liokatis, P.; Malenova, Y.; Fegg, F.N.; Haidari, S.; Probst, M.; Boskov, M.; Cornelius, C.-P.; Troeltzsch, M.; Probst, F.A. Digital planning and individual implants for secondary reconstruction of midfacial deformities: A pilot study. Laryngoscope Investig. Otolaryngol. 2022, 7, 369–379. [Google Scholar] [CrossRef] [PubMed]
| Reconstruction Method | Region | Material/Technique | Key Considerations |
|---|---|---|---|
| Reconstruction without bone | Cranium |
|
|
| Midface |
|
| |
| Mandible |
|
| |
| Reconstruction with bone | Cranium |
|
|
| Midface/Mandible |
|
| |
| Fibula |
|
| |
| DCIA Flap |
|
| |
| Scapula |
|
|
| Category | Details |
|---|---|
| Advantages | |
| Visualization |
|
| Accessibility |
|
| Data Processing |
|
| Surgical Simulation |
|
| Precision Manufacturing |
|
| Safety and Control |
|
| Time Efficiency |
|
| Results Optimization |
|
| Interdisciplinary Use |
|
| Education and Documentation |
|
| Disadvantages | |
| Costs |
|
| Dependencies |
|
| Planning Gaps |
|
| Complexity |
|
| Time Constraints |
|
| Radiation Exposure |
|
| Expertise Distribution |
|
| Future Developments | |
| Technological Innovation |
|
| Cost Reduction |
|
| Improved Data Integration |
|
| Radiation-Free Solutions |
|
| Data Protection |
|
| Outcome Tracking |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license https://creativecommons.org/licenses/by/4.0/.
Share and Cite
Rana, M.; Buchbinder, D.; Aniceto, G.S.; Mast, G. Patient-Specific Solutions for Cranial, Midface, and Mandible Reconstruction Following Ablative Surgery: Expert Opinion and a Consensus on the Guidelines and Workflow. Craniomaxillofac. Trauma Reconstr. 2025, 18, 15. https://doi.org/10.3390/cmtr18010015
Rana M, Buchbinder D, Aniceto GS, Mast G. Patient-Specific Solutions for Cranial, Midface, and Mandible Reconstruction Following Ablative Surgery: Expert Opinion and a Consensus on the Guidelines and Workflow. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(1):15. https://doi.org/10.3390/cmtr18010015
Chicago/Turabian StyleRana, Majeed, Daniel Buchbinder, Gregorio Sánchez Aniceto, and Gerson Mast. 2025. "Patient-Specific Solutions for Cranial, Midface, and Mandible Reconstruction Following Ablative Surgery: Expert Opinion and a Consensus on the Guidelines and Workflow" Craniomaxillofacial Trauma & Reconstruction 18, no. 1: 15. https://doi.org/10.3390/cmtr18010015
APA StyleRana, M., Buchbinder, D., Aniceto, G. S., & Mast, G. (2025). Patient-Specific Solutions for Cranial, Midface, and Mandible Reconstruction Following Ablative Surgery: Expert Opinion and a Consensus on the Guidelines and Workflow. Craniomaxillofacial Trauma & Reconstruction, 18(1), 15. https://doi.org/10.3390/cmtr18010015






